Densities of Agrilus auroguttatus and Other Borers in California and Arizona Oaks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites: Southern California
2.2. Study Sites: Southeastern Arizona
| Location | Site names | Host species (Section) | Coordinates (°N, °W) | Elevation (m) | n | DBH a (cm) |
|---|---|---|---|---|---|---|
| CA | Roberts’ Ranch | Q. agrifolia (Lobatae)/Q. engelmannii (Quercus) | 32.82920, 116.61699 | 1051 | 17 | 74.2 ± 8.2 |
| Thing Valley | Q. kelloggii (Lobatae)/Q. chrysolepis (Protobalanus) | 32.85440, 116.42055 | 1801 | 20 | 72.0 ± 6.9 | |
| Cuyamaca State Park | Q. agrifolia (Lobatae) | 32.86501, 116.59295 | 1085 | 10 | 69.7 ± 7.7 | |
| Pine Creek | 32.83640, 116.54304 | 1106 | 10 | 82.5 ± 8.5 | ||
| Kitchen Creek Canyon | 32.81052, 116.44904 | 1528 | 10 | 67.8 ± 6.2 | ||
| Noble Canyon | 32.85044, 116.52192 | 1134 | 11 | 78.1 ± 8.7 | ||
| Corral Canyon | 32.72927, 116.54258 | 955 | 10 | 104.3 ± 13.3 | ||
| Guatay | 32.84879, 116.55009 | 1215 | 10 | 62.7 ± 6.9 | ||
| Long Valley | 32.81453, 116.53244 | 1189 | 10 | 63.4 ± 6.6 | ||
| Cottonwood | 32.70152, 116.48983 | 953 | 10 | 72.2 ± 8.1 | ||
| Warner Springs b | 33.31826, 116.69494 | 900 | 10 | 97.4 ± 8.7 | ||
| Wooded Hill Road | Q. kelloggii (Lobatae) | 32.86470, 116.45683 | 1692 | 10 | 89.5 ± 7.1 | |
| Wooded Hill CG | 32.84978, 116.43376 | 1822 | 10 | 84.1 ± 11.2 | ||
| Kitchen Creek Road | 32.84705, 116.44854 | 1788 | 10 | 81.6 ± 9.0 | ||
| Horse Heaven | 32.89007, 116.44107 | 1728 | 10 | 66.9 ± 8.4 | ||
| Sunrise Highway | 32.84897, 116.48450 | 1534 | 10 | 45.7 ± 3.9 | ||
