1. Introduction
Soil treatments with non-repellent slow-acting liquid termiticides have been the dominant method of subterranean termite treatment [
1]. Although termite baiting systems are typically aimed to suppress or eliminate termite colony populations from an area, the success of termite baits is often unpredictable as termites may not find and forage on the baits but may still find their way into the structure needing protection. Our earlier study [
2] showed that a spot treatment of soil with a liquid and dust formulation of fipronil effectively killed termites that were not in the treatment site, suggesting that this termiticide is transferred among the members of the foraging population.
Although subterranean termites live underground by nature, construction of above-ground nests is not uncommon for
Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae), but it is for native subterranean termites [
3]. Typically Formosan subterranean termites will move from the soil carton nest to above-ground nests in a home or a tree, maintaining satellite nests connected via shelter tubes both above and below ground. True aerial nests, defined as an above-ground nest that has no connection to the ground [
4], are less common. Aerial nests are most commonly the result of a connection break from the ground colony caused by a disturbance. Aerial colonies can also begin from the winged king and queen starting an aerial nest without ever having been in contact with the ground, or by worker termites bringing the queen and king to an above-ground site before breaking ground contact [
4,
5]. However, determining that the colony never had ground contact is not usually possible and only anecdotal estimates of such events are provided in the literature.
Control of termites in above-ground nests is very challenging, as it is difficult to find them for treatment. The options that became available in the mid-1990s were to treat the soil with either bait, or a slow-acting non-repellent termiticide with the likelihood of toxicant transfer through trophallaxis, mutual grooming or both. Previous studies focused on this aspect have had mixed results. Some studies [
6,
7,
8,
9,
10] have reported success in suppressing termite populations well beyond the treated areas, while some [
11,
12,
13] cast doubt on the long-distance impact of these termiticides. Despite differences in interpretation, it is established that slow-acting non-repellent termiticides have an impact beyond the treated site. No documented information, however, is available that
C. formosanus in above-ground nests will not be impacted by a ground soil treatment with a non-repellent termiticide. The aim of this study is to determine the effects of ground-applied termiticides (fipronil, imidacloprid and chlorantraniliprole) on the above-ground foraging behavior of
C. formosanus in artificial arenas in the laboratory.
2. Materials and Methods
Commercial formulations of three termiticides, fipronil (Termidor
® SC, 9.1% active ingredient (a.i.), BASF Corp., Ludwigshafen, Germany), imidacloprid (Premise
® 75 WP, 75% a.i., Bayer, Leverkusen, Germany) and chlorantraniliprole (Altriset™, 18.4% a.i., previously DuPont and now Syngenta, Basel, Switzerland) were tested in this study. These termiticides were already available in the lab and had been used for other experiments. Foraging groups of
Coptotermes formosanus Shiraki were collected in October 2014, from Brechtel Park, New Orleans, LA, USA using milk crate traps [
14]. In short, milk crates loaded with wood sticks arranged in lattice structure were placed in a known termite infested area and checked after two months. The infested crates were retrieved and brought to the lab and held in trash cans. Water was added when necessary to maintain the wood moisture and high relative humidity inside the cans. One to two days prior to the bioassays, termites were dislodged from the wood and collected in a clean container lined with moist paper towels. Termites collected from a single colony were used for this study.
