Nuclear Immunolocalization of Hexamerins in the Fat Body of Metamorphosing Honey Bees
Abstract
1. Introduction
2. Experimental Section
2.1. Bee Sampling
2.2. Sample Preparation for SDS-PAGE and Western Blot
2.3. Immunolocalization of the Hexamerins in the Fat Body Cells
2.4. Injection of Antibodies against Hexamerins
3. Results
3.1. Dynamics of Hexamerin Expression in the Hemolymph and Fat Body during and after Metamorphosis
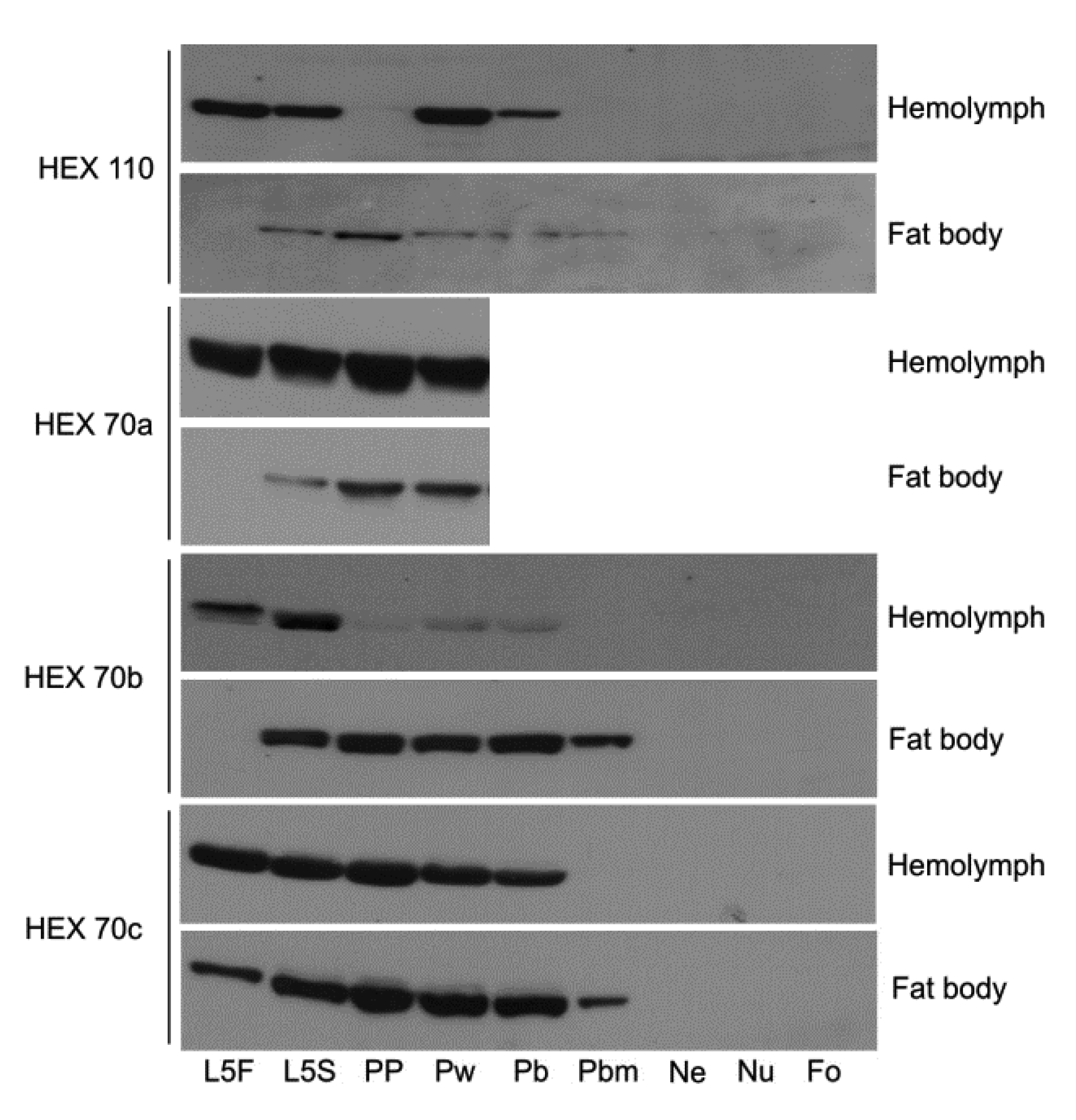
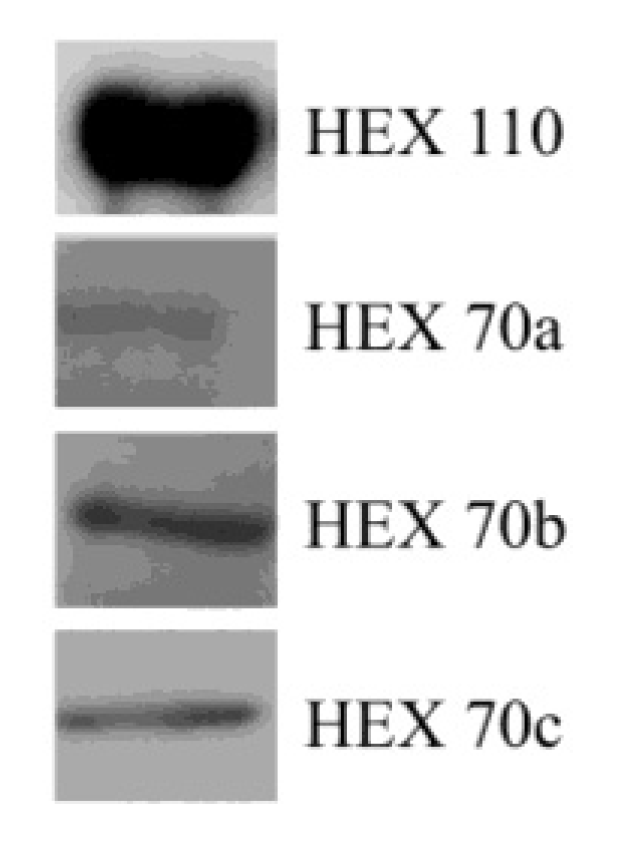
3.2. Detection of Hexamerins in Isolated Fat Body Cell Nuclei
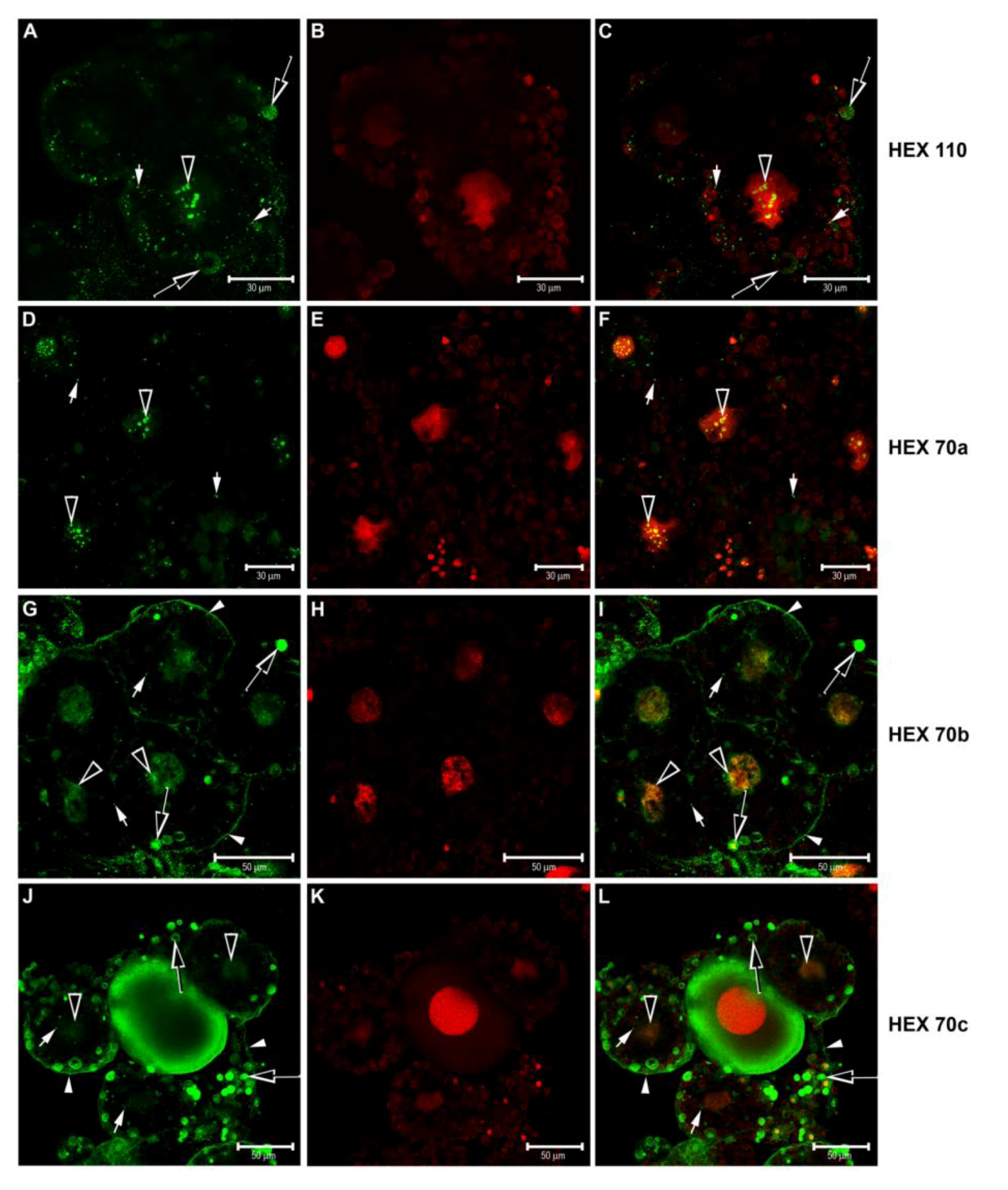
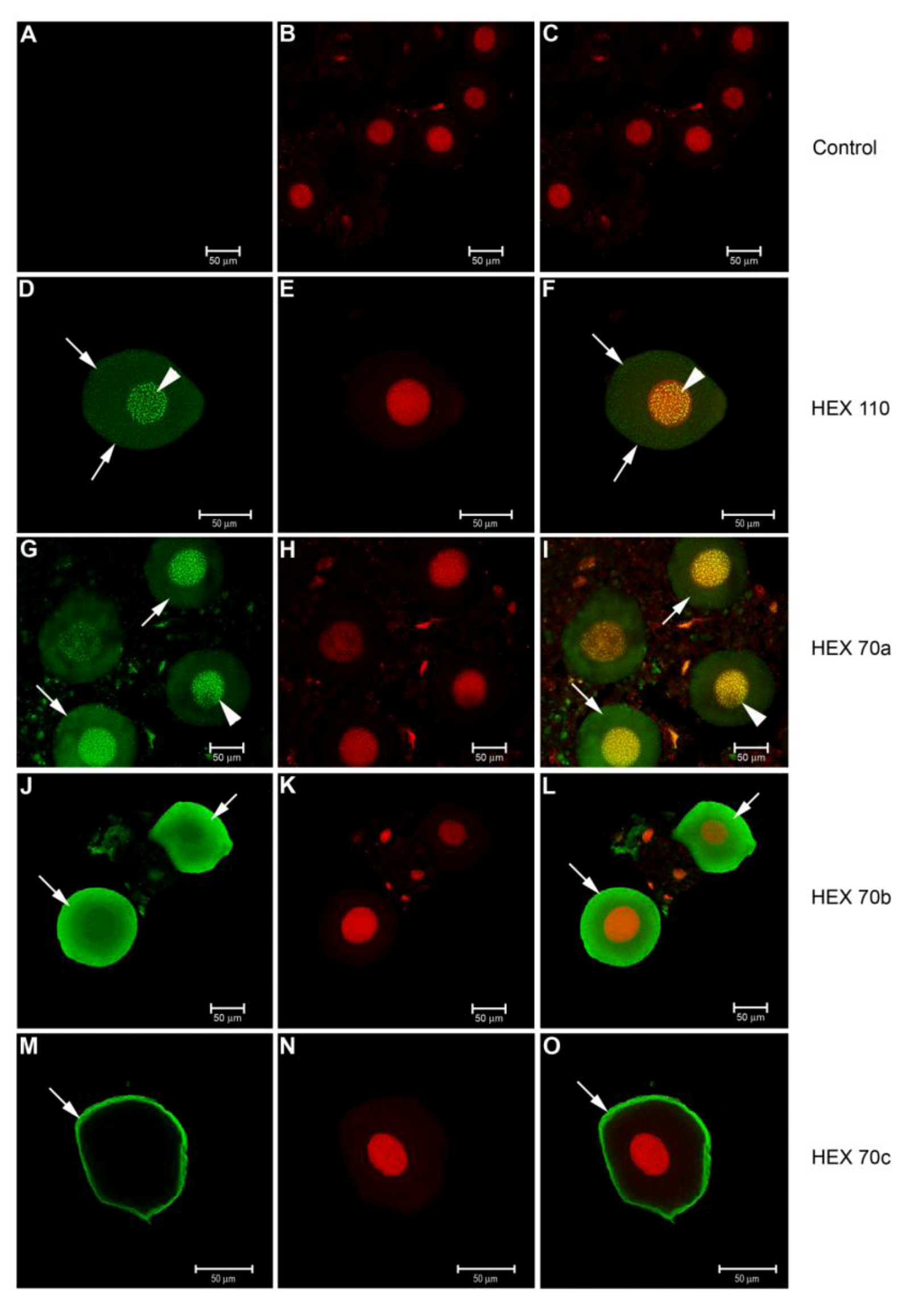
3.3. Immunolocalization of Hexamerins in the Cytoplasm and Nucleus of Fat Body Cells
3.4. Injection of Antibodies against Hexamerins in Pharate Adults: Effect on Survival and Timing to Adult Ecdysis
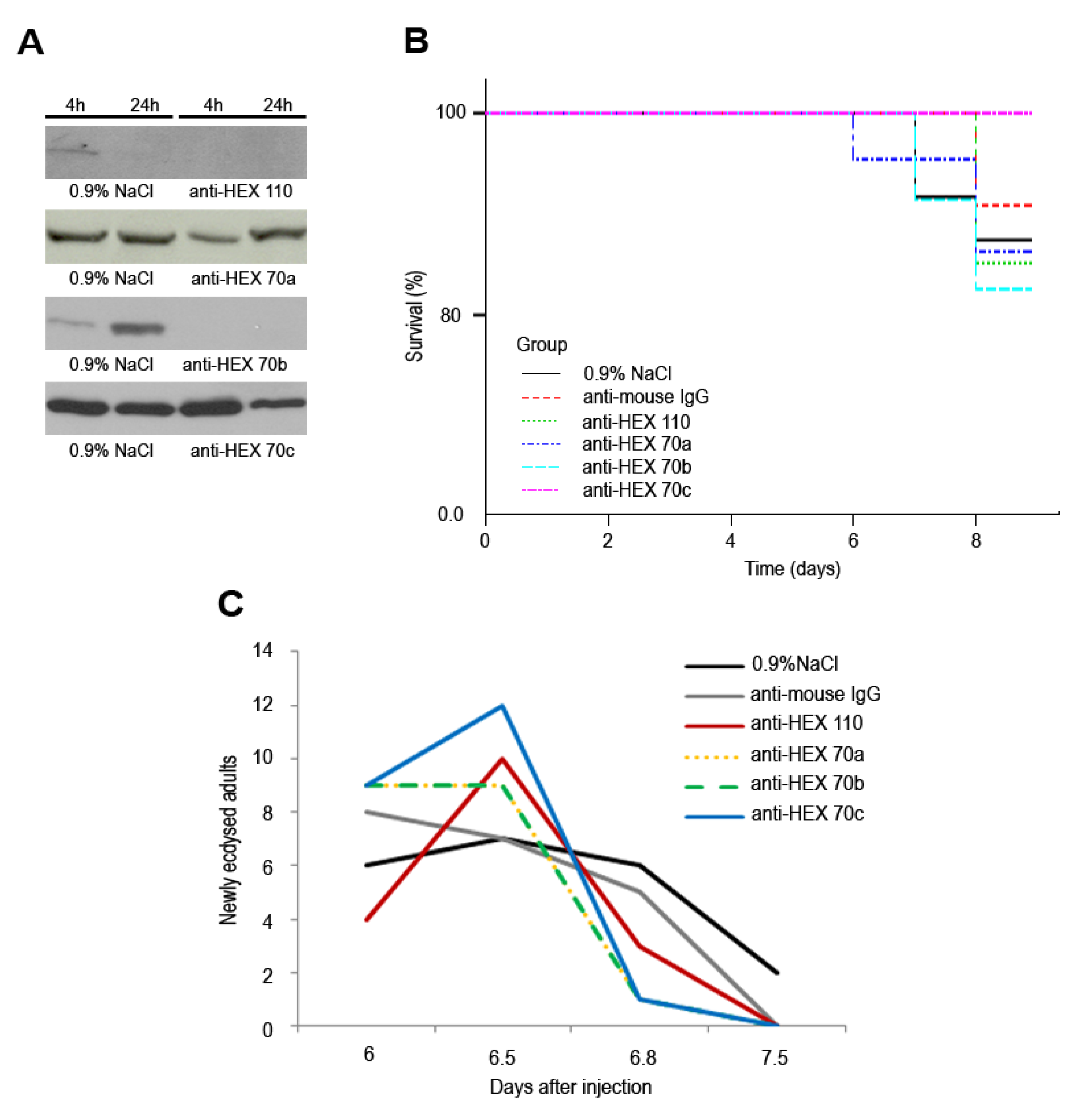
4. Discussion and Conclusions
Acknowledgments
References
- Keeley, L.L. Physiology and biochemistry of the fat body. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert., L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 3, pp. 211–248. [Google Scholar]
- Dean, R.L.; Locke, M.; Collins, J.V. Structure of the fat body. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 3, pp. 155–210. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect Fat body: Energy, metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Munn, E.A.; Feinstein, A.; Greville, G.D. A major protein constituent of pupae of the blowfly Calliphora erythrocephala (Diptera). Biochem. J. 1967, 102, 5–6. [Google Scholar]
- Munn, E.A.; Price, G.M.; Greville, G.D. The synthesis in vitro of the protein calliphorin by fat body from the larva of the blowfly, Calliphora erythrocephala. J. Insect Physiol. 1969, 15, 1601–1605. [Google Scholar] [CrossRef]
- Munn, E.A.; Feinstein, A.; Greville, G.D. The isolation and properties of the protein calliphorin. Biochem. J. 1971, 124, 367–374. [Google Scholar]
- Munn, E.; Greville, G. The soluble proteins of developing Calliphora erythrocephala, particularly calliphorin, and similar proteins in other insects. J. Insect Physiol. 1969, 15, 1935–1950. [Google Scholar] [CrossRef]
- Wyatt, G.; Pan, M. Insect plasma proteins. Annu. Rev. Biochem. 1978, 47, 779–817. [Google Scholar]
- Levenbook, L. Insect storage proteins. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 9, pp. 307–346. [Google Scholar]
- Kanost, M.R.; Kawooya, J.K.; Law, J.H.; Ryan, R.O.; Van Heusden, M.C.; Ziegler, R. Insect hemolymph proteins. Adv. Insect Physiol. 1990, 22, 299–369. [Google Scholar] [CrossRef]
- Burmester, T. Origin and evolution of arthropod hemocyanins and related proteins. J. Comp. Physiol. 2002, 172, 95–107. [Google Scholar]
- Locke, M.; Collins, J.V. Protein uptake into multivesicular bodies and storage granule in the fat body of an insect. J. Cell Biol. 1968, 36, 453–483. [Google Scholar] [CrossRef]
- Tojo, S.; Betchaku, T.; Ziccardi, V.; Wyatt, G. Fat body protein granules and storage proteins in the silkmoth, Hyalophora cecropia. J. Cell Biol. 1978, 78, 823–838. [Google Scholar] [CrossRef]
- Tojo, S.; Nagata, M.; Kobayashi, M. Storage proteins of the silkworm, Bombyx mori. Insect Biochem. 1980, 10, 289–303. [Google Scholar] [CrossRef]
- Butterworth, F.M.; Tysell, B.; Waclawski, I. The effect of 20-hydroxyecdysone and protein on granule formation in the in vitro cultured fat body of Drosophila. J. Insect Physiol. 1979, 25, 855–860. [Google Scholar] [CrossRef]
- Koopmanschap, A.B.; deKort, C.A.D. Isolation and characterization of a high molecular weight JH-III transport protein in the hemolymph of Locusta migratoria. Arch. Insect Biochem. Physiol. 1988, 7, 105–118. [Google Scholar] [CrossRef]
- Braun, R.P.; Wyatt, G.R. Sequence of the hexameric juvenile hormone binding protein from the hemolymph of Locusta migratoria. J. Biol. Chem. 1996, 271, 31756–31762. [Google Scholar]
- Ismail, S.M.; Gillott, C. Identification, characterization, and developmental profile of a high molecular weight, juvenile hormone binding protein in the hemolymph of the migratory grasshopper, Melanoplus sanguinipes. Arch. Insect Biochem. Physiol. 1995, 29, 415–430. [Google Scholar] [CrossRef]
- Zalewska, M.; Kochman, A.; Esteve, J.P.; Lopez, F.; Chaoui, K.; Susini, C.; Ozyhar, A.; Kochman, M. Juvenile hormone binding protein traffic—Interaction with ATP synthase and lipid transfer proteins. BBA Biomembranes 2009, 1788, 1695–1705. [Google Scholar] [CrossRef]
- Zhou, X.; Oi, F.M.; Scharf, M.E. Social exploitation of hexamerin, RNAi reveals a major caste-regulatory factor in termites. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 4499–4504. [Google Scholar]
- Zhou, X.; Tarver, M.R.; Bennett, G.W.; Oi, F.M.; Scharf, M.E. Two hexamerin genes from the termite Reticulitermes flavipes, sequence, expression, and proposed functions in caste regulation. Gene 2006, 376, 47–58. [Google Scholar] [CrossRef]
- Zhou, X.; Tarver, M.R.; Scharf, M.E. Hexamerin-based regulation of juvenile hormone dependent gene expression underlies phenotypic plasticity in a social insect. Development 2007, 134, 601–610. [Google Scholar]
- Scharf, M.E.; Buckspan, C.E.; Grzymala, T.L.; Zhou, X. Regulation of polyphenic caste differentiation in the termite Reticulitermes flavipes by interaction of intrinsic and extrinsic factors. J. Exp. Biol. 2007, 210, 4390–4398. [Google Scholar] [CrossRef]
- Ryan, R.O.; Schmidt, J.O.; Law, J.H. Arylphorin from the haemolymph of the larval honey bee. Insect Biochem. 1984, 14, 515–520. [Google Scholar] [CrossRef]
- Danty, E.; Arnold, G.; Burmester, T.; Huet, J.C.; Huet, D.; Pernollet, J.C.; Masson, C. Identification and developmental profiles of hexamerins in antenna and hemolymph of the honeybee, Apis mellifera. Insect Biochem. Mol. Biol. 1998, 28, 387–397. [Google Scholar] [CrossRef]
- Cunha, A.D.; Nascimento, A.M.; Guidugli, K.R.; Simões, Z.L.P.; Bitondi, M.M.G. Molecular cloning and expression of a hexamerin cDNA from the honey bee, Apis mellifera. J. Insect Physiol. 2005, 51, 1135–1147. [Google Scholar] [CrossRef]
- Bitondi, M.M.G.; Nascimento, A.M.; Cunha, A.D.; Guidugli, K.R.; Nunes, F.M.F.; Simões, Z.L.P. Characterization and expression of the hex 110 gene encoding a glutamine-rich hexamerin in the honey bee, Apis mellifera. Arch. Insect Biochem. Physiol. 2006, 63, 57–72. [Google Scholar] [CrossRef]
- Martins, J.R.; Nunes, F.M.F.; Simões, Z.L.P.; Bitondi, M.M.G. A honeybee storage protein gene, hex 70a, expressed in developing gonads and nutritionally regulated in adult fat body. J. Insect Physiol. 2008, 54, 867–877. [Google Scholar] [CrossRef]