| Camp Ole | 32.88230, 116.43046 | 1755 | 10 | 82.5 ± 6.8 | ||
| Desert View | 32.87011, 116.41417 | 1800 | 10 | 46.9 ± 4.2 | ||
| Penny Pines | 32.90527, 116.45949 | 1660 | 10 | 65.9 ± 9.1 | ||
| AZ | Miller Canyon c | Q. hypoleucoides (Lobatae)/Q. arizonica (Quercus) | 31.41546, 110.27553 | 1744 | 20 | 32.6 ± 3.3 |
| Carr Canyon c | 31.44143, 110.28586 | 1658 | 20 | 22.6 ± 1.0 | ||
| Cochise Stronghold | Q. emoryi/Q. hypoleucoides (both Lobatae)/Q. arizonica (Quercus) | 31.92567, 109.96637 | 1483 | 15 | ±1.7 | |
| Chiricahua Nat’l Mon c | Q. emoryi (Lobatae) | 32.00880, 109.37302 | 1585 | 10 | 50.9 ± 3.2 | |
| Pinery Canyon c | 31.96989, 109.32278 | 1727 | 10 | 27.5 ± 2.5 | ||
| Gardner Canyon | 31.72206, 110.71739 | 1496 | 10 | 47.4 ± 4.4 | ||
| Madera Canyon | Q. hypoleucoides (Lobatae) | 30.72500, 110.87944 | 1524 | 10 | 18.4 ± 1.3 |
2.3. Evaluation of Borer Density
2.4.Data Analyses
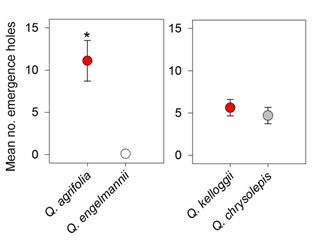
3. Results
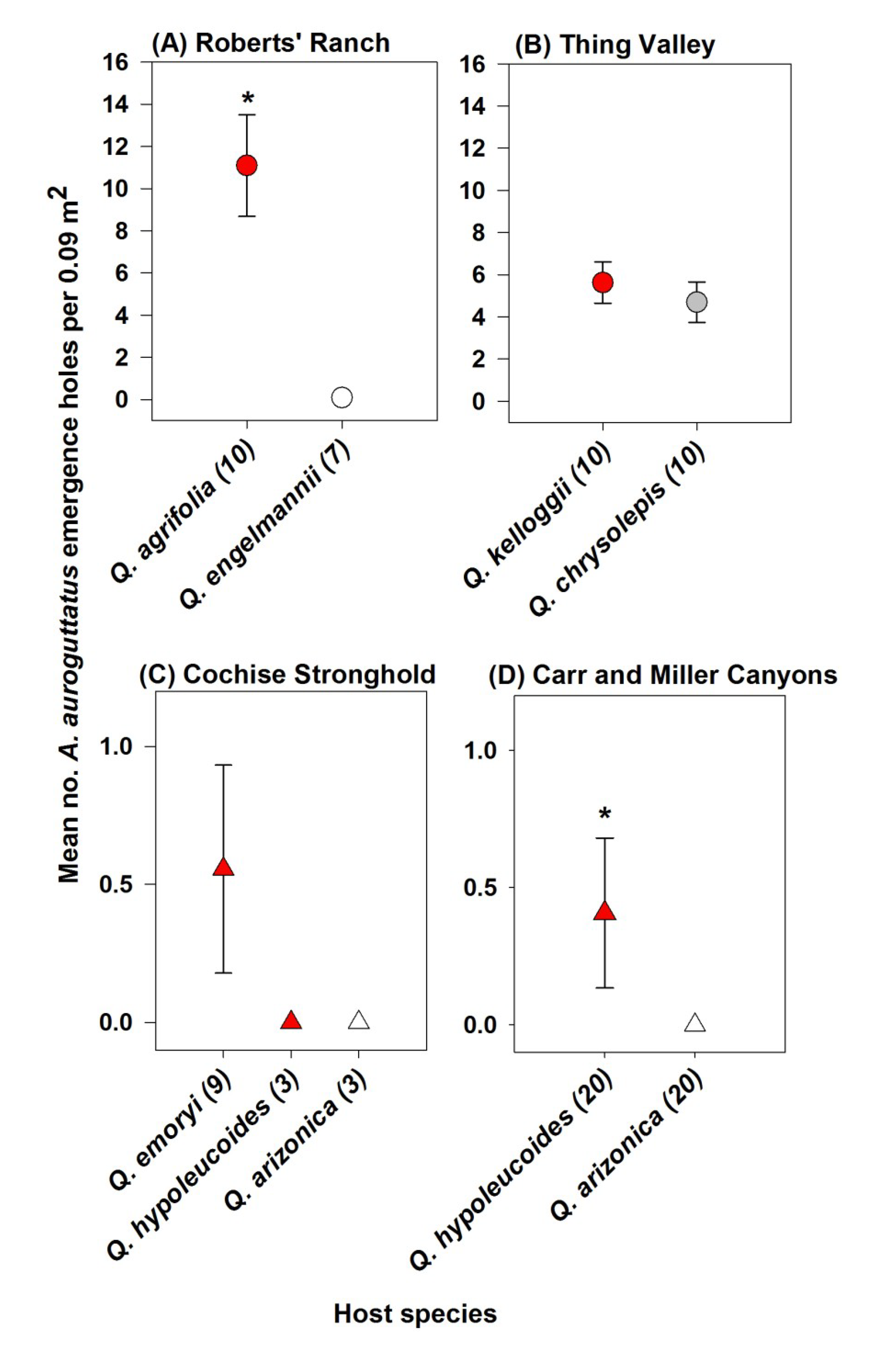
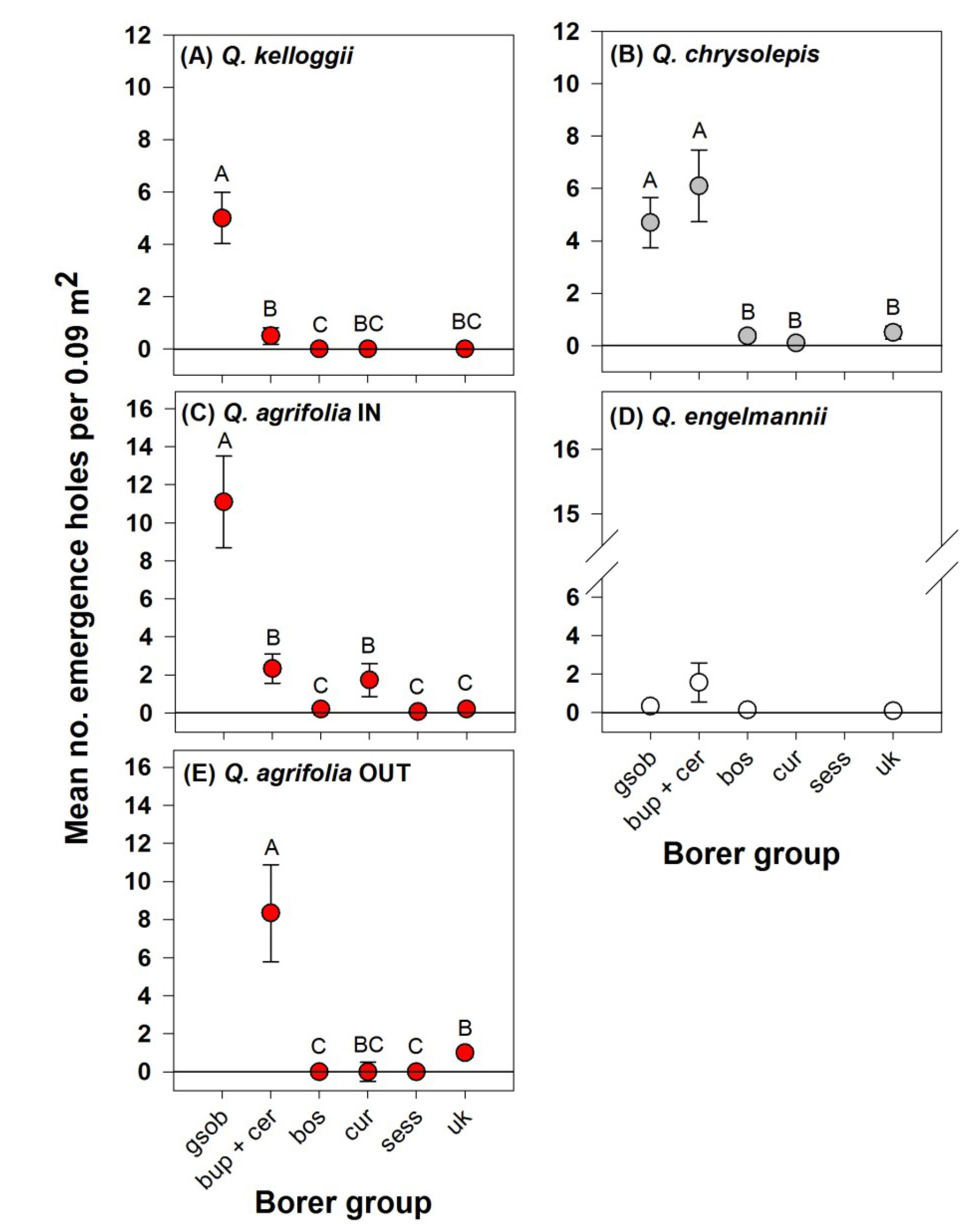
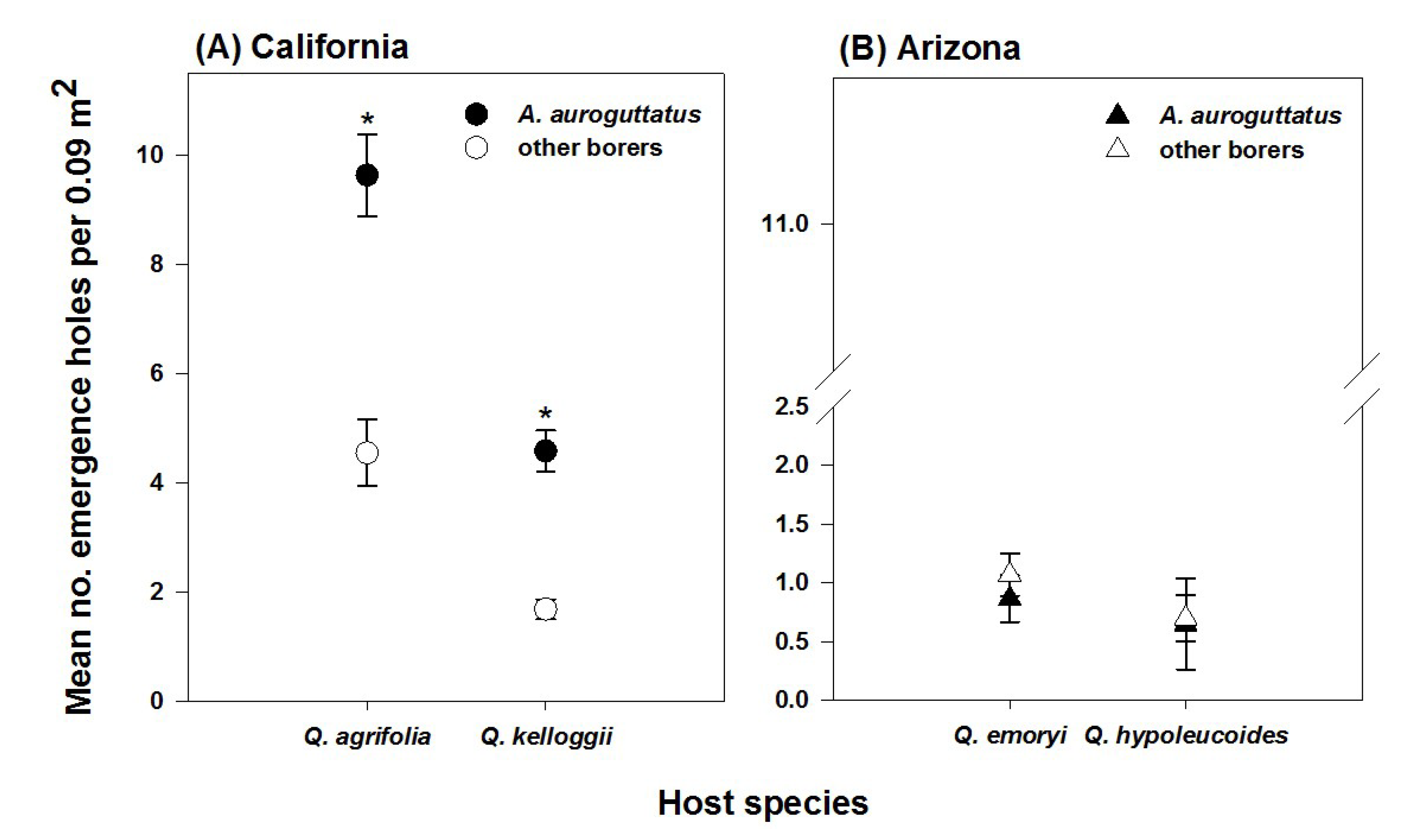
4. Discussion
| Agrilus spp. | Host spp. | Relationship | Density per 0.09 m2 of bark | Density per m2 of bark | Reference |
|---|---|---|---|---|---|
| A. auroguttatus Schaeffer, goldspotted oak borer | Quercus agrifolia | naïve | 10(1–50) | 111(11–556) | This study a |
| Q. kelloggii (both Fagaceae) | naïve | 5(0–16) | 56(0–178) | ||
| A. planipennis Fairmaire, emerald ash borer | Fraxinus pennsylvanica, F. americana (Oleaceae) | naïve | 8(2–15) | 89(17–170) | [31] b |
| A. anxius Gory, bronze birch borer | Betula pendula (Betulaceae) | naïve | 21(N/A) | 232(N/A) | [32] c |