The bioassay arena consisted of two foraging sites, a bottom site and top site, connected by a 21 cm wooden ramp so that termites could readily move from one site to another (
Figure 1). To prepare the top foraging site, a round clear acrylic container (11 cm diameter × 4 cm high, Pioneer Plastics
©, North Dixon, KY, USA) with a hole on bottom one side was lined with a single layer of filter paper. The container was partly filled with autoclaved sand (~400 g) and distilled water was added to make the moisture content ~10%. A block of
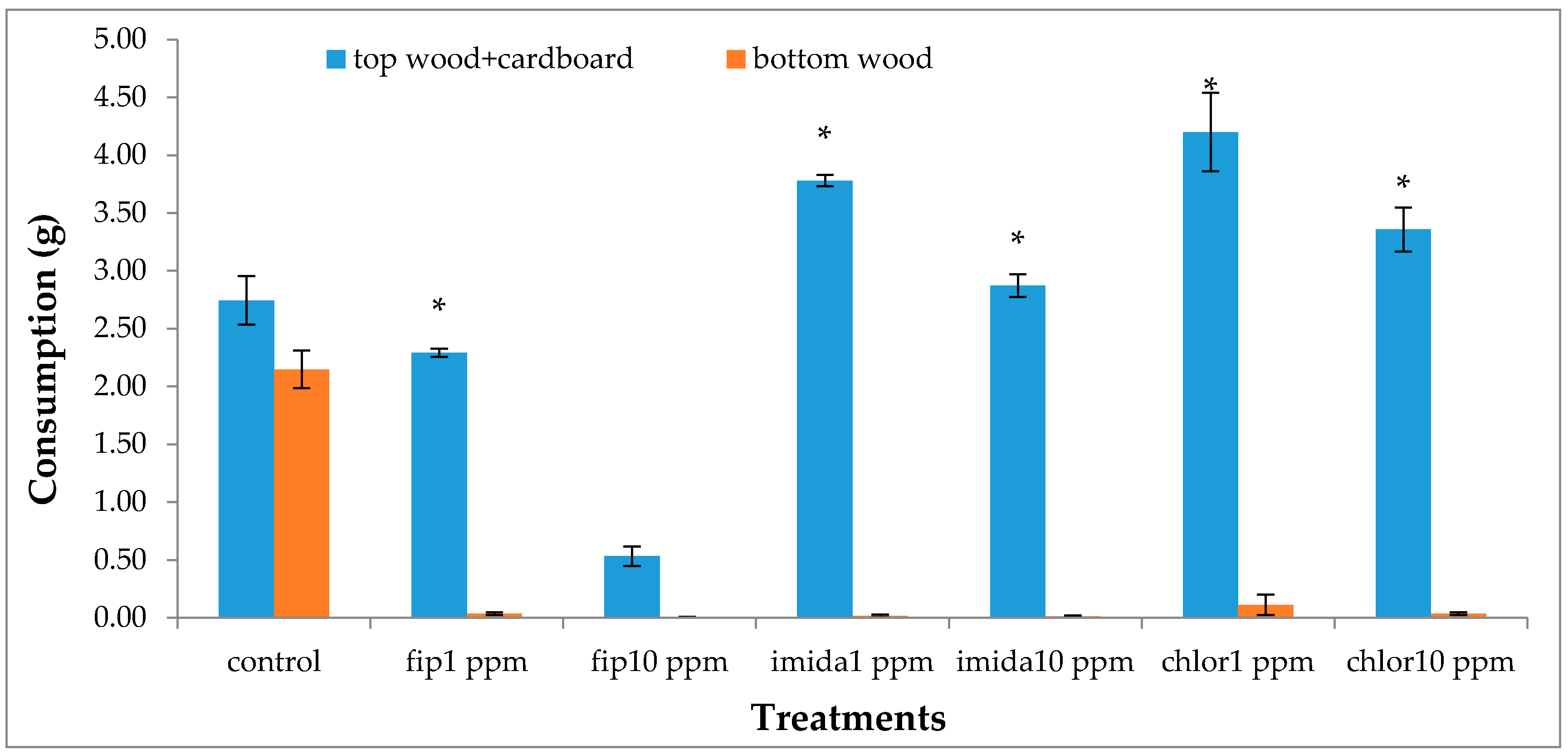
Pinus wood (3 cm × 4 cm× 1 cm) followed by a disc of corrugated cardboard, both autoclaved and weighed, were placed on sand surface. The total amount of food materials provided (top wood, top cardboard, ramp and bottom wood) was identical (≈30 g) for all the bioassay arenas. The cardboard was moistened by adding 2 mL of distilled water. A small hole was made on the lid of the top container to facilitate in adding water whenever deemed necessary.