- Martins, J.R.; Nunes, F.M.F.; Cristino, A.S.; Simões, Z.L.P.; Bitondi, M.M.G. The four hexamerin genes in the honey bee: Structure, molecular evolution and function deduced from expression patterns in queens, workers and drones. BMC Mol. Biol. 2010, 11, 23. [Google Scholar] [CrossRef]
- Martins, J.R.; Anhezini, L.; Dallacqua, R.P.; Simões, Z.L.P.; Bitondi, M.M.G. A honey bee hexamerin, HEX 70a, is likely to play an intranuclear role in developing and mature ovarioles and testioles. PLoS One 2011, 6, e29006. [Google Scholar]
- Kirankumar, N.; Ismail, S.M.; Dutta-Gupta, A. Uptake of storage protein in the Rice Moth Corcyra cephalonica: Identification of storage protein binding proteins in the fat body cell membranes. Insect Biochem. Mol. Biol. 1997, 27, 671–679. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–682. [Google Scholar] [CrossRef]
- Robbs, S.L.; Ryan, R.O.; Schmidt, J.O.; Keim, P.S.; Law, J.H. Lipophorin of the larval honeybee, Apis mellifera L. J. Lipid Res. 1985, 26, 241–247. [Google Scholar]
- Larsen, W.J. Cell remodeling in the fat body of an insect. Tissue Cell 1976, 8, 73–92. [Google Scholar] [CrossRef]
- de Priester, W.; van der Molen, L.G. Premetamorphic changes in the ultrastructure of Calliphora fat cells. Cell Tissue Res. 1979, 198, 79–93. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation by Statistical Computing: Viena, Austria, 2011. Available online: http://www.r-project.org (accessed on 19 October 2012).
- Hoshizaki, D.K. Fat cell development. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Elsevier: Oxford, UK, 2005; Volume 2.9, pp. 315–345. [Google Scholar]