| A. auroguttatus | Q. emoryi | co-evolved | 1(0–7) | 11(0–78) | This study |
| Q. hypoleucoides | co-evolved | 1(0–12) | 1(0–133) | ||
| A. bilineatus (Weber), twolined chestnut borer | Q. rubra, Q. velutina | co-evolved | 4(N/A) | 41(N/A) | [33] b |
| A. biguttatus (Fabricius), oak buprestid beetle | Quercus spp. | co-evolved | Max = 7 | Max = 76 | [34] d |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coleman, T.W.; Seybold, S.J. Previously unrecorded damage to oak, Quercus spp., in southern California by the goldspotted oak borer, Agrilus coxalis Waterhouse (Coleoptera: Buprestidae). Pan. Pac. Entomol. 2008, 84, 288–300. [Google Scholar] [CrossRef]
- Westcott, R.L. A new species of Chrysobothris Eschscholtz from Oregon and Washington, with notes on other Buprestidae (Coleoptera) occurring the United States and Canada. Zootaxa 2005, 1044, 1–15. [Google Scholar]
- Coleman, T.W.; Seybold, S.J. Collection history and comparison of the interactions of the goldspotted oak borer, Agrilus auroguttatus Schaeffer (Coleoptera: Buprestidae), with host oaks in Southern California and Southeastern Arizona, U.S.A. Coleopts. Bull. 2011, 65, 93–108. [Google Scholar] [CrossRef]
- Coleman, T.W.; Lopez, V.; Rugman-Jones, P.; Stouthamer, R.; Seybold, S.J.; Reardon, R.; Hoddle, M.S. Can the destruction of California’s oak woodlands be prevented? Potential for biological control of the goldspotted oak borer, Agrilus auroguttatus. BioControl 2012, 57, 211–225. [Google Scholar] [CrossRef]
