General Stress Responses in the Honey Bee
Abstract
:1. Introduction
1.1. Concept of Stress
1.2. Why Study Stress in Honey Bees?
1.3. How Does an Organism React to Stress?
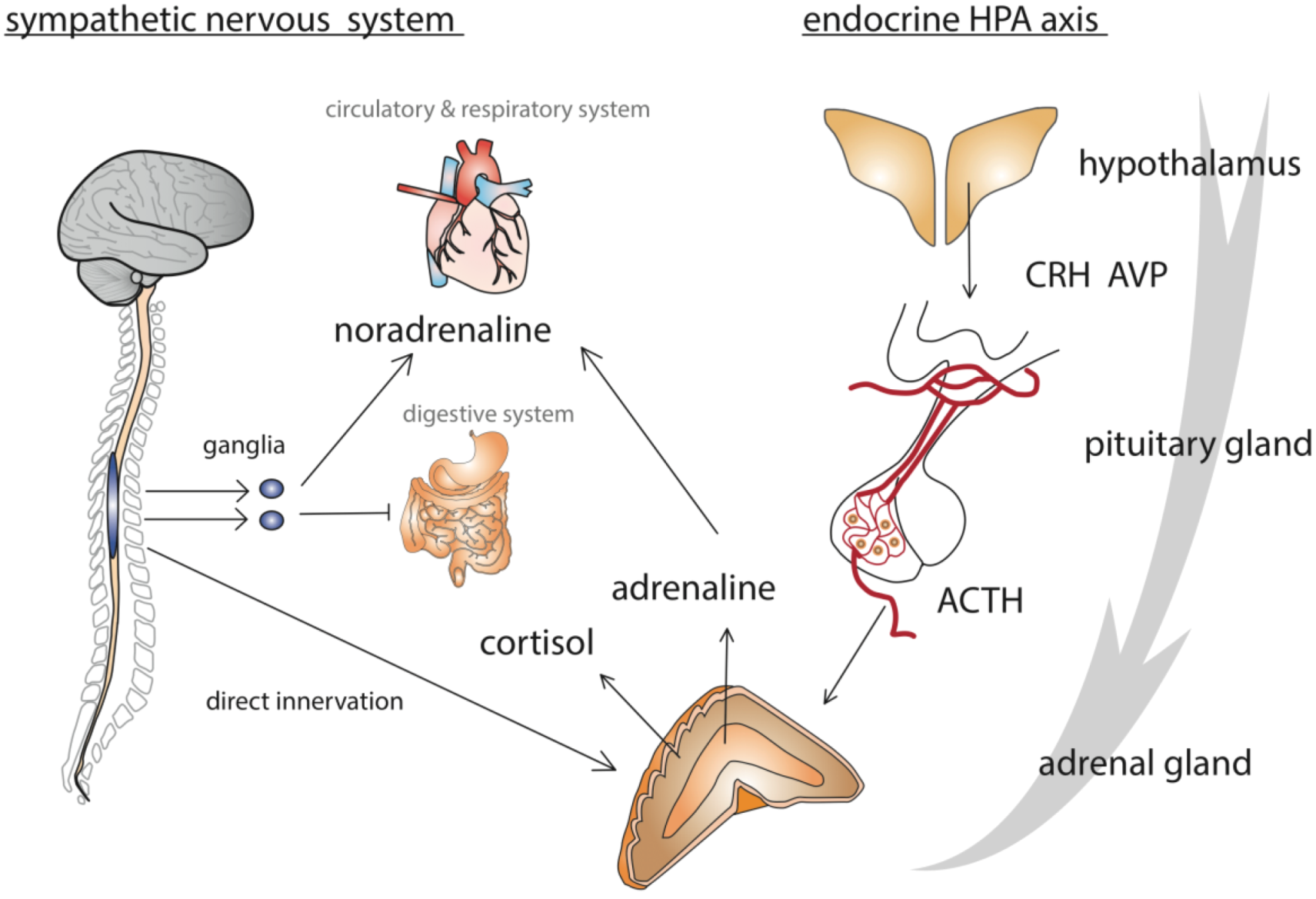
2. Model of the Honey Bee Stress Response
2.1. How Has Stress Been Assessed in Honey Bees?
| stress response measure | stressor | acute/chronic | variable | references |
|---|---|---|---|---|
| physiological responses | ||||
| juvenile hormone (RIA) | cold anesthesia, caging | A | task specialization, duration after treatment | Lin et al., 2004 [34] |
| brain biogenic amines (HPLC) | spinning, caging, chilling, CO2 | A | spinning speed, duration of stressor | Chen et al., 2008 [35] |
| brain biogenic amines (HPLC) | leg pinch | A | duration of stressor, age, season, patriline | Harris and Woodring, 1992 [36] |
| cellular stress responses | ||||
| HSP 70 (Elisa) | capture, transport, chilling, harnessing | A/C | ethanol concentration, duration of harness | Hranitz et al., 2010 [31] |
| HSP70 (western) | heat | A | duration of stressor, age body part | Elekonich, 2009 [28] |
| hsp70, hsc70 (q PCR) | ||||
| CRH-BP (qPCR) | cold, heat, UV light | A | intensity of stressor, caste, development stage, body part | Liu et al., 2011 [37] |
| behavioral response | ||||
| stinging response | electric shock | A | patriline | Lenoir et al., 2006 [38] |
| stinging response (delay) | electric shock | A | genotype, housing conditions, | Uribe-Rubio et al., 2008 [39] |
| task specialization | ||||
| sting extension | electric shock | A | genotype, exposure to alarm pheromone | Balderrama et al., 1987, 2002 Roussel et al., 2009 [40,41,42] |
| task specialization | ||||
| sting extension | electric shock | A | morphine and opioid peptides treatment | Núñez et al., 1983, 1997 [43,44] |
| proboscis extension | soil-borne pollutants | C | treatment concentration | Hladun et al., 2012 [45] |
| survival | ||||
| survival | hyperoxia | C | learning performance | Amdam et al., 2010 [46] |
| survival | paraquat injection | C | vitellogenin level, reproductive castes | Seehuus et al., 2006; Corona et al., 2007 [47,48] |
| (oxidative stressor), hyperoxia |
| chemicals | abbreviation | nature | stress-related action | references |
|---|---|---|---|---|
| biogenic amines | ||||
| octopamine | OA | neurotransmitter | enhances arousal, increases heart rate, modulates muscle activity | Corbet, 1991; Farooqui, 2012, |
| Papaefthimiou and Theophilidis, 2011, | ||||
| Pflüger et al., 2004 [49,50,51,52] | ||||
| neurohormone | ||||
| dopamine | DA | neurotransmitter | modulates arousal | Mustard et al., 2010 [53] |
| peptides | ||||
| adipokinetic hormone | AKH | hormone | mobilize energy in the fat body | Kodrik, 2008 [54] |
| cortico releasing hormone-binding protein | CRH-BP | chaperone? | potentiates or inhibits hormonal release ? | Liu et al., 2011 [37] |
| diuretic hormone-I | DH-I | hormone | stimulates diuresis induced by crop draining into hindgut after energy mobilization. | Coast et al., 2002 [55] |
| corazonin | Crz | neurohormone | activates metabolism? | Veenstra, 2009 [56] |
| allatostatin-A | AST-A | neurohormone | activates gut contraction ? inhibits corazonin neurosecretion ? | Veenstra, 2009 [56] |
| insulin-like peptide | ILP | ? | regulates energy stores ? | Corona et al., 2007 [48] |
| proteins | ||||
| heat shock proteins | HSP70 | chaperone | protects cells against oxidative stress and excess protein misfolding | Hranitz et al., 2010, Elekonich, 2009 [28,31] |
| ERK2 | ERK2 | ? | protects cells against damage ? | Li et al., 2012 [30] |
| vitellogenin | Vg | antioxidant | protects cells against damage | Seehuus et al., 2006 [47] |
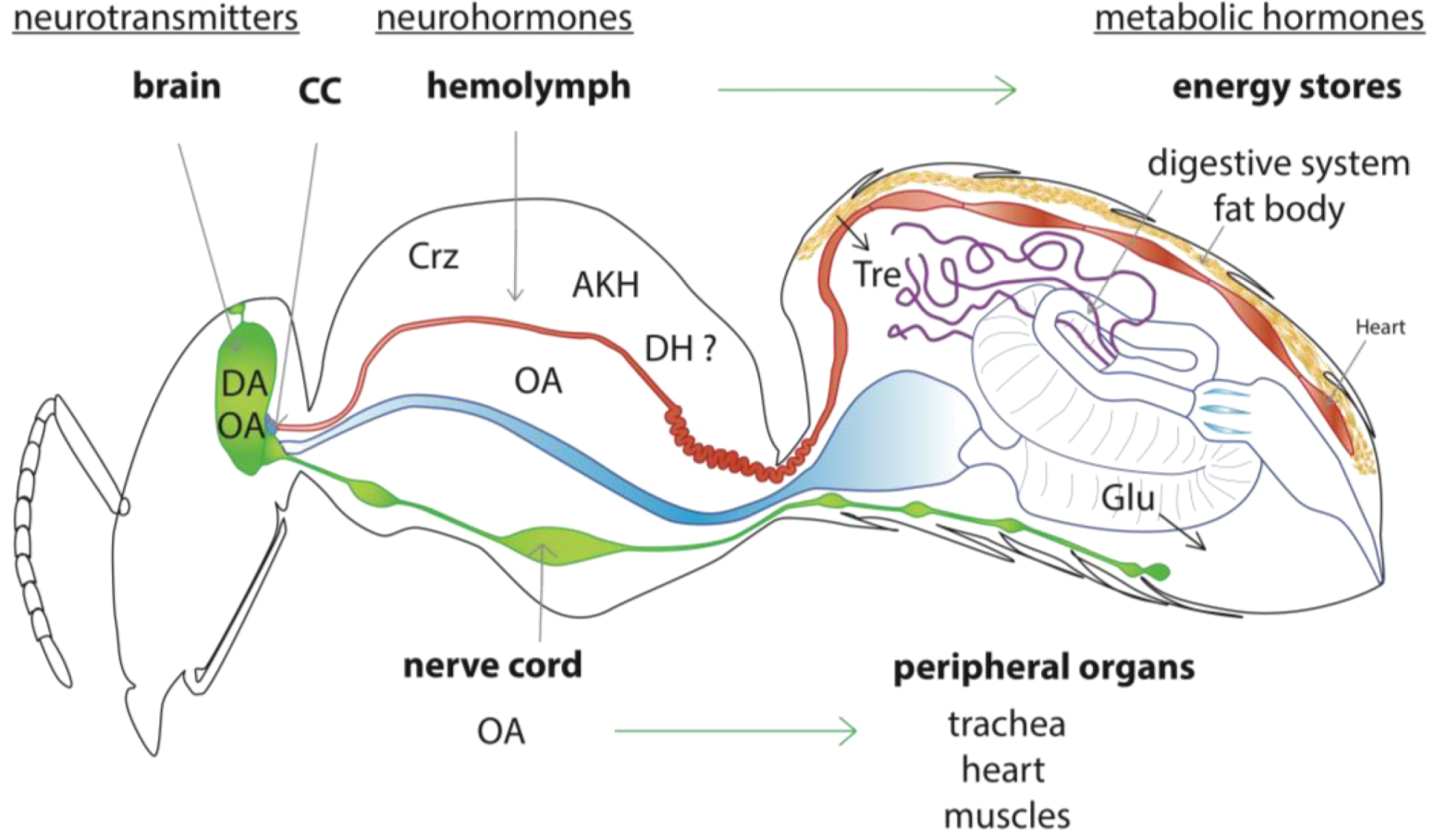
2.2. Model of the Honey Bee Stress Response
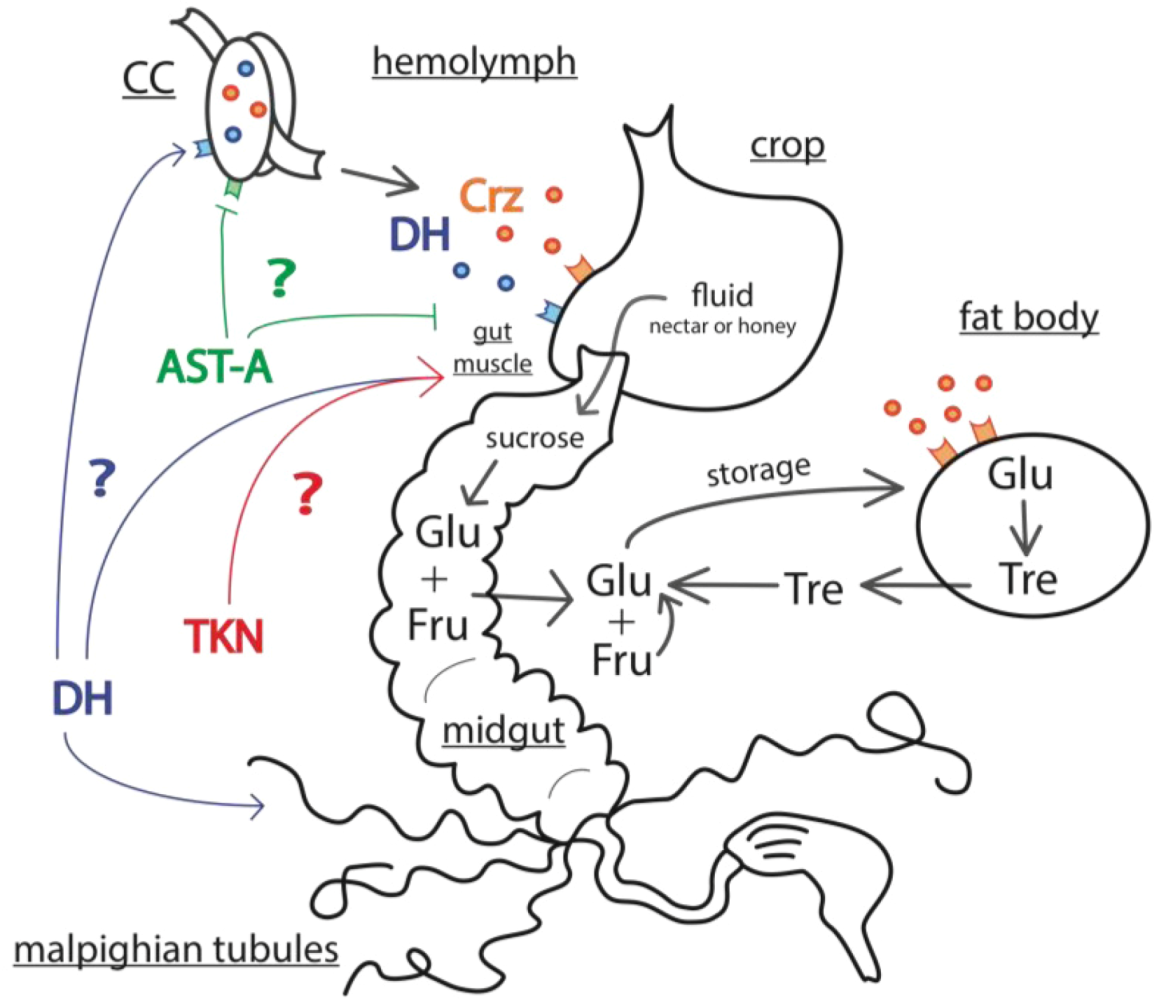
3. Molecular Signals of the Honey Bee Stress Response
3.1. Stress Indicators within the Brain
3.1.1. Biogenic Amines
3.1.2. Cortico-Releasing Hormone-Binding Protein (CRH-BP) and Its Putative Diuretic Hormone Ligand (DH-I)
3.2. Coordinated Peripheral Stress Responses
3.2.1. Octopamine
3.2.2. Corazonin
3.2.3. Allatostatins
3.2.4. Adipokinetic Hormone (AKH)
3.3. Mechanisms of Energy Mobilization in Honey Bees
4. Gaps in Knowledge and Urgent Questions
4.1. What Is the Role of JH in the Stress Response?
4.2. Can Dopamine Be Considered As a Stress Hormone?
4.3. Neuropeptides in the CC?
4.4. Stress Responses and Immune System
4.5. Task Specialization and Sensitivity to Stress
5. Conclusions
Acknowledgments
References
- Selye, H. The Stress of Life, 2nd ed.; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stressors, Stress, and Neuroendocrine Integration of the Adaptive Response: The 1997 Hans Selye Memorial Lecture. Ann. NY Acad. Sci. 1998, 851, 311–335. [Google Scholar] [CrossRef]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138. [Google Scholar]