- Snodgrass, R.E. The fat body, urate cells and oenocytes. In Anatomy of the Honey Bee; Cornell University Press: Ithaca, NY, USA, 1956. [Google Scholar]
- Cruz-Landim, C. Abelhas—Morfologia e Função de Sistemas (in Portuguese); Editora Unesp: São Paulo, Brazil, 2009; pp. 159–160. [Google Scholar]
- Bishop, G.H. Cell metabolism in the insect fat body.I. Cytological changes accompanying growth and histolysis of the fat body of Apis mellifica. J. Morphol. 1922, 36, 567–601. [Google Scholar] [CrossRef]
- Bishop, G.H. Cell metabolism in the insect fat body. II. A functional interpretation of the changes in structure in the fat body cells of the honey bee. J. Morphol. 1923, 37, 533–553. [Google Scholar] [CrossRef]
- Poiani, S.B.; Cruz-Landim, C. Storaged products and presence of acid phosphatase in fat body cells at pre-pupal worker stage of Apis mellifera Linnaeus, 1758 (Hymenoptera, Apidae). Micron 2012, 43, 475–478. [Google Scholar] [CrossRef]
- Tysell, B.; Butterworth, F.M. Different rate of protein granule formation in the larval fat body of Drosophila melanogaster. J. Insect Physiol. 1978, 24, 201–206. [Google Scholar] [CrossRef]
- Burns, K.A.; Gutzwiller, L.M.; Tomoyasu, Y.; Gebelein, B. Oenocyte development in the red flour beetle Tribolium castaneum. Dev. Gene Evol. 2012, 222, 77–88. [Google Scholar] [CrossRef]
- Loeb, M.J.; Hakim, R.S. Insect midgut epithelium in vitro: An insect stem cell system. J. Insect Physiol. 1996, 42, 1103–1111. [Google Scholar] [CrossRef]