- Haavik, L.J.; Graves, A.D.; Coleman, T.W.; Flint, M.L.; Venette, R.C.; Seybold, S.J. Suitability of native and ornamental oak species in California for Agrilus auroguttatus. Entomol. Exp. Appl. 2014, 150, 86–97. [Google Scholar] [CrossRef]
- Chen, Y.; Coleman, T.W.; Jones, M.I.; Flint, M.L.; Seybold, S.J. Foliar nutrients explain goldspotted oak borer, Agrilus auroguttatus Schaeffer (Coleoptera: Buprestidae), adult feeding preference among four California oak species. Entomol. Exp. Appl. 2013, 149, 57–66. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Orians, C.M.; Ward, D. Evolution of plant defenses in nonindigenous environments. Annu. Rev. Entomol. 2010, 55, 439–459. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M.; Jactel, H. The ecology of forest insect invasions and advances in their management. Can. J. For. Res. 2006, 36, 263–268. [Google Scholar] [CrossRef]
- Lovett, G.M.; Canham, C.D.; Arthur, M.A.; Weathers, K.C.; Fitzhugh, R.D. Forest ecosystem response to exotic pests and pathogens in eastern North America. BioScience 2006, 56, 395–405. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar] [CrossRef]
- USDA Forest Service, Forest Health Monitoring (FHM). Aerial Survey Region 5 Database. Available online: http://www.fs.usda.gov/detail/r5/forest-grasslandhealth/?cid=fsbdev3_046696/ (accessed on 5 December 2013).
- Coleman, T.W.; Graves, A.D.; Hoddle, M.; Heath, Z.; Chen, Y.; Flint, M.L.; Seybold, S.J. Forest stand composition and impacts associated with Agrilus auroguttatus Schaeffer (Coleoptera: Buprestidae) and Agrilus coxalis Waterhouse in oak woodlands. For. Ecol. Manage. 2012, 276, 104–117. [Google Scholar] [CrossRef]
