Eicosanoids: Exploiting Insect Immunity to Improve Biological Control Programs
Abstract
:1. Introduction
2. A Sketch of Insect Immunity
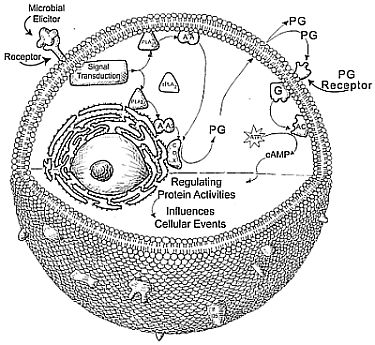
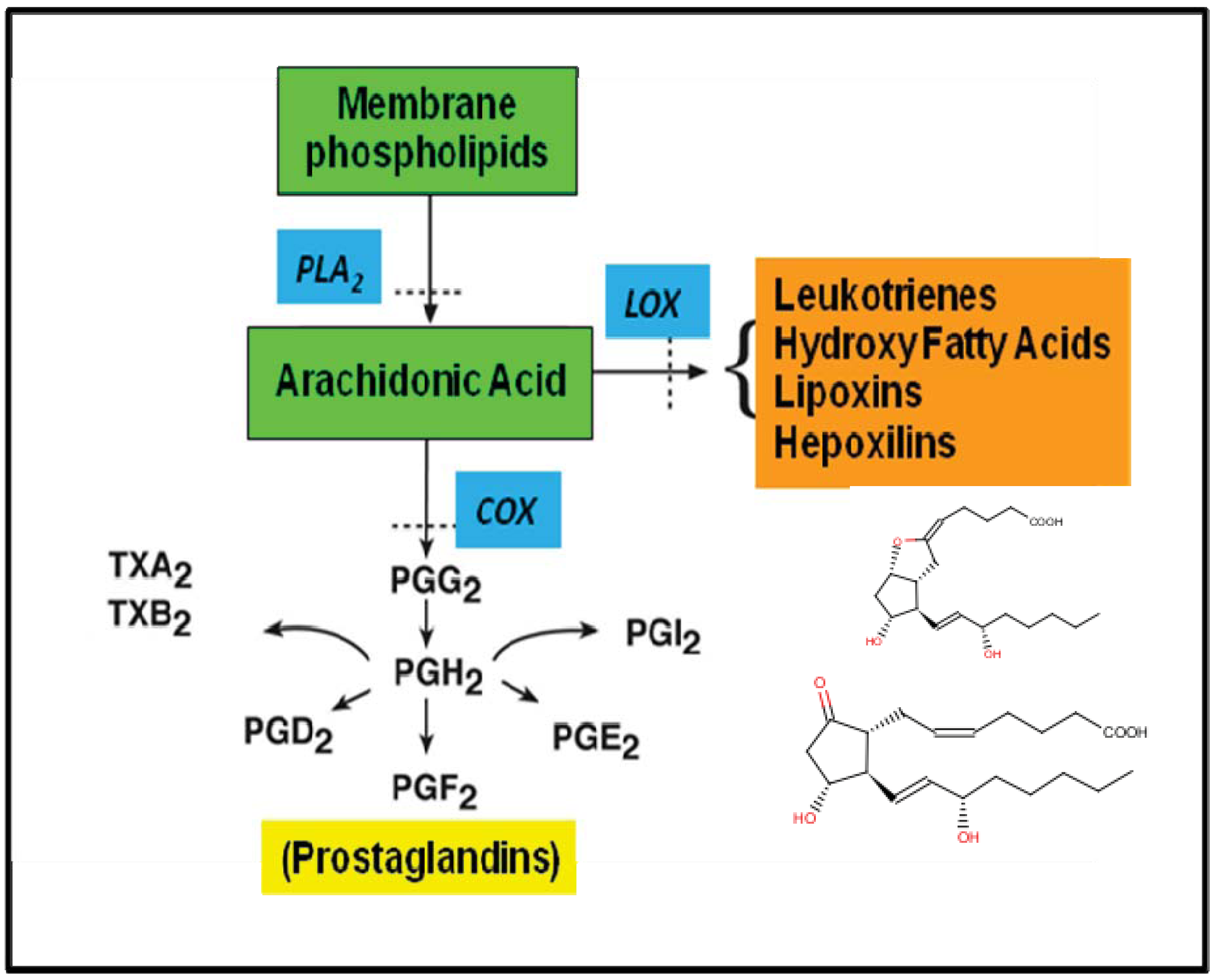
3. An Outline of Eicosanoid Biosynthesis and Action
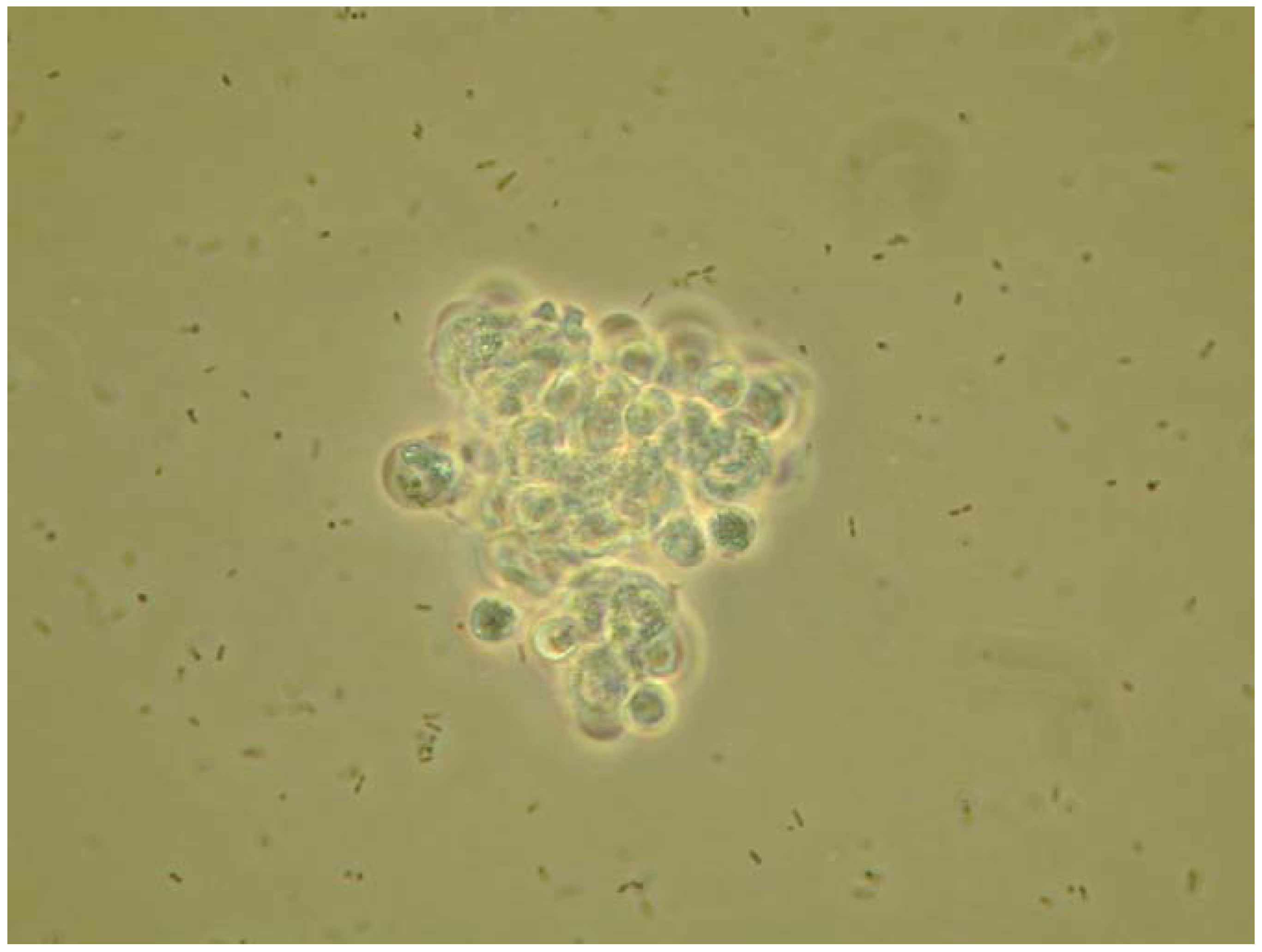
4. Eicosