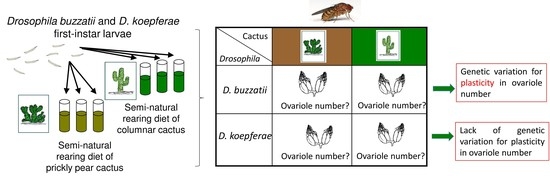
Contrasting Plasticity in Ovariole Number Induced by A Dietary Effect of the Host Plants between Cactophilic Drosophila Species
Abstract
:1. Introduction
2. Experimental Section
2.1. Fly Collections and Stock Maintenance
2.2. Oviposition Preferences of D. buzzatii and D. koepferae Isofemale Lines
2.3. Effect of Semi-Natural Diets on Ovariole Number
2.3.1. Semi-Natural Diets
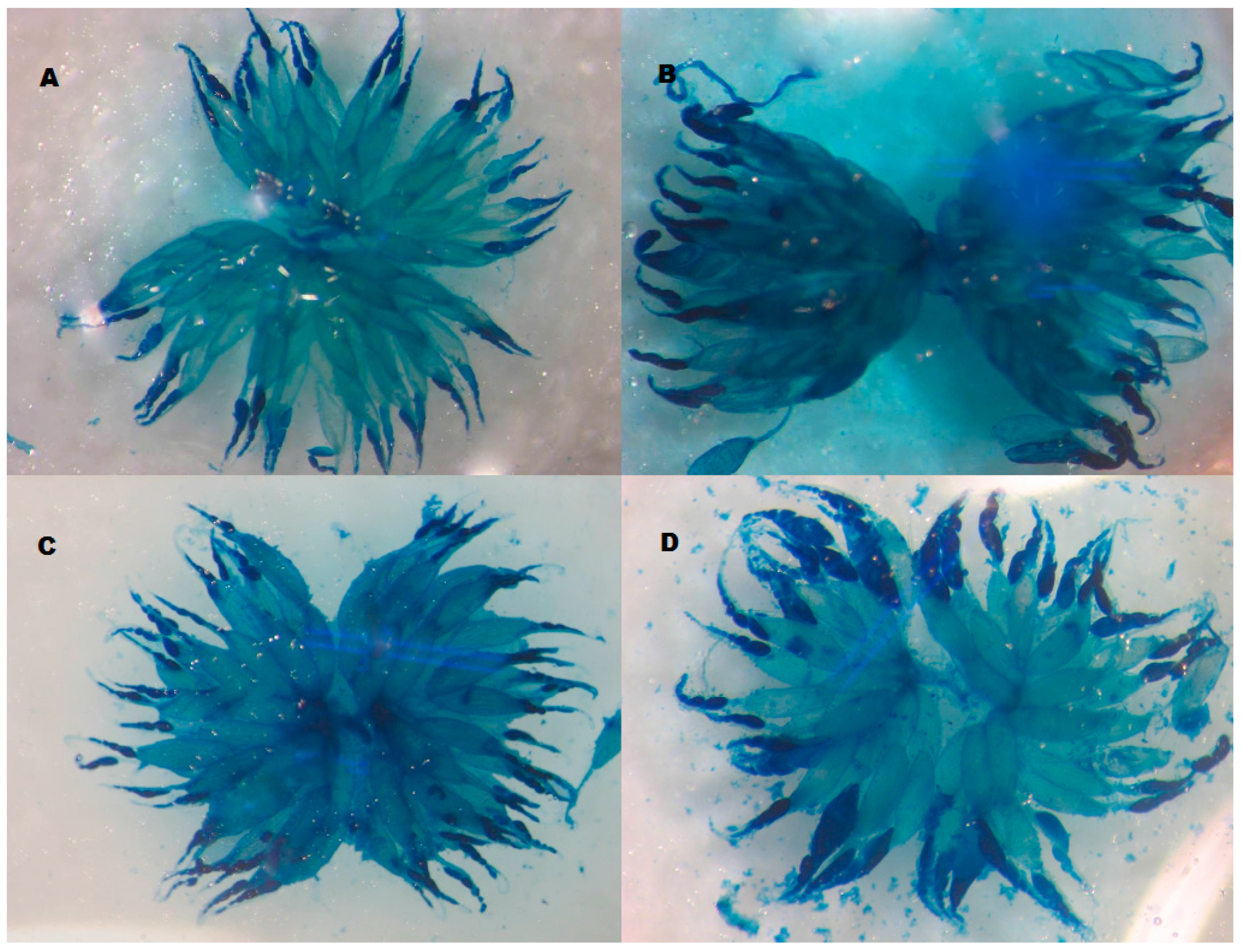
2.3.2. Ovariole Number
2.3.3. Wing Length
2.4. Plasticity Index
2.5. Effect of A Standard Diet (with No Cactus Additive) on Ovariole Number
2.6. Effect of Natural Substrates on Ovariole Number
2.7. Statistical Analysis
3. Results
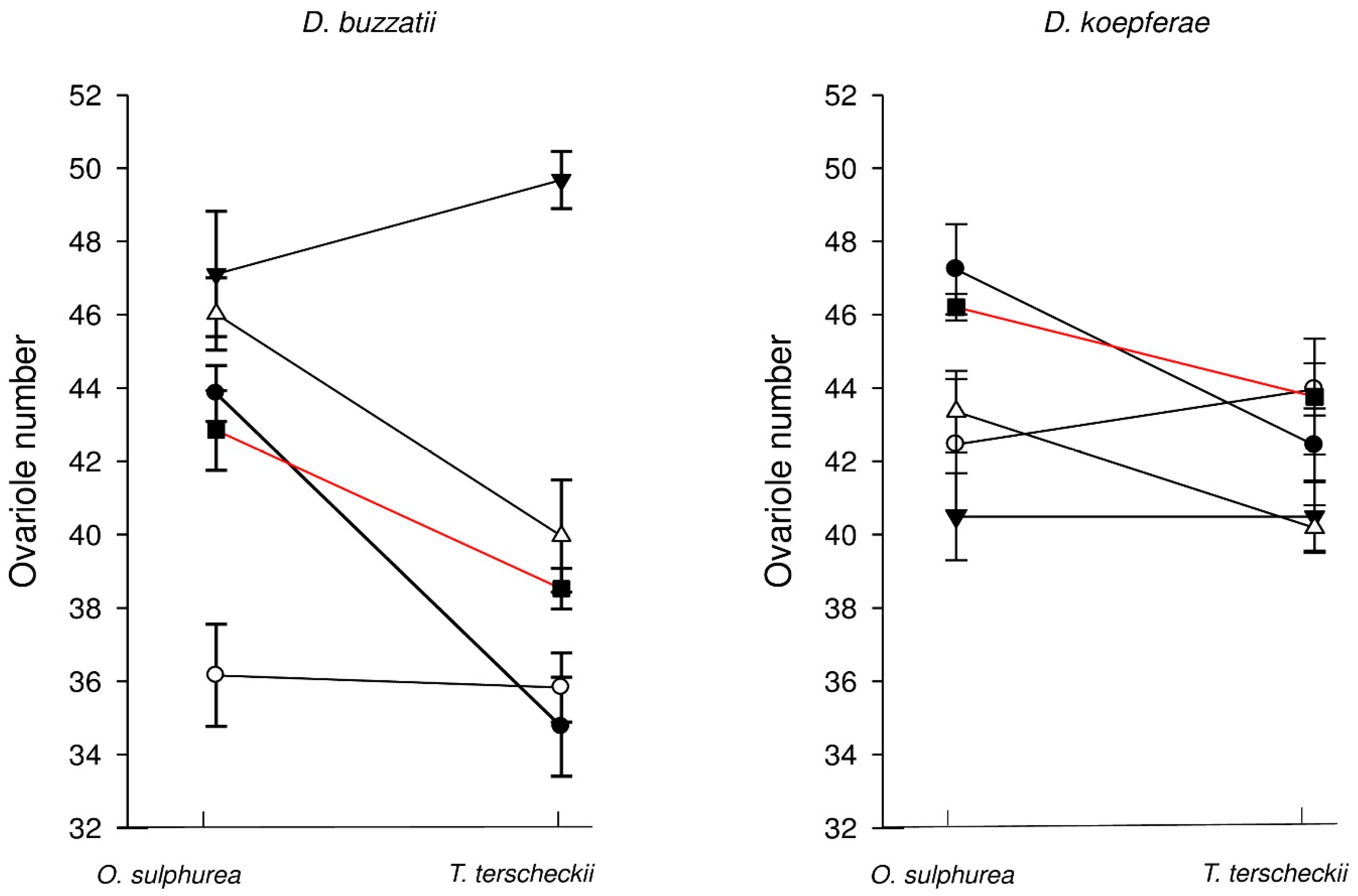
3.1. Effect of Semi-Natural Diets on Ovariole Number
3.2. Effect of Standard Diet (with No Cactus Additive) on Ovariole Number
3.3. Effect of Natural Substrates on Ovariole Number
4. Discussion
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Kambysellis, M.P.; Heed, W.B. Studies of oogenesis in natural populations of Drosophilidae. I. Relation of ovarian development and ecological habitats of the Hawaiian species. Am. Nat. 1971, 105, 31–49. [Google Scholar] [CrossRef]
- Kambysellis, M.P. Comparative studies of oogenesis and egg morphology among species of the genus Drosophila. Univ. Tex. Publ. Stud. Genet. 1968, 4, 71–92. [Google Scholar]
- Markow, T. Evolution of Drosophila mating systems. Evol. Biol. 1996, 29, 73–106. [Google Scholar]
- Lavista-Llanos, S.; Svatoš, A.; Kai, M.; Riemensperger, T.; Birman, S.; Stensmyr, M.C.; Hansson, B.S. Dopamine drives Drosophila sechellia adaptation to its toxic host. Elife 2014. [Google Scholar] [CrossRef] [PubMed]
- Faille, A.; Pluot-Sigwalt, D. Convergent Reduction of Ovariole Number Associated with Subterranean Life in Beetles. PLoS ONE 2015, 10, e0131986. [Google Scholar] [CrossRef] [PubMed]