- Flint, M.L.; Jones, M.I.; Coleman, T.W.; Seybold, S.J. Goldspotted oak borer. In Agriculture and Natural Resources Pest Notes Publication 74163; University of California Statewide Integrated Pest Management Program: Oakland, CA, USA, 2013; p. 7. [Google Scholar]
- Brown, L.R.; Eads, C.O. A technical study of insects affecting the oak tree in southern California. In California Agricultural Experiment Station Bulletin 810; California Experimental Station: Berkeley, CA, USA, 1965; p. 106. [Google Scholar]
- Furniss, R.L.; Carolin, V.M. Western Forest Insects; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 1977; p. 654. [Google Scholar]
- Swiecki, T.J.; Bernhardt, E.A. A Field Guide to Insects and Diseases of California Oaks. Gen. Tech. Rep. PSW-GTR-197; U.S. Department of Agriculture Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2006; p. 151. [Google Scholar]
- Fairweather, M.L.; McMillin, J.; Rogers, T.; Conklin, D.; Fitzgibbon, B. Field Guide to Insects and Diseases of Arizona and New Mexico Forests; U.S. Department of Agriculture Forest Service Southwestern Region MR-R3-16-3: Albuquerque, NM, USA, 2006; p. 269. [Google Scholar]
- Haavik, L.J.; Coleman, T.W.; Flint, M.L.; Venette, R.C.; Seybold, S.J. Agrilus auroguttatus exit hole distribution on Quercus agrifolia boles and a sampling method to estimate their density on individual trees. Can. Entomol. 2012, 144, 1–12. [Google Scholar] [CrossRef]
- Western Regional Climate Center (WRCC). 1981–2010 Monthly Normals. Available online: http://www.wrcc.dri.edu/summary/climsmsca.html/ (accessed on 5 December 2013).