- McEwen, B.S. The brain is the central organ of stress and adaptation. NeuroImage 2009, 47, 911–913. [Google Scholar] [CrossRef]
- Boerjan, B.; Verleyen, P.; Huybrechts, J.; Schoofs, L.; de Loof, A. In search for a common denominator for the diverse functions of arthropod corazonin: A role in the physiology of stress? Gen. Comp. Endocr. 2010, 166, 222–233. [Google Scholar] [CrossRef]
- Ivanovic, J. Metabolic response to stressor. In Hormones and Metabolism in Insect stress; Ivanovic, J., Jankovic-Hlandni, M., Eds.; CRC Press: Boca Raton, FL, USA, 1991; p. 27. [Google Scholar]
- Roeder, T. Tyramine and octopamine: Ruling Behavior and Metabolism. Annu. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef]
- Johnson, E.C.; White, M.P. Stressed-Out Insects: Hormonal Actions and Behavioral Modifications. In Horm. Brain Behav.; Pfaff, D.W., Arnold, A.P., Fahrbach, S.E., Etgen, A.M., Rubin, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1069–1096. [Google Scholar]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS One 2009, 4, e6481. [Google Scholar]
- Ratnieks, F.L.W.; Carreck, N.L. Clarity on Honey Bee Collapse? Science 2010, 327, 152–153. [Google Scholar]
- Neumann, P.; Carreck, N. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Oldroyd, B.P. What’s Killing American Honey Bees? PLoS Biol. 2007, 5, e168. [Google Scholar] [CrossRef]
- Khoury, D.S.; Myerscough, M.R.; Barron, A.B. A Quantitative Model of Honey Bee Colony Population Dynamics. PLoS One 2011, 6, e18491. [Google Scholar]
- Wilson, E.O. The insect societies (Belknap Press); Belknap Press of Harvard University Press: Cambridge, UK, 1971. [Google Scholar]
- Bamberger, C.M.; Schulte, H.M.; Chrousos, G.P. Molecular Determinants of Glucocorticoid Receptor Function and Tissue Sensitivity to Glucocorticoids. Endocr. Rev. 1996, 17, 245–261. [Google Scholar]
- McEwen, B.S. The neurobiology of stress: From serendipity to clinical relevance. Brain Res. 2000, 886, 172–189. [Google Scholar] [CrossRef]
- Stratakis, C.A.; Chrousos, G.P. Neuroendocrinology and Pathophysiology of the Stress System. Ann. NY Acad. Sci. 1995, 771, 1–18. [Google Scholar] [CrossRef]
- Santoro, M.G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000, 59, 55–63. [Google Scholar] [CrossRef]
- Takeda, K.; Noguchi, T.; Naguro, I.; Ichijo, H. Apoptosis Signal-Regulating Kinase 1 in Stress and Immune Response. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 199–225. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Gibney, E.; Gault, J.; Williams, J. The use of stress proteins as a biomarker of sub-lethal toxicity: Induction of heat shock protein 70 by 2-isobutyl piperidine and transition metals at sub-lethal concentrations. Biomarkers 2001, 6, 204–217. [Google Scholar] [CrossRef]
- Nazir, A.; Saxena, D.K.; Kar Chowdhuri, D. Induction of hsp70 in transgenic Drosophila: Biomarker of exposure against phthalimide group of chemicals. Biochim. Biophys. Acta Gen. Subj. 2003, 1621, 218–225. [Google Scholar] [CrossRef]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Range; D’Appleton and company: New York, NY, USA, 1915. [Google Scholar]
- Gregorc, A.; Bowen, I.D. In situ localization of heat-shock and histone proteins in honey-bee (Apis mellifera l.) larvae infected with paenibacillus larvae. Cell Biol. Int. 1999, 23, 211–218. [Google Scholar]
- Elekonich, M. Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones 2009, 14, 219–226. [Google Scholar] [CrossRef]
- Severson, D.W.; Erickson, E.H.; Williamson, J.L.; Aiken, J.M. Heat stress induced enhancement of heat shock protein gene activity in the honey bee (Apis mellifera). Cell Mol. Life Sci. 1990, 46, 737–739. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Kang, M.; Guo, X.; Baohua, X. AccERK2, a map kinase gene from Apis cerana cerana, plays roles in stress responses, developmental processes, and the nervous system. Arch. Insect Biochem. Physiol. 2012, 79, 121–134. [Google Scholar] [CrossRef]
- Hranitz, J.M.; Abramson, C.I.; Carter, R.P. Ethanol increases HSP70 concentrations in honeybee (Apis mellifera L.) brain tissue. Alcohol 2010, 44, 275–282. [Google Scholar]
- Corona, M.; Hughes, K.A.; Weaver, D.B.; Robinson, G.E. Gene expression patterns associated with queen honey bee longevity. Mech. Ageing Dev. 2005, 126, 1230–1238. [Google Scholar] [CrossRef]
- Duell, M.E.; Abramson, C.I.; Wells, H.; Aptes, T.E.; Hall, N.M.; Pendergraft, L.J.; Zuniga, E.M.; Oruç, H.H.; Sorucu, A.; Çakmak, I.; et al. An Integrative Model of Cellular Stress and Environmental Stressors in the Honey Bee. Insects 2012. submited. [Google Scholar]
- Lin, H.; Dusset, C.; Huang, Z.Y. Short-term changes in juvenile hormone titers in honey bee workers due to stress. Apidologie 2004, 35, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Hung, Y.S.; Yang, E.C. Biogenic amine levels change in the brains of stressed honeybees. Arch. Insect Biochem. Physiol. 2008, 68, 241–250. [Google Scholar] [CrossRef]