- Sadrud-Din, S.Y.; Hakim, R.S.; Loeb, M. Proliferation and differentiation of midgut epithelial cells from Manduca sexta, in vitro. Invert. Reprod. Dev. 1994, 26, 197–204. [Google Scholar] [CrossRef]
- Sadrud-Din, S.Y.; Loeb, M.J.; Hakim, R.S. In vitro differentiation of isolated stem cells from the midgut of Manduca sexta larvae. J. Exp. Biol. 1996, 199, 319–325. [Google Scholar] [CrossRef]
- Blackburn, M.B.; Loeb, M.J.; Clark, E.; Jaffe, H. Stimulation of midgut stem cell proliferation by Manduca sexta α-arylphorin. Arch. Insect Biochem. Physiol. 2004, 55, 26–32. [Google Scholar] [CrossRef]
- Hakim, R.S.; Blackburn, M.B.; Corti, P.; Gelman, D.B.; Goodman, C.; Elsen, K.; Loeb, M.J.; Lynn, D.; Soin, T.; Smagghe, G. Growth and mitogenic effects of arylphorin in vivo and in vitro. Arch. Insect Biochem. Physiol. 2007, 64, 63–73. [Google Scholar] [CrossRef]
- Cermenati, G.; Corti, P.; Caccia, S.; Giordana, B.; Casartelli, M. A morphological and functional characterization of Bombyx mori larval midgut cells in culture. Invert. Suv. J. 2007, 4, 119–126. [Google Scholar]
- Tanaka, E.D.; Hartfelder, K. The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes. Arthropod Struct. Dev. 2004, 33, 431–442. [Google Scholar] [CrossRef]
- Rhee, W.J.; Lee, E.H.; Park, J.H.; Lee, J.E.; Park, T.H. Inhibition of HeLa Cell apoptosis by storage-protein 2. Biotechnol. Prog. 2007, 23, 1441–1446. [Google Scholar] [CrossRef]
- Guo, C.; Yu, S.; Davis, A.T.; Wang, H.; Green, J.E.; Ahmed, K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J. Biol. Chem. 2001, 276, 5992–5999. [Google Scholar]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Trembley, J.H.; Wang, G.; Unger, G.; Slaton, J.; Ahmed, K. Protein kinase CK2 in health and disease: CK2: A key player in cancer biology. Cell Mol. Life Sci. 2009, 66, 1858–1867. [Google Scholar]