- Pryde, P.R. San Diego: An Introduction to the Region; Kendall/Hunt Publishing Co.: Dubuque, IA, USA, 1984; p. 297. [Google Scholar]
- U.S. Department of Agriculture Forest Service, Remote Sensing Applications Center. Fire Detection Maps. Available online: http://firemapper.sc.egov.usda.gov/activefiremaps.php?sensor=modis&op=maps&rCode=swx/ (accessed on 5 December 2013).
- Martin, S.C.; Reynolds, H.G. The Santa Rita Experimental Range: Your facility for research on semidesert ecosystems. J. Ariz. Acad. Sci. 1973, 8, 56–67. [Google Scholar] [CrossRef]
- Hishinuma, S.; Coleman, T.W.; Flint, M.L.; Seybold, S.J. Goldspotted Oak Borer Field Identification Guide; University of California Agriculture and Natural Resources Statewide Integrated Pest Management Program: Oakland, CA, USA, 2011; p. 6. [Google Scholar]
- Fisher, W.S. A revision of the north American species of buprestid beetles belonging to the genus Agrilus. In Smithsonian Institution, United States National Museum; Bulletin 145; U.S. Govt. Print. Off.: Washington, DC, USA, 1928; p. 347. [Google Scholar]
- Solomon, J.D. Guide to insect borers in North American broadleaf trees and shrubs. In USDA Forest Service Agriculture Handbook AH 706; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 1995; p. 747. [Google Scholar]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012.
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar]
- Box, G.E.; Cox, D.R. An analysis of transformations (with discussion). J. Roy. Stat. Soc. B 1964, 26, 211–246. [Google Scholar]
- Haack, R.A.; Benjamin, D.M.; Haack, K.D. Buprestidae, Cerambycidae, and Scolytidae associated with successive stages of Agrilus bilineatus (Coleoptera: Buprestidae) infestation of oaks in Wisconsin. Great Lakes Entomol. 1983, 16, 47–55. [Google Scholar]
- McCullough, D.G.; Siegert, N.W. Estimating potential emerald ash borer (Coleoptera: Buprestidae) populations using ash inventory data. J. Econ. Entomol. 2007, 100, 1577–1586. [Google Scholar] [CrossRef]
- Akers, R.C.; Nielsen, D.G. Spatial emergence pattern of bronze birch borer, (Coleoptera: Buprestidae) from European white birch. J. Entomol. Sci. 1990, 25, 150–157. [Google Scholar]
- Haack, R.A.; Benjamin, D.M. The biology and ecology of the twolined chestnut borer, Agrilus bilineatus (Coleoptera: Buprestidae), on oaks, Quercus spp., in Wisconsin. Can. Entomol. 1982, 114, 385–396. [Google Scholar] [CrossRef]
- Moraal, L.G.; Hilszczański, J. The oak buprestid beetle, Agrilus biguttatus (F.) (Col., Buprestidae), a recent factor in oak decline in Europe. J. Pest. Sci. 2000, 73, 134–138. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haavik, L.J.; Coleman, T.W.; Flint, M.L.; Venette, R.C.; Seybold, S.J. Densities of Agrilus auroguttatus and Other Borers in California and Arizona Oaks. Insects 2014, 5, 287-300. https://doi.org/10.3390/insects5010287
Haavik LJ, Coleman TW, Flint ML, Venette RC, Seybold SJ. Densities of Agrilus auroguttatus and Other Borers in California and Arizona Oaks. Insects. 2014; 5(1):287-300. https://doi.org/10.3390/insects5010287
Chicago/Turabian StyleHaavik, Laurel J., Tom W. Coleman, Mary Louise Flint, Robert C. Venette, and Steven J. Seybold. 2014. "Densities of Agrilus auroguttatus and Other Borers in California and Arizona Oaks" Insects 5, no. 1: 287-300. https://doi.org/10.3390/insects5010287
APA StyleHaavik, L. J., Coleman, T. W., Flint, M. L., Venette, R. C., & Seybold, S. J. (2014). Densities of Agrilus auroguttatus and Other Borers in California and Arizona Oaks. Insects, 5(1), 287-300. https://doi.org/10.3390/insects5010287