- Harris, J.W.; Woodring, J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L.) brain. J. Insect Physiol. 1992, 38, 29–35. [Google Scholar]
- Liu, L.; Yu, X.; Meng, F.; Guo, X.; Xu, B. Identification and characterization of a novel corticotropin-releasing hormone-binding protein (CRH-BP) gene from Chinese honeybee (Apis cerana cerana). Arch. Insect Biochem. Physiol. 2011, 78, 161–175. [Google Scholar] [CrossRef]
- Lenoir, J.C.; Laloi, D.; Dechaume-Moncharmont, F.X.; Solignac, M.; Pham, M.H. Intra-colonial variation of the sting extension response in the honey bee Apis mellifera. Insectes Soc. 2006, 53, 80–85. [Google Scholar] [CrossRef]
- Uribe-Rubio, J.; Guzmán-Novoa, E.; Vázquez-Peláez, C.; Hunt, G. Genotype, Task Specialization, and Nest Environment Influence the Stinging Response Thresholds of Individual Africanized and European Honeybees to Electrical Stimulation. Behav. Genet. 2008, 38, 93–100. [Google Scholar] [CrossRef]
- Balderrama, N.; Diaz, H.; Sequeda, A.; Nunez, J.; Maldonato, H. Behavioral and Pharmacological Analysis of the Stinging Response in Africanized and Italian Bees. In Neurobiology and behavior of honeybees; Menzel, R., Mercer, A., Eds.; Springer: Berlin, Germany, 1987; pp. 121–128. [Google Scholar]
- Balderrama, N.; Núñez, J.; Guerrieri, F.; Giurfa, M. Different functions of two alarm substances in the honeybee. J. Comp. Physiol. A 2002, 188, 485–491. [Google Scholar] [CrossRef]
- Roussel, E.; Carcaud, J.; Sandoz, J.C.; Giurfa, M. Reappraising Social Insect Behavior through Aversive Responsiveness and Learning. PLoS One 2009, 4, e4197. [Google Scholar]
- Núñez, J.; Almeida, L.; Balderrama, N.; Giurfa, M. Alarm Pheromone Induces Stress Analgesia via an Opioid System in the Honeybee. Physiol. Behav. 1997, 63, 75–80. [Google Scholar] [CrossRef]
- Núñez, J.; Maldonado, H.; Miralto, A.; Balderrama, N. The stinging response of the honeybee: Effects of morphine, naloxone and some opioid peptides. Pharmacol. Biochem. Behav. 1983, 19, 921–924. [Google Scholar] [CrossRef]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium Toxicity to Honey Bee (Apis mellifera L.) Pollinators: Effects on Behaviors and Survival. PLoS ONE 2012, 7, e34137. [Google Scholar]
- Amdam, G.V.; Fennern, E.; Baker, N.; Rascon, B. Honeybee Associative Learning Performance and Metabolic Stress Resilience Are Positively Associated. PLoS One 2010, 5, e9740. [Google Scholar]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar]
- Corbet, S.A. A Fresh Look at the Arousal Syndrome of Insects. Adv. Insect Physiol. 1991, 23, 81–116. [Google Scholar] [CrossRef]
- Farooqui, T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Papaefthimiou, C.; Theophilidis, G. Octopamine, a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): Acceleration vs. inhibition. J. Insect Physiol. 2011, 57, 316–325. [Google Scholar] [CrossRef]
- Pflüger, H.J.; Duch, C.; Heidel, E. Neuromodulatory octopaminergic neurones and their functions during insect motor behaviour. Acta Biol. Hung. 2004, 55, 3–12. [Google Scholar] [CrossRef]
- Mustard, J.A.; Pham, P.M.; Smith, B.H. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect Physiol. 2010, 56, 422–430. [Google Scholar] [CrossRef]
- Kodrík, D. Adipokinetic hormone functions that are not associated with insect flight. Physiol. Entomol. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Coast, G.M.; Orchard, I.; Phillips, J.E.; Schooley, D.A. Insect diuretic and antidiuretic hormones. Adv. Insect Physiol. 2002, 29, 279–409. [Google Scholar] [CrossRef]
- Veenstra, J.A. Does corazonin signal nutritional stress in insects? Insect Biochem. Mol. Biol. 2009, 39, 755–762. [Google Scholar] [CrossRef]
- Davenport, A.P.; Evans, P.D. Stress-induced changes in the octopamine levels of insect haemolymph. Insect Biochem. 1984, 14, 135–143. [Google Scholar] [CrossRef]
- Kreissl, S.; Eichmüller, S.; Bicker, G.; Rapus, J.; Eckert, M. Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honeybee. J. Comp. Neurol. 1994, 348, 583–595. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.J.; Blenau, W.; Broeck, J.V. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef]
- Crailsheim, K. Intestinal transport of sugars in the honeybee (Apis mellifera L.). J. Insect Physiol. 1988, 34, 839–845. [Google Scholar]
- Blatt, J.; Roces, F. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): Dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J. Exp. Biol. 2001, 204, 2709–2716. [Google Scholar]
- Blatt, J.; Roces, F. The control of the proventriculus in the honeybee (Apis mellifera carnica L.) II. Feedback mechanisms. J. Insect Physiol. 2002, 48, 683–691. [Google Scholar]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Bicker, G.; Menzel, R. Chemical codes for the control of behaviour in arthropods. Nature 1989, 337, 33–39. [Google Scholar] [CrossRef]
- Evans, P.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef]
- Scheiner, R.; Baumann, A.; Blenau, W. Aminergic control and modulation of honeybee behaviour. Curr. Neuropharmacol. 2006, 4, 259–276. [Google Scholar] [CrossRef]