- Begna, D.; Han, B.; Feng, M.; Fang, Y.; Li, J. Differential expressions of nuclear proteomes between honeybee (Apis mellifera L.) queen and worker larvae: A deep insight into caste pathway decisions. J. Proteome Res. 2012, 11, 1317–1329. [Google Scholar] [CrossRef]
- Callier, V.; Nijhout, H.F. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14664–14669. [Google Scholar]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. Critical weight in the development of insect body size. Evol. Dev. 2003, 5, 188–197. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Davidowitz, G.; Roff, D.A. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 2006, 5, 16. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, J.R.; Bitondi, M.M.G. Nuclear Immunolocalization of Hexamerins in the Fat Body of Metamorphosing Honey Bees. Insects 2012, 3, 1039-1055. https://doi.org/10.3390/insects3041039
Martins JR, Bitondi MMG. Nuclear Immunolocalization of Hexamerins in the Fat Body of Metamorphosing Honey Bees. Insects. 2012; 3(4):1039-1055. https://doi.org/10.3390/insects3041039
Chicago/Turabian StyleMartins, Juliana Ramos, and Márcia Maria Gentile Bitondi. 2012. "Nuclear Immunolocalization of Hexamerins in the Fat Body of Metamorphosing Honey Bees" Insects 3, no. 4: 1039-1055. https://doi.org/10.3390/insects3041039
APA StyleMartins, J. R., & Bitondi, M. M. G. (2012). Nuclear Immunolocalization of Hexamerins in the Fat Body of Metamorphosing Honey Bees. Insects, 3(4), 1039-1055. https://doi.org/10.3390/insects3041039