To prepare the bottom foraging site, a tall round clear acrylic container (11 cm diameter × 21 cm high, Pioneer Plastics©, North Dixon, KY, USA) was partly filled with ~400 g of autoclaved treated sand and a wood block of similar size as on the top container was placed on the sand surface. Three chemicals each with two concentrations, 1 ppm and 10 ppm were tested. To treat the sand, first autoclaved sand was held in a sealable plastic bag and the required amount of chemical mixed with distilled water (the amount of distilled water was calculated to make the moisture content of sand 10%) was added. The bag was then sealed and kneaded thoroughly to mix the chemicals uniformly in the substrate. Controls received moist sand with no added termiticide. A small hole was made about the middle height on one side of the container to add water when necessary. A slit was made on the lid in a way that it was aligned with the hole of the top container. A 21-cm-long wooden stake was used as a ramp connecting the top and the bottom foraging sites. One end of the stake was placed on the surface of the bottom substrate and the other end was inserted through the hole to reach the substrate on the top container so that the ramp connects at an angle of ~60°.
Fourteen hundred termites (in their collected worker/soldier/nymph ratio) were introduced on the top container and the lid placed back on. Three replications were prepared for each treatment and control, for a total of 21 bioassay arenas and ~30,000 termites in this study. The test arenas were placed undisturbed in the lab at 23 ± 2 °C. All the bioassays ran for one month, unless mortality appeared to be 100% prior to that date.
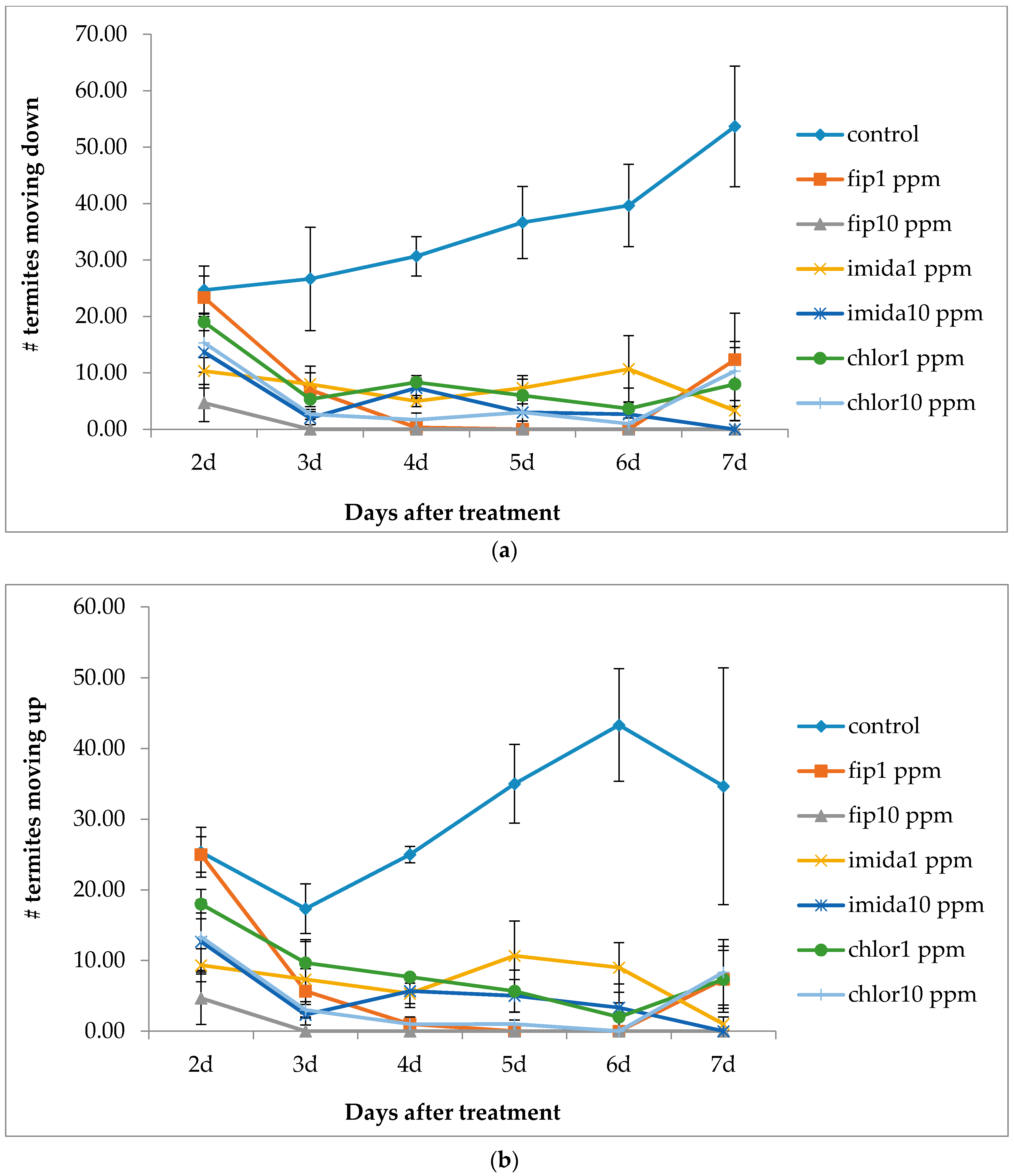