- Sombati, S.; Hoyle, G. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J. Neurobiol. 1984, 15, 481–506. [Google Scholar] [CrossRef]
- Andretic, R.; van Swinderen, B.; Greenspan, R.J. Dopaminergic Modulation of Arousal in Drosophila. Curr. Biol. 2005, 15, 1165–1175. [Google Scholar] [CrossRef]
- Van Swinderen, B.; Andretic, R. Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. R. Soc. B 2011, 278, 906–913. [Google Scholar] [CrossRef]
- Crocker, A.; Shahidullah, M.; Levitan, I.B.; Sehgal, A. Identification of a Neural Circuit that Underlies the Effects of Octopamine on Sleep:Wake Behavior. Neuron 2010, 65, 670–681. [Google Scholar] [CrossRef]
- Lebestky, T.; Chang, J.S.C.; Dankert, H.; Zelnik, L.; Kim, Y.C.; Han, K.A.; Wolf, F.W.; Perona, P.; Anderson, D.J. Two Different Forms of Arousal in Drosophila Are Oppositely Regulated by the Dopamine D1 Receptor Ortholog DopR via Distinct Neural Circuits. Neuron 2009, 64, 522–536. [Google Scholar] [CrossRef]
- Fussnecker, B.L.; Smith, B.H.; Mustard, J.A. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J. Insect Physiol. 2006, 52, 1083–1092. [Google Scholar] [CrossRef]
- Pribbenow, B.; Erber, J. Modulation of Antennal Scanning in the Honeybee by Sucrose Stimuli, Serotonin, and Octopamine: Behavior and Electrophysiology. Neurobiol. Learn. Mem. 1996, 66, 109–120. [Google Scholar] [CrossRef]
- Barron, A.B.; Maleszka, R.; Vander Meer, R.K.; Robinson, G.E. Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 1703–1707. [Google Scholar]
- McQuillan, H.; Barron, A.; Mercer, A. Age- and behaviour-related changes in the expression of biogenic amine receptor genes in the antennae of honey bees (Apis mellifera). J. Comp. Physiol. A 2012, 198, 753–761. [Google Scholar] [CrossRef]
- Menzel, R.; Heyne, A.; Kinzel, C.; Gerber, B.; Fiala, A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav. Neurosci. 1999, 113, 744–754. [Google Scholar] [CrossRef]
- Heisenberg, M. Mushroom body memoir: From maps to models. Nat. Rev. Neurosci. 2003, 4, 266–275. [Google Scholar] [CrossRef]
- Vergoz, V.; Roussel, E.; Sandoz, J.C.; Giurfa, M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One 2007, 2, e288. [Google Scholar] [CrossRef]
- Agarwal, M.; Giannoni Guzmán, M.; Morales-Matos, C.; Del Valle Díaz, R.A.; Abramson, C.I.; Giray, T. Dopamine and Octopamine Influence Avoidance Learning of Honey Bees in a Place Preference Assay. PLoS One 2011, 6, e25371. [Google Scholar]
- Roozendaal, B.F.; McGaugh, J.L. Memory modulation. Behav. Neurosci. 2011, 125, 797–824. [Google Scholar] [CrossRef]
- Huising, M.O.; Metz, J.R.; van Schooten, C.; Taverne-Thiele, A.J.; Hermsen, T.; Kemenade, B.M.; Flik, G. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J. Mol. Endocrinol. 2004, 32, 627–648. [Google Scholar]
- Westphal, N.J.; Seasholtz, A.F. CRH-BP: The regulation and function of a phylogenetically conserved binding protein. Front. Biosci. 2006, 11, 1878–1891. [Google Scholar] [CrossRef]
- Chang, C.L.; Hsu, S.Y.T. Ancient evolution of stress-regulating peptides in vertebrates. Peptides 2004, 25, 1681–1688. [Google Scholar] [CrossRef]
- Huising, M.O.; Flik, G. The Remarkable Conservation of Corticotropin-Releasing Hormone (CRH)-Binding Protein in the Honeybee (Apis mellifera) Dates the CRH System to a Common Ancestor of Insects and Vertebrates. Endocrinology 2005, 146, 2165–2170. [Google Scholar] [CrossRef]
- Lovejoy, D.A.; Balment, R.J. Evolution and Physiology of the Corticotropin-Releasing Factor (CRF) Family of Neuropeptides in Vertebrates. Gen. Comp. Endocr. 1999, 115, 1–22. [Google Scholar] [CrossRef]
- Zandawala, M. Calcitonin-like diuretic hormones in insects. Insect Biochem. Mol. Biol. 2012, 42, 816–825. [Google Scholar] [CrossRef]
- Boerjan, B.; Cardoen, D.; Bogaerts, A.; Landuyt, B.; Schoofs, L.; Verleyen, P. Mass spectrometric profiling of (neuro)-peptides in the worker honeybee, Apis mellifera. Neuropharmacology 2010, 58, 248–258. [Google Scholar] [CrossRef]
- Scharrer, B. The neurosecretory neuron in neuroendocrine regulatory mechanisms. Am. Zool. 1967, 7, 161–169. [Google Scholar]
- De Loof, A.; Lindemans, M.; Liu, F.; de Groef, B.; Schoofs, L. Endocrine archeology: Do insects retain ancestrally inherited counterparts of the vertebrate releasing hormones GnRH, GHRH, TRH, and CRF? G. Comp. Endocr. 2012, 177, 18–27. [Google Scholar]
- Malamud, J.G.; Mizisin, A.P.; Josephson, R.K. The effects of octopamine on contraction kinetics and power output of a locust flight muscle. J. Comp. Physiol. A 1988, 162, 827–835. [Google Scholar] [CrossRef]
- Orchard, I.; Lange, A.B. Evidence for octopaminergic modulation of an insect visceral muscle. J. Neurobiol. 1985, 16, 171–181. [Google Scholar] [CrossRef]
- Luffy, D.; Dorn, A. Immunohistochemical demonstration in the stomatogastric nervous system and effects of putative neurotransmitters on the motility of the isolated midgut of the stick insect, Carausius morosus. J. Insect Physiol. 1992, 38, 287–299. [Google Scholar] [CrossRef]