- Delpuech, J.M.; Moreteau, B.; Chiche, J.; Pla, E.; Vouidibio, J.; David, J.R. Phenotypic plasticity and reaction norms in temperate and tropical populations of Drosophila melanogaster: Ovarian size and developmental temperature. Evolution 1995, 49, 670–675. [Google Scholar] [CrossRef]
- Hodin, J.; Riddiford, L.M. Different mechanisms underlie phenotypic plasticity and interspecific variation for a reproductive character in drosophilids (Insecta: Diptera). Evolution 2000, 54, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Bergland, A.O.; Genissel, A.; Nuzhdin, S.V.; Tatar, M. Quantitative trait loci affecting phenotypic plasticity and the allometric relationship of ovariole number and thorax length in Drosophila melanogaster. Genetics 2008, 180, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.P.; Tatar, M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell 2003, 2, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Extavour, C.G. Insulin signaling underlies both plasticity and divergence of a reproductive trait in Drosophila. Proc. Biol. Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.; Almeida, F.C.; O’Grady, P.M.; Armella, M.A.; DeSalle, R.; Etges, W.J. Monophyly, divergence times, and evolution of host plant use inferred from a revised phylogeny of the Drosophila repleta species group. Mol. Phylogenet. Evol. 2012, 64, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M. Evolution of the Replete Group. In The Genetics and Biology of Drosophila; Ashburner, M., Carson, H., Thompson, J.N., Eds.; Academic Press: London, UK, 1982; pp. 61–139. [Google Scholar]
- Pfeiler, E.; Markow, T.A. Phylogeography of the Cactophilic Drosophila and Other Arthropods Associated with Cactus Necroses in the Sonoran Desert. Insects 2011, 2, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Carreira, V.P.; Padró, J.; Moniardino Koch, N.; Fontanarrosa, P.; Alonso, J.I.; Soto, I.M. Nutritional composition of Opuntiasulphurea (G. Don in Loudon) cladodes. Haseltonia 2014, 19, 38–45. [Google Scholar] [CrossRef]
- Padró, J.; Soto, I.M. Exploration of the nutritional profile of Trichocereus terscheckii (Parmentier) Britton & Rose stems. J. Prof. Assoc. Cactus 2013, 15, 1–12. [Google Scholar]