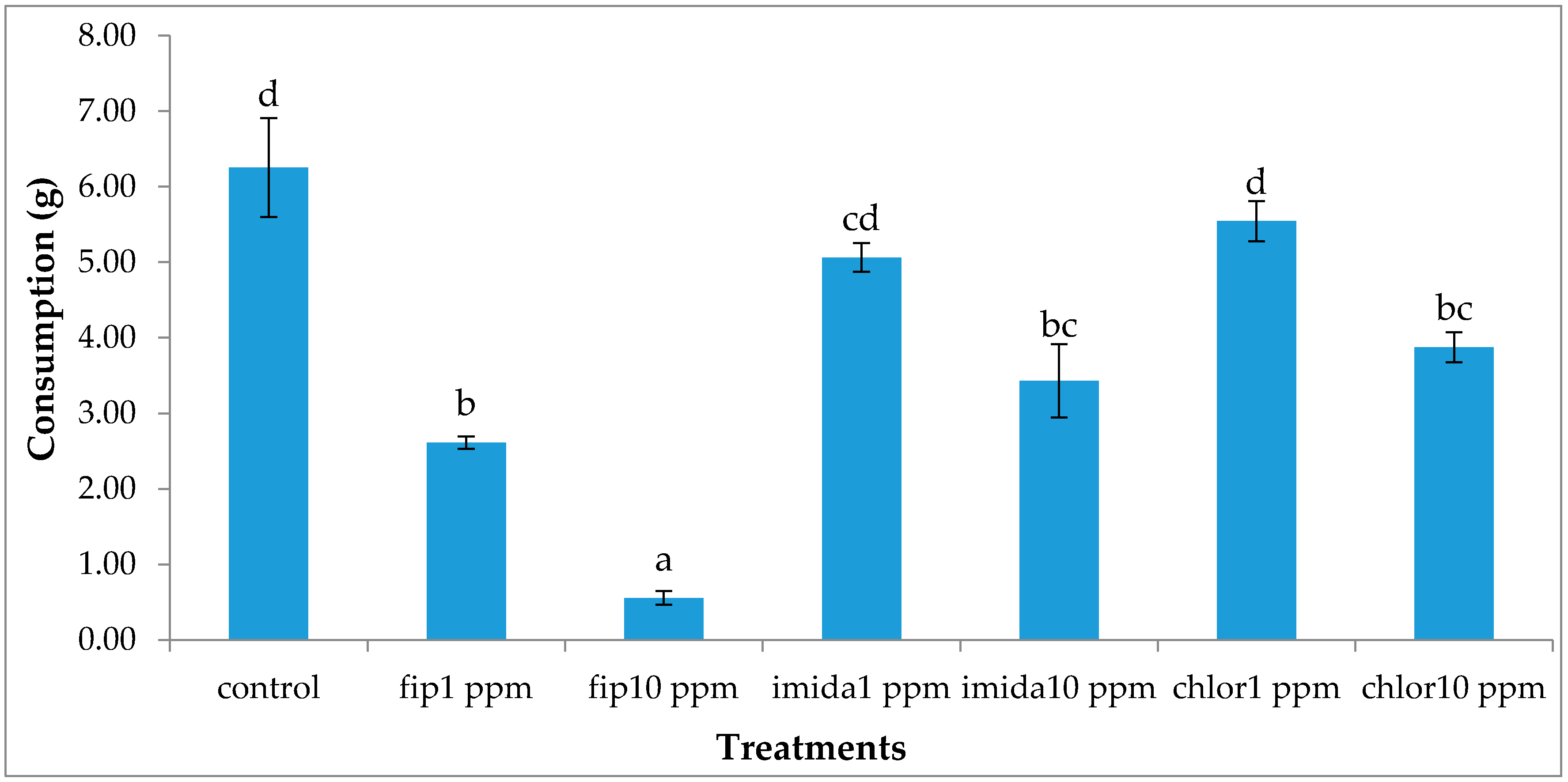
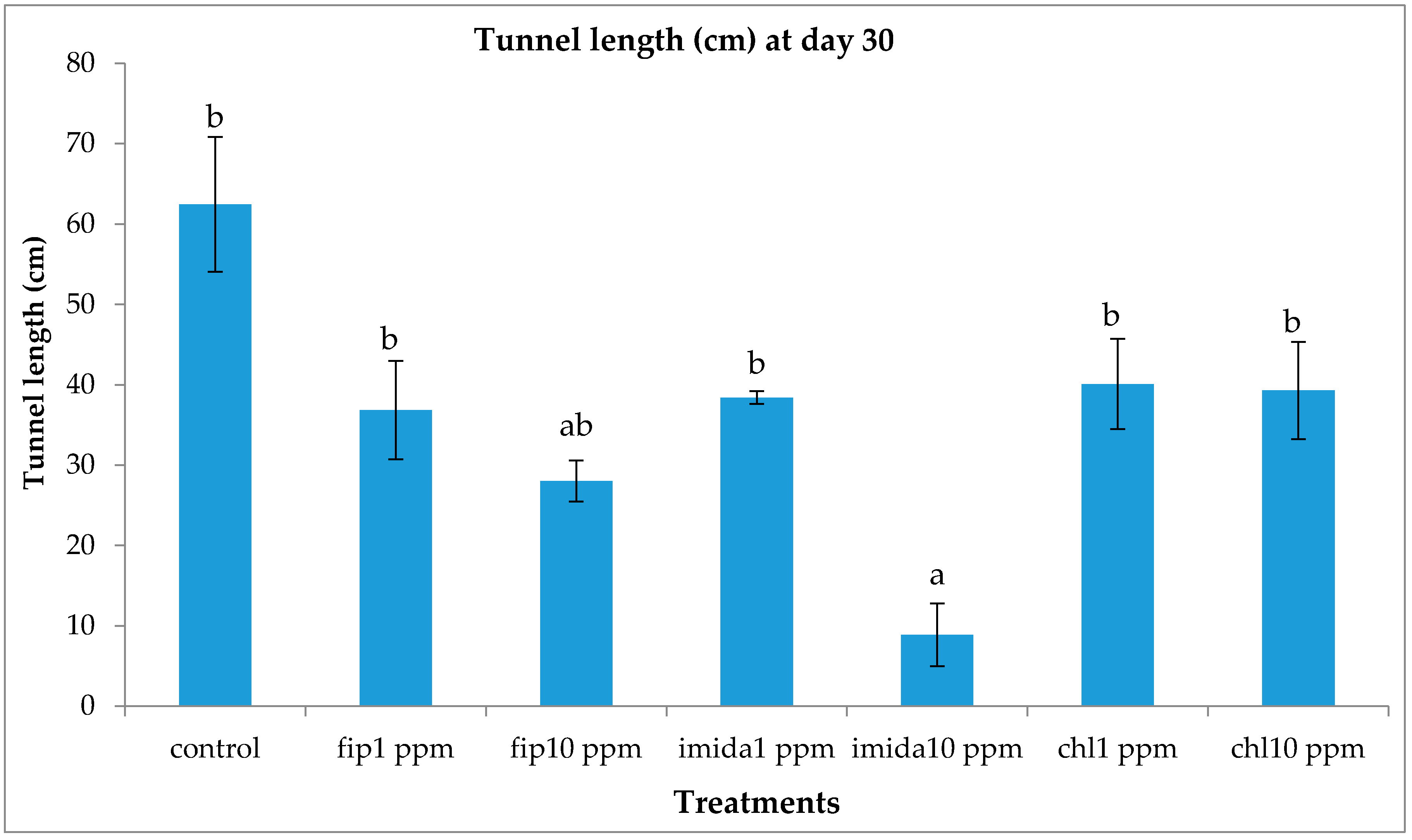
Termite movement between the top and the bottom foraging sites was recorded. For this, the number of termites making upward and downward movement in a 5 min period was recorded every day from day 2 to day 7 and then at day 20 and day 30. The bottom side of the bottom foraging container was scanned at day 2, day 7 and day 30 to measure progress in tunneling over time if any was present. During the test period, notable observations like aggregation of termites and fungus growth on dead termites were recorded. After one month, we measured the length of tunnels (using a scanner) in the lower end of the bottom foraging site, dismantled arenas and counted live termites in all sections of the arenas. Wood and cardboard were cleaned and dried to calculate the consumption.