- Lange, A.B.; Orchard, I. Identified octopaminergic neurons modulate contractions of locust visceral muscle via adenosine 3',5'-monophosphate (cyclic AMP). Brain Res. 1986, 363, 340–349. [Google Scholar] [CrossRef]
- Orchard, I.; Lange, A.B. Cockroach oviducts: The presence and release of octopamine and proctolin. J. Insect Physiol. 1987, 33, 265–268. [Google Scholar] [CrossRef]
- Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 38–49. [Google Scholar] [CrossRef]
- Avila, F.W.; Bloch Qazi, M.C.; Rubinstein, C.D.; Wolfner, M.F. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc. Natl. Acad. Sci. USA 2012, 109, 4562–4567. [Google Scholar]
- Stevenson, P.A.; Pflüger, H.J.; Eckert, M.; Rapus, J. Octopamine-like immunoreactive neurones in locust genital abdominal ganglia. Cell Tissue Res. 1994, 275, 299–308. [Google Scholar] [CrossRef]
- Prier, K.R.; Beckman, O.H.; Tublitz, N.J. Modulating a modulator: Biogenic amines at subthreshold levels potentiate peptide-mediated cardioexcitation of the heart of the tobacco hawkmoth Manduca sexta. J. Exp. Biol. 1994, 197, 377–391. [Google Scholar]
- Hertel, W.; Penzlin, H. Function and modulation of the antennal heart of Periplaneta americana (L.). Acta Biol. Hung. 1992, 43, 113–125. [Google Scholar]
- Collins, C.; Miller, T. Studies on the action of biogenic amines on cockroach heart. J. Exp. Biol. 1977, 67, 1–15. [Google Scholar]
- Johnson, E.; Ringo, J.; Dowse, H. Modulation of Drosophila heartbeat by neurotransmitters. J. Comp. Physiol. B 1997, 167, 89–97. [Google Scholar] [CrossRef]
- Zeng, H.; Loughton, B.G.; Jennings, K.R. Tissue specific transduction systems for octopamine in the locust (Locusta migratoria). J. Insect Physiol. 1996, 42, 765–769. [Google Scholar] [CrossRef]
- Farooqui, T. Octopamine-Mediated Neuromodulation of Insect Senses. Neurochem. Res. 2007, 32, 1511–1529. [Google Scholar] [CrossRef]
- Downer, R.G.H. Trehalose production in isolated fat body of the american cockroach, Periplaneta americana. Comp. Biochem. Physiol. C Comp. Pharmacol. 1979, 62, 31–34. [Google Scholar] [CrossRef]
- Gole, J.W.D.; Downer, R.G.H. Elevation of adenosine 3',5'-monophosphate by octopamine in fat body of the american cockroach, Periplaneta americana L. Comp. Biochem. Physiol. C Comp. Pharmacol. 1979, 64, 223–226. [Google Scholar]
- Orchard, I.; Carlisle, J.A.; Loughton, B.G.; Gole, J.W.D.; Downer, R.G.H. In vitro studies on the effects of octopamine on locust fat body. Gen. Comp. Endocr. 1982, 48, 7–13. [Google Scholar] [CrossRef]
- Meyer-Fernandes, J.R.; Gondim, K.C.; Wells, M.A. Developmental changes in the response of larval Manduca sexta fat body glycogen phosphorylase to starvation, stress and octopamine. Insect Biochem. Mol. Biol. 2000, 30, 415–422. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Goosey, M.W.; Candy, D.J. The release and removal of octopamine by tissues of the locust Schistocerca americana gregaria. Insect Biochem. 1982, 12, 681–685. [Google Scholar] [CrossRef]
- Martin, R.J.; Jahagirdar, A.P.; Downer, R.G.H. Partial characterization of N-acetyltransferase activity from cerebral ganglia and malpighian tubules of Periplaneta americana. Insect Biochem. 1989, 19, 351–359. [Google Scholar] [CrossRef]
- David, J.C.; Lafon-Cazal, M. Octopamine distribution in the Locusta migratoria nervous and non-nervous, systems. Comp. Biochem. Physiol. C Comp. Pharmacol. 1979, 64, 161–164. [Google Scholar]
- Lam, F.; McNeil, J.N.; Donly, C. Octopamine receptor gene expression in three lepidopteran species of insect. Peptides. 2012, in press. [Google Scholar]
- Adamo, S.A.; Baker, J.L. Conserved features of chronic stress across phyla: The effects of long-term stress on behavior and the concentration of the neurohormone octopamine in the cricket, Gryllus texensis. Horm. Behav. 2011, 60, 478–483. [Google Scholar] [CrossRef]
- Duch, C.; Pflüger, H.J. DUM neurons in locust flight: A model system for amine-mediated peripheral adjustments to the requirements of a central motor program. J. Comp. Physiol. A 1999, 184, 489–499. [Google Scholar] [CrossRef]
- Orchard, I.; Ramirez, J.M.; Lange, A.B. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 1993, 38, 227–249. [Google Scholar] [CrossRef]
- Bellah, K.L.; Fitch, G.K.; Kammer, A.E. A central action of octopamine on ventilation frequency in Corydalus cornutus. J. Exp. Zool. 1984, 231, 289–292. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Pearson, K.G. Octopamine induces bursting and plateau potentials in insect neurones. Brain Res. 1991, 549, 332–337. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.F.; Li, X.H.; Adamo, S.A.; Ye, G.Y. The characterization of a concentration-sensitive adrenergic-like octopamine receptor found on insect immune cells and its possible role in mediating stress hormone effects on immune function. Brain Behav. Immun. 2012, 26, 942–950. [Google Scholar] [CrossRef]