- Koch, N.M.; Soto, I.M.; Hasson, E.; Galvano, M.; Ianonne, L. Biodiversity of cactophilic microorganisms in western Argentina: Community structure and species composition in the necroses of two sympatric cactus hosts. Fungal Ecol. 2015, 13, 167–180. [Google Scholar] [CrossRef]
- Hurtado, J.P.; Soto, E.M.; Orellana, L.; Hasson, E. Mating success depends on rearing substrate in cactophilic Drosophila. Evol. Ecol. 2012, 26, 733–743. [Google Scholar] [CrossRef]
- Soto, E.M.; Goenaga, J.; Hurtado, J.P.; Hasson, E. Oviposition and performance in natural host in cactophilic Drosophila. Evol. Ecol. 2012, 26, 975–990. [Google Scholar] [CrossRef]
- Corio, C.; Soto, I.M.; Carreira, V.; Padró, J.; Betti, M.I.L.; Hasson, E. An alkaloid fraction extracted from the cactus Trichocereus terscheckii affects fitness in the cactophilic fly Drosophila buzzatii (Diptera: Drosophilidae). Biol. J. Linn. Soc. 2013, 109, 342–353. [Google Scholar] [CrossRef]
- Soto, I.M.; Carreira, V.P.; Corio, C.; Padró, J.; Soto, E.M.; Hasson, E. Differences in tolerance to host cactus alkaloids in Drosophila koepferae and D. buzzatii. PLoS ONE 2014, 9, e88370. [Google Scholar] [CrossRef] [PubMed]
- Hasson, E.; Naveira, H.; Fontdevila, A. The breeding sites of the Argentinian species of the Drosophila mulleri complex (subgenus Drosophila repleta group). Rev. Chil. Hist. Nat. 1992, 65, 319–326. [Google Scholar]
- Fanara, J.J.; Mensch, J.; Folguera, G.; Hasson, E. Developmental time and thorax length differences between the cactophilic sibling species Drosophila buzzatii and D. koepferae reared in different natural hosts. Evol. Ecol. 2004, 18, 203–214. [Google Scholar] [CrossRef]