Data analysis was done using Proc GLM in SAS 9.3. (SAS Institute Inc., Cary, NC, USA) Mortality data were arcsine of the square root transformed to improve normality for the analysis. Termite movement observed for 5 min period on the ramp each day was analyzed separately and compared among the treatments for the same day. Post-ANOVA means comparison was done using Tukey’s honestly significant difference at α = 0.05.
4. Discussion
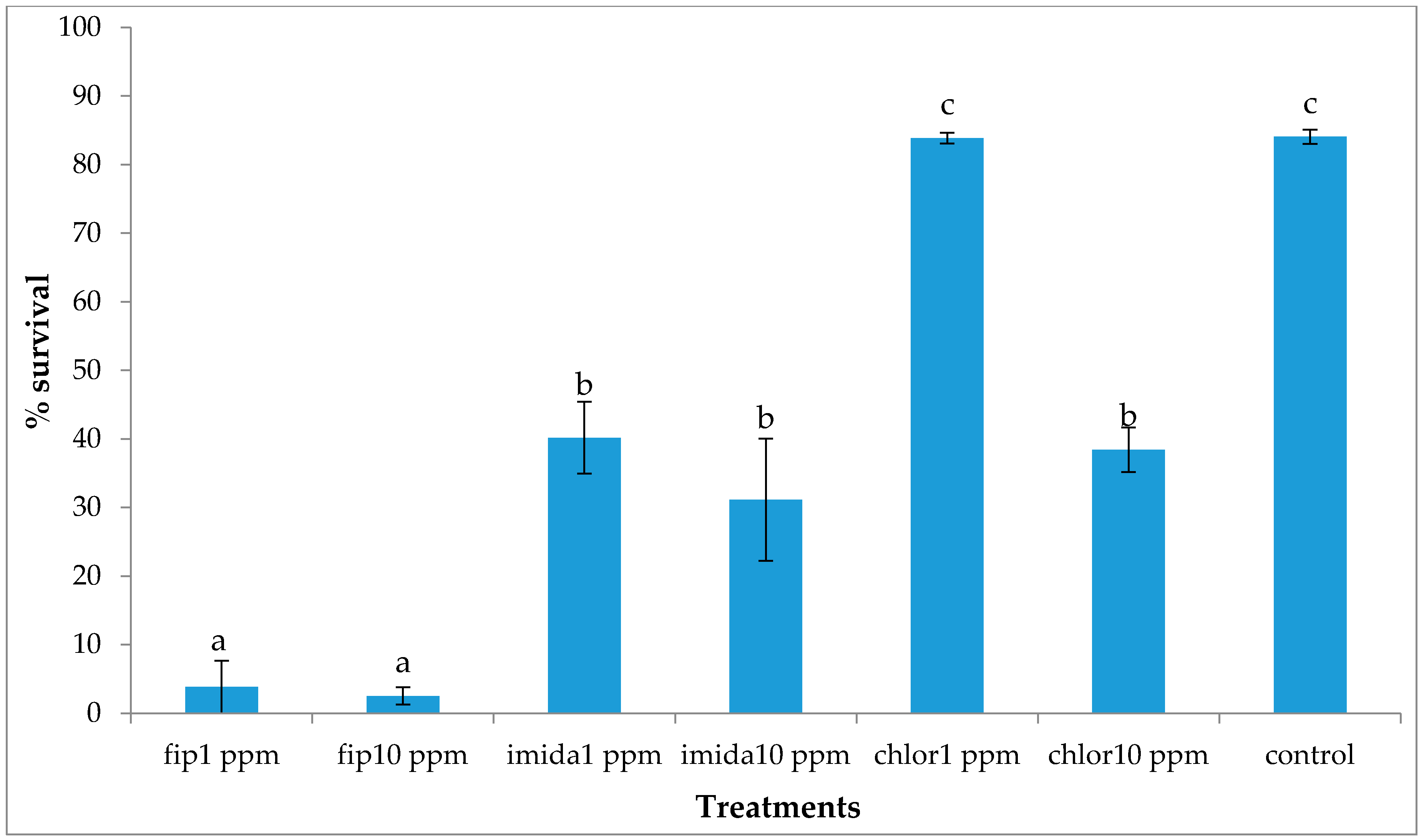
In the present study we found that all colony units attempted to make ground contact despite the treatments that were in place. Most treatments caused repellency prior to colony death and only fipronil was able to induce a high mortality to an above-ground colony making ground contact.
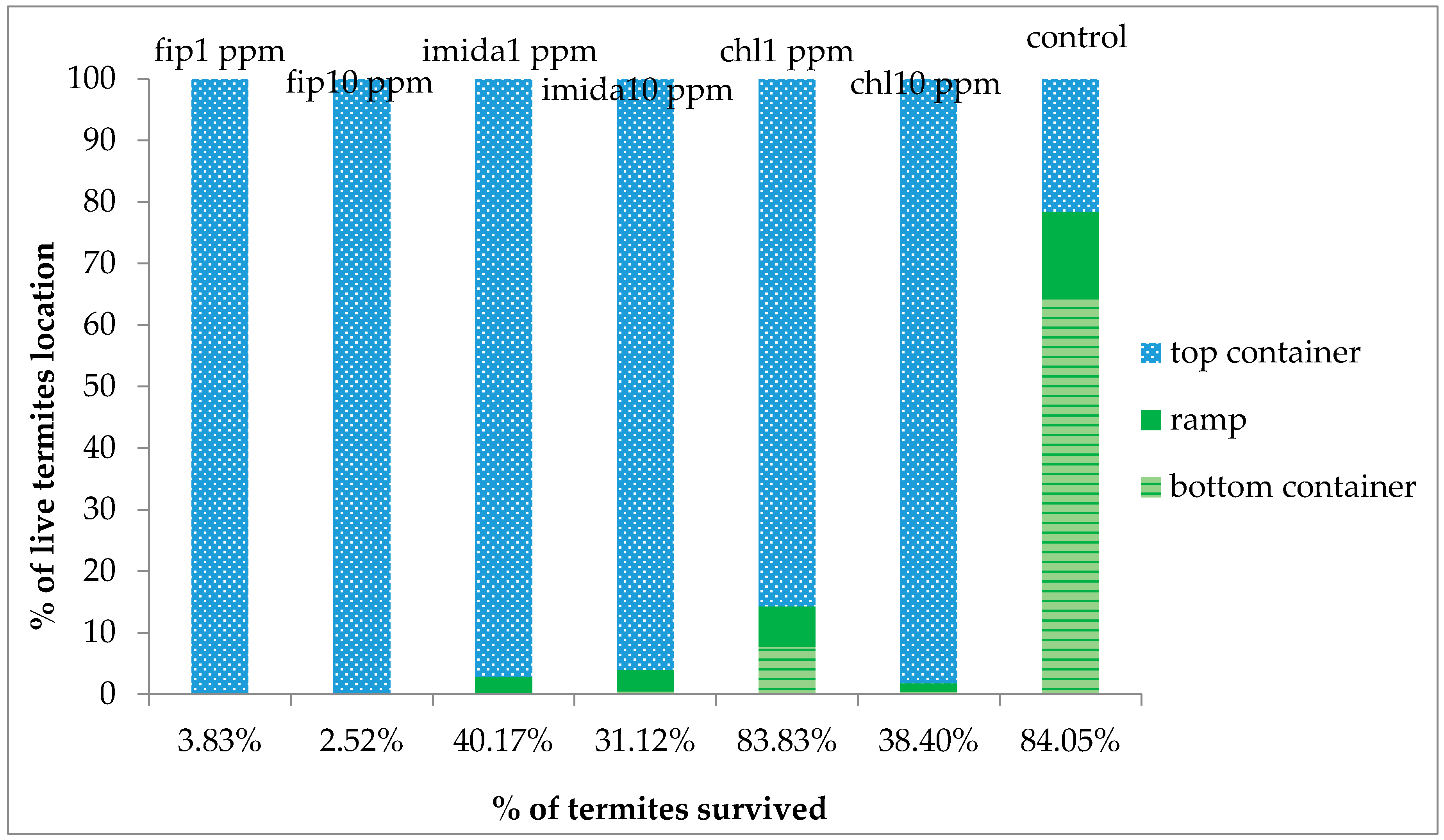
The delayed action property of termiticides is usually considered an advantage over fast action as it allows termites to live longer, thereby allowing more time to interact with other nestmates in the colony. However, based on our results, the comparatively faster action of fipronil (comparison made within the same concentration) may be desirable in some situations as termites would be killed before they get a chance to avoid the piles of dead nestmates, usually covered with fungus. Even with the relatively fast action of fipronil, a small proportion of termites were alive at the end of the experiment and looked healthy and active. These live termites were living at the bottom of the top substrate, apparently avoiding the area where dead termites were present and fungus growth was observed, suggesting that C. formosanus manages to avoid the mortality-causing factors. It was likely that the termites that were not dead at the end of the one-month tests would have survived normally had we extended the test period.
The lower concentration of the chlorantraniliprole (1 ppm) treatment did not cause significant mortality in termites and termite activities, such as shelter tube construction on ramps, were unaffected. Previous studies have reported that chlorantraniliprole has a more delayed action compared to fipronil or imidacloprid [
15,
16]. At 10 ppm, however, chlorantraniliprole had a similar mortality as imidacloprid. Buczkowski et al. [
17] reported that 5 ppm chlorantraniliprole treatments caused significant mortality in donors but not in the recipients, suggesting that the chemical has a dose-dependent toxicity and delayed action.
For all three chemicals tested, a proportion of the released termites were alive at the end of the experiment and they looked healthy and active. The live termites were all on the top foraging sites, except a small proportion that was retrieved from the upper part of the ramps. None were collected from the bottom foraging sites which had the treated substrate. We suspect that different impacts may be seen between termites traveling a vertical distance versus a horizontal distance. Previous researchers [
10,
12,
13] conducted laboratory studies to test the mortality of termites beyond the treated site based on horizontal distance alone. There are no reports of using vertical distance measures. Su [
12] reported the clogging/blockage of tunnels by dead termites, thus severing the contact after a certain percentage of termites were dead. There was no clogging/blockage in our study as no shelter tubes were constructed on ramps in the treatments except in the 1 ppm chlorantraniliprole treatment. However, the number of termites moving down the ramp was fewer and fewer as the days passed by and almost no termite movement was observed at or after 20 days of treatment. We suggest that the cessation of termite movement to the bottom container was due to the repellent effects from dead termites covered with fungus.
Imidacloprid treatments seemed to show an immediate effect on termite behavior, especially at 10 ppm, as the tunneling on the bottom foraging substrate was significantly lower compared to other termiticide treatments. Interestingly, while termite mortality at this treatment was slower compared to the fipronil treatments (1 or 10 ppm), they almost immediately aggregated and stopped activities such as movement or tunnel construction. Although such an aggregation on the bottom container was observed in the 10 ppm chlorantraniliprole treatments also, some termites were observed digging into the substrate, apparently engaging in tunnel construction. The finding that 10 ppm imidacloprid treatment impacted termite activity quickly is consistent with the finding by the author of [
18] who reported that imidacloprid treatments bring about an immediate change in
C. formosanus behavior.