- Bicker, G. Biogenic amines in the brain of the honeybee: Cellular distribution, development, and behavioral functions. Micros. Res. Tech. 1999, 44, 166–178. [Google Scholar] [CrossRef]
- Sinakevitch, I.; Niwa, M.; Strausfeld, N.J. Octopamine-like immunoreactivity in the honey bee and cockroach: Comparable organization in the brain and subesophageal ganglion. J. Comp. Neurol. 2005, 488, 233–254. [Google Scholar] [CrossRef]
- Schröter, U.; Malun, D.; Menzel, R. Innervation pattern of suboesophageal ventral unpaired median neurones in the honeybee brain. Cell Tissue Res. 2007, 327, 647–667. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Sporhase-Eichmann, U. Localization of octopaminergic neurones in insects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1995, 110, 203–215. [Google Scholar]
- Bräunig, P. The peripheral branching pattern of identified dorsal unpaired median (DUM) neurones of the locust. Cell Tissue Res. 1997, 290, 641–654. [Google Scholar] [CrossRef]
- Bräunig, P.; Pflüger, H.J. The unpaired median neurons of insects. Adv. Insect Physiol. 2001, 28, 185–266. [Google Scholar] [CrossRef]
- Field, L.H.; Duch, C.; Pflüger, H.J. Responses of efferent octopaminergic thoracic unpaired median neurons in the locust to visual and mechanosensory signals. J. Insect Physiol. 2008, 54, 240–254. [Google Scholar] [CrossRef]
- Bräunig, P. Dorsal unpaired median (DUM) neurones with neurohaemal functions in the locust, Locusta migratoria. Acta Biol. Hung. 1995, 46, 471–479. [Google Scholar]
- Bräunig, P.; Stevenson, P.A.; Evans, P.D. A locust octopamine-immunoreactive dorsal unpaired median neurone forming terminal networks on sympathetic nerves. J. Exp. Biol. 1994, 192, 225–238. [Google Scholar]
- Veenstra, J.A. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989, 250, 231–234. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Tanaka, S.; de Loof, A.; Schoofs, L.; Baggerman, G.; Waelkens, E.; Derua, R.; Milner, Y.; Yerushalmi, Y.; Pener, M.P. Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc. Natl. Acad. Sci. USA 1999, 96, 7083–7087. [Google Scholar]
- Kim, Y.J.; Spalovska-Valachova, I.; Cho, K.H.; Zitnanova, I.; Park, Y.; Adams, M.E.; Zitnan, D. Corazonin receptor signaling in ecdysis initiation. Proc. Natl. Acad. Sci. USA 2004, 101, 6704–6709. [Google Scholar]
- Roller, L.; Tanaka, S.; Kimura, K.; Satake, H.; Tanaka, Y. Molecular cloning of [Thr4], [His7]-corazonin (Apime-corazonin) and its distribution in the central nervous system of the honey bee Apis mellifera (Hymenoptera: Apidae). Appl. Entomol. Zool. 2006, 41, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Verleyen, P.; Baggerman, G.; Mertens, I.; Vandersmissen, T.; Huybrechts, J.; Lommel, A.V.; de Loof, A.; Schoofs, L. Cloning and characterization of a third isoform of corazonin in the honey bee Apis mellifera. Peptides 2006, 27, 493–499. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Wang, J.; Dow, J.A.T. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007, 39, 715–720. [Google Scholar] [CrossRef]
- Johnson, E.C.; Shafer, O.T.; Trigg, J.S.; Park, J.; Schooley, D.A.; Dow, J.A.; Taghert, P.H. A novel diuretic hormone receptor in Drosophila: Evidence for conservation of CGRP signaling. J. Exp. Biol. 2005, 208, 1239–1246. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.J.; Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, Corazonin, and AKH supports a theory of lignad-receptor coevolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11423–11428. [Google Scholar] [CrossRef]
- Hansen, K.K.; Stafflinger, E.; Schneider, M.; Hauser, F.; Cazzamali, G.; Williamson, M.; Kollmann, M.; Schachtner, J.; Grimmelikhuijzen, C.J.P. Discovery of a Novel Insect Neuropeptide Signaling System Closely Related to the Insect Adipokinetic Hormone and Corazonin Hormonal Systems. J. Biol. Chem. 2010, 285, 10736–10747. [Google Scholar]
- Bendena, W.G.; Donly, B.C.; Tobe, S.S. Allatostatins: A Growing Family of Neuropeptides with Structural and Functional Diversity. Ann. NY Acad. Sci. 1999, 897, 311–329. [Google Scholar] [CrossRef]
- Stay, B.; Tobe, S.S. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 2007, 52, 277–299. [Google Scholar] [CrossRef]
- Audsley, N.; Weaver, R.J. Neuropeptides associated with the regulation of feeding in insects. Gen. Comp. Endocr. 2009, 162, 93–104. [Google Scholar] [CrossRef]
- Veenstra, J.A. Allatostatin C and its paralog allatostatin double C: The arthropod somatostatins. Insect Biochem. Mol. Biol. 2009, 39, 161–170. [Google Scholar] [CrossRef]
- Meyering-Vos, M.; Woodring, J. A-type allatostatins and sulfakinins as satiety effectors in the Mediterranean field cricket Gryllus bimaculatus. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2008, 16, 409–412. [Google Scholar]
- Veenstra, J.; Agricola, H.J.; Sellami, A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008, 334, 499–516. [Google Scholar] [CrossRef]