- Carreira, V.P.; Soto, I.M.; Hasson, E.; Fanara, J.J. Patterns of variation in wing morphology in the cactophilic Drosophila buzzatii and its sibling D. koepferae. J. Evol. Biol. 2006, 19, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.M.; Carreira, V.P.; Soto, E.M.; Hasson, E. Wing morphology and fluctuating asymmetry depend on the host plant in cactophilic Drosophila. J. Evol. Biol. 2008, 21, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Fanara, J.J.; Folguera, G.; Fernandez Iriarte, P.; Mensch, J.; Hasson, E. Genotype environment interactions in viability and developmental time in populations of cactophilic Drosophila. J. Evol. Biol. 2006, 19, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Fontdevila, A.; Ruiz, A.; Alonso, G.; Ocaña, J. Evolutionary History of Drosophila buzzatii. I. Natural Chromosomal Polymorphism in Colonized Populations of the Old World. Evolution 1981, 35, 148–157. [Google Scholar] [CrossRef]
- Barker, J.S.F. Genetic history of a colonizing population: Drosophila buzzatii (Diptera: Drosophilidae) in Australia. Biol. J. Linn. Soc. 2013, 109, 682–698. [Google Scholar] [CrossRef]
- Fanara, J.J.; Soto, I.M.; Lipko, P.; Hasson, E. First Record of Drosophila buzzatii (Patterson & Wheeler) (Diptera: Drosophilidae) Emerging from a Non-Cactus Host. Neotrop. Entomol. 2016. [Google Scholar] [CrossRef]
- Soto, E.M.; Hasson, E.; Mensch, J.; Universidad de Buenos Aires; Buenos Aires, Argentina. Unpublished data. 2015.
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Fanara, J.J.; Hasson, E. Oviposition acceptance and fecundity schedule in the cactophilic sibling species Drosophila buzzatii and D. koepferae on their natural hosts. Evolution 2001, 55, 2615–2619. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.A. A revision of the Drosophila species group (Diptera-Drosophilidae). Rev. Brasil. Entomol. 1983, 27, 1–114. [Google Scholar]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Loeschcke, V.; Bundgaard, J.; Barker, J. Variation in body size and life history traits in Drosophila aldrichi and D. buzzatii from a latitudinal cline in eastern Australia. Heredity 2000, 85, 423–433. [Google Scholar] [PubMed]
- Rohlf, F.J. TPSDig. Available online: http://life.bio.sunysb.edu/morph/index.html (accessed on 9 July 2016).
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Jaenike, J. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 1990, 21, 243–273. [Google Scholar] [CrossRef]
- Courtney, S.P.; Kibota, T.T. Mother Doesnt Know Best: Selection of Hosts by Ovipositing Insects. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 96, pp. 161–188. [Google Scholar]
- Craig, T.P.; Itami, J.K. Evolution of preference and performance relationships. In Specialization, Speciation, and Radiation. The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; University of California Press: Berkeley, CA, USA, 2008; pp. 20–28. [Google Scholar]
- Fox, C.W. A Quantitative Genetic Analysis of Oviposition Preference and Larval Performance on Two Hosts in the Bruchid Beetle, Callosobruchusmaculatus. Evolution 1993, 47, 166–175. [Google Scholar] [CrossRef]
- Werenkraut, V.; Hasson, E.; Oklander, L.; Fanara, J.J. A comparative study of competitive ability between two cactophilic species in their natural hosts. Austral. Ecol. 2008, 33, 663–671. [Google Scholar] [CrossRef]
- Matzkin, L.M.; Johnson, S.; Paight, C.; Bozinovic, G.; Markow, T.A. Dietary protein and sugar differentially affect development and metabolic pools in ecologically diverse Drosophila. J. Nutr. 2011, 41, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Beldade, P.; Mateus, A.R.; Keller, R.A. Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 2011, 20, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. USA 2005, 102, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Inauer Associates Inc.: Massachusetts, MA, USA, 1998. [Google Scholar]
- Jha, A.R.; Miles, C.M.; Lippert, N.R.; Brown, C.D.; White, K.P.; Kreitman, M. Whole-Genome Resequencing of Experimental Populations Reveals Polygenic Basis of Egg-Size Variation in Drosophila melanogaster. Mol. Biol. Evol. 2015, 32, 2616–2632. [Google Scholar] [CrossRef] [PubMed]
- R’kha, S.; Moreteau, B.; Coyne, J.A.; David, J.R. Evolution of a lesser fitness trait: Egg production in the specialist Drosophila sechellia. Genet. Res. 1997, 69, 17–23. [Google Scholar] [CrossRef]
- Markow, T.A.; Beall, S.; Matzkin, L.M. Egg size, embryonic development time and ovoviviparity in Drosophila species. J. Evol. Biol. 2009, 22, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Vijendravarma, R.K.; Narasimha, S.; Kawecki, T.J. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biol. Lett. 2010, 6, 238–241. [Google Scholar] [CrossRef] [PubMed]

| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| Species | 1 | 0.001 | 0.001 | 0.995 |
| Cactus | 1 | 0.021 | 2.859 | 0.140 |
| Species × Cactus | 1 | 0.001 | 0.171 | 0.693 |
| Line (S) | 6 | 0.022 | 2.996 | 0.101 |
| Line (S) × Cactus | 6 | 0.007 | 4.235 | 0.005 |
| Wing Length | 1 | 0.002 | 1.596 | 0.209 |
| Error | 117 | 0.001 |
| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| Line | 3 | 0.048 | 3.723 | 0.150 |
| Cactus | 1 | 0.014 | 1.273 | 0.336 |
| Line × Cactus | 3 | 0.014 | 7.539 | 0.001 |
| Wing Length | 1 | 0.001 | 0.806 | 0.372 |
| Error | 79 | 0.002 |
| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| Line | 3 | 0.006 | 2.119 | 0.269 |
| Cactus | 1 | 0.004 | 1.145 | 0.365 |
| Line × Cactus | 3 | 0.003 | 2.623 | 0.067 |
| Wing Length | 1 | 0.001 | 1.048 | 0.309 |
| Error | 71 | 0.001 |
| Species-by-Cactus | Natural Host (X ± SD) | Semi-Natural (X ± SD) | F | P |
|---|---|---|---|---|
| D. buzzatii in O. sulphurea | 41.1 ± 2.50 | 43.4 ± 5.48 | 1.954 | 0.167 |
| D. buzzatii in T. terscheckii | 38.9 ± 6.38 | 40.4 ± 7.50 | 0.391 | 0.534 |
| D. koepferae in T. terscheckii | 43.1 ± 3.26 | 42.5 ± 3.27 | 0.255 | 0.615 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peluso, D.; Soto, E.M.; Kreiman, L.; Hasson, E.; Mensch, J. Contrasting Plasticity in Ovariole Number Induced by A Dietary Effect of the Host Plants between Cactophilic Drosophila Species. Insects 2016, 7, 21. https://doi.org/10.3390/insects7020021
Peluso D, Soto EM, Kreiman L, Hasson E, Mensch J. Contrasting Plasticity in Ovariole Number Induced by A Dietary Effect of the Host Plants between Cactophilic Drosophila Species. Insects. 2016; 7(2):21. https://doi.org/10.3390/insects7020021
Chicago/Turabian StylePeluso, Daniela, Eduardo M. Soto, Lucas Kreiman, Esteban Hasson, and Julián Mensch. 2016. "Contrasting Plasticity in Ovariole Number Induced by A Dietary Effect of the Host Plants between Cactophilic Drosophila Species" Insects 7, no. 2: 21. https://doi.org/10.3390/insects7020021
APA StylePeluso, D., Soto, E. M., Kreiman, L., Hasson, E., & Mensch, J. (2016). Contrasting Plasticity in Ovariole Number Induced by A Dietary Effect of the Host Plants between Cactophilic Drosophila Species. Insects, 7(2), 21. https://doi.org/10.3390/insects7020021