- Wilson, C.H.; Christie, A.E. Distribution of C-type allatostatin (C-AST)-like immunoreactivity in the central nervous system of the copepod Calanus finmarchicus. Gen. Comp. Endocr. 2010, 167, 252–260. [Google Scholar] [CrossRef]
- Robertson, L.; Rodriguez, E.P.; Lange, A.B. The neural and peptidergic control of gut contraction in Locusta migratoria: The effect of an FGLa/AST. J. Exp. Biol. 2012, 215, 3394–3402. [Google Scholar] [CrossRef]
- Wang, C.; Chin-Sang, I.; Bendena, W.G. The FGLamide-Allatostatins Influence Foraging Behavior in Drosophila melanogaster. PLoS One 2012, 7, 36059. [Google Scholar]
- Audsley, N.; Matthews, J.; Nachman, R.J.; Weaver, R.J. Transepithelial flux of an allatostatin and analogs across the anterior midgut of Manduca sexta larvae in vitro. Peptides 2008, 29, 286–294. [Google Scholar] [CrossRef]
- Mayoral, J.G.; Nouzova, M.; Brockhoff, A.; Goodwin, M.; Hernandez-Martinez, S.; Richter, D.; Meyerhof, W.; Noriega, F.G. Allatostatin-C receptors in mosquitoes. Peptides 2010, 31, 442–450. [Google Scholar] [CrossRef]
- Hergarden, A.C.; Tayler, T.D.; Anderson, D.J. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 3967–3972. [Google Scholar]
- Kreissl, S.; Strasser, C.; Galizia, C.G. Allatostatin immunoreactivity in the honeybee brain. J. Comp. Neurol. 2010, 518, 1391–1417. [Google Scholar] [CrossRef]
- Gade, G.; Auerswald, L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocr. 2003, 132, 10–20. [Google Scholar] [CrossRef]
- Bharucha, K.N.; Tarr, P.; Zipursky, S.L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 2008, 211, 3103–3110. [Google Scholar] [CrossRef]
- Isabel, G.; Martin, J.R.; Chidami, S.; Veenstra, J.A.; Rosay, P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 531–538. [Google Scholar]
- Lee, G.; Park, J.H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef]
- Wicher, D. Metabolic Regulation and Behavior: How Hunger Produces Arousal—An Insect Study. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 304–310. [Google Scholar] [CrossRef]
- Pannabecker, T.; Orchard, I. Octopamine and cyclic AMP mediate release of adipokinetic hormone I and II from isolated locust neuroendocrine tissue. Mol. Cell Endocrinol. 1986, 48, 153–159. [Google Scholar] [CrossRef]
- Passier, P.C.C.M.; Vullings, H.G.B.; Diederen, J.H.B.; van der Horst, D.J. Modulatory Effects of Biogenic Amines on Adipokinetic Hormone Secretion from Locust Corpora Cardiaca in Vitro. Gen. Comp. Endocr. 1995, 97, 231–238. [Google Scholar] [CrossRef]
- Pannabecker, T.; Orchard, I. Regulation of adipokinetic hormone release from locust neuroendocrine tissue: Participation of calcium and cyclic AMP. Brain Res. 1987, 423, 13–22. [Google Scholar] [CrossRef]
- Candy, D.J. Adipokinetic hormones concentrations in the haemolymph of Schistocerca gregaria, measured by radioimmunoassay. Insect Biochem. Mol. Biol. 2002, 32, 1361–1367. [Google Scholar] [CrossRef]
- Kodrík, D.; Socha, R. The effect of insecticide on adipokinetic hormone titre in the insect body. Pest Manage. Sci. 2005, 61, 1077–1082. [Google Scholar] [CrossRef]
- Velki, M.; Kodrík, D.; Vecera, J.; Hackenberger, B.K.; Socha, R. Oxidative stress elicited by insecticides: A role for the adipokinetic hormone. Gen. Comp. Endocr. 2011, 172, 77–84. [Google Scholar] [CrossRef]
- Večeřa, J.; Krishnan, N.; Mithöfer, A.; Vogel, H.; Kodrík, D. Adipokinetic hormone-induced antioxidant response in Spodoptera littoralis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 389–395. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Kellner, R.; Woodring, J.; Hoffmann, K.H.; Gade, G. Hypertrehalosaemic peptides in the honeybee (Apis mellifera): Purification, identification and function. J. Insect Physiol. 1999, 45, 647–653. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—The Insect “Blood” Sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar] [CrossRef]
- Van der Horst, D.J. Insect adipokinetic hormones: Release and integration of flight energy metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 217–226. [Google Scholar] [CrossRef]
- Panzenbock, U.; Crailsheim, K. Glycogen in honeybee queens, workers and drones (Apis mellifera carnica Pollm.). J. Insect Physiol. 1997, 43, 155–165. [Google Scholar]
- Woodring, J.; Das, S.; Gade, G. Hypertrehalosemic factors from the corpora cardiaca of the honeybee (Apis mellifera) and the paper wasp (Polistes exclamans). J. Insect Physiol. 1994, 40, 685–692. [Google Scholar] [CrossRef]
- Kunieda, T.; Fujiyuki, T.; Kucharski, R.; Foret, S.; Ament, S.A.; Toth, A.L.; Ohashi, K.; Takeuchi, H.; Kamikouchi, A.; Kage, E.; et al. Carbohydrate metabolism genes and pathways in insects: insights from the honey bee genome. Insect Mol. Biol. 2006, 15, 563–576. [Google Scholar] [CrossRef]
- Nassel, D.R. Tachykinin-related peptides in invertebrates: a review. Peptides 1999, 20, 141–158. [Google Scholar] [CrossRef]
- Hauser, F.; Cazzamali, G.; Williamson, M.; Blenau, W.; Grimmelikhuijzen, C.J.P. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 2006, 80, 1–19. [Google Scholar] [CrossRef]
- Broughton, S.J.; Piper, M.D.W.; Ikeya, T.; Bass, T.M.; Jacobson, J.; Driege, Y.; Martinez, P.; Hafen, E.; Withers, D.J.; Leevers, S.J.; et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 3105–3110. [Google Scholar]
- Pinto, L.Z.; Bitondi, M.M.G.; Simões, Z.L.P. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000, 46, 153–160. [Google Scholar] [CrossRef]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocr. 2012, in press. [Google Scholar]
- Gruntenko, N.E.; Bogomolova, E.V.; Adonyeva, N.V.; Karpova, E.K.; Menshanov, P.N.; Alekseev, A.A.; Romanova, I.V.; Li, S.; Rauschenbach, I.Y. Decrease in juvenile hormone level as a result of genetic ablation of the Corpus allatum cells affects the synthesis and metabolism of stress related hormones in Drosophila. J. Insect Physiol. 2012, 58, 49–55. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of honey bee age polyethism by juvenile hormone. Behav. Ecol. Sociobiol. 1987, 20, 329–338. [Google Scholar] [CrossRef]
- Schulz, D.J.; Sullivan, J.P.; Robinson, G.E. Juvenile Hormone and Octopamine in the Regulation of Division of Labor in Honey Bee Colonies. Horm. Behav. 2002, 42, 222–231. [Google Scholar] [CrossRef]
- Shimizu, T.; Mihara, M.; Takeda, N. High-performance liquid chromatography of biogenic amines in the corpus cardiacum of the American cockroach, Periplaneta americana. J. Chromatogr. A 1991, 539, 193–197. [Google Scholar] [CrossRef]
- Bateson, M.; Desire, S.; Gartside, S.E.; Wright, G.A. Agitated Honeybees Exhibit Pessimistic Cognitive Biases. Curr. Biol. 2011, 21, 1070–1073. [Google Scholar] [CrossRef]
- Adamo, S.A. The effects of the stress response on immune function in invertebrates: An evolutionary perspective on an ancient connection. Horm. Behav. 2012, 62, 324–330. [Google Scholar] [CrossRef]
- Adamo, S.A.; Parsons, N.M. The emergency life-history stage and immunity in the cricket, Gryllus texensis. Anim. Behav. 2006, 72, 235–244. [Google Scholar] [CrossRef]
- Baines, D.; DeSantis, T.; Downer, R.G.H. Octopamine and 5-hydroxytryptamine enhance the phagocytic and nodule formation activities of cockroach (Periplaneta americana) haemocytes. J. Insect Physiol. 1992, 38, 905–914. [Google Scholar] [CrossRef]
- Mowlds, P.; Barron, A.; Kavanagh, K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microbes Infect. 2008, 10, 628–634. [Google Scholar] [CrossRef]
- Adamo, S.A.; Roberts, J.L.; Easy, R.H.; Ross, N.W. Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J. Exp. Biol. 2008, 211, 531–538. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar]
- Köhler, A.; Pirk, C.W.W.; Nicolson, S.W. Simultaneous stressors: Interactive effects of an immune challenge and dietary toxin can be detrimental to honeybees. J. Insect Physiol. 2012, 58, 918–923. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar]
- Robinson, G.E. Regulation of Division of Labor in Insect Societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
- Slessor, K.; Winston, M.; Le Conte, Y. Pheromone Communication in the Honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar]
- Winston, M.L. The biology of the honey bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Wagener-Hulme, C.; Kuehn, J.C.; Schulz, D.J.; Robinson, G.E. Biogenic amines and division of labor in honey bee colonies. J. Comp. Physiol. A 1999, 184, 471–479. [Google Scholar] [CrossRef]
- Fahrbach, S.E.; Robinson, G.E. Juvenile hormone, behavioral maturation, and brain structure in the honey bee. Dev. Neurosci. 1996, 18, 102–114. [Google Scholar] [CrossRef]
- Williams, J.B.; Roberts, S.P.; Elekonich, M.M. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp. Gerontol. 2008, 43, 538–549. [Google Scholar] [CrossRef]
- Schulz, D.J.; Barron, A.B.; Robinson, G.E. A role for octopamine in honey bee division of labor. Brain Behav. Evolut. 2002, 60, 350–359. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar]
- Hauser, F.; Neupert, S.; Williamson, M.; Predel, R.; Tanaka, Y.; Grimmelikhuijzen, C.J.P. Genomics and Peptidomics of Neuropeptides and Protein Hormones Present in the Parasitic Wasp Nasonia vitripennis. J. Proteome Res. 2010, 9, 5296–5310. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Even, N.; Devaud, J.-M.; Barron, A.B. General Stress Responses in the Honey Bee. Insects 2012, 3, 1271-1298. https://doi.org/10.3390/insects3041271
Even N, Devaud J-M, Barron AB. General Stress Responses in the Honey Bee. Insects. 2012; 3(4):1271-1298. https://doi.org/10.3390/insects3041271
Chicago/Turabian StyleEven, Naïla, Jean-Marc Devaud, and Andrew B. Barron. 2012. "General Stress Responses in the Honey Bee" Insects 3, no. 4: 1271-1298. https://doi.org/10.3390/insects3041271
APA StyleEven, N., Devaud, J.-M., & Barron, A. B. (2012). General Stress Responses in the Honey Bee. Insects, 3(4), 1271-1298. https://doi.org/10.3390/insects3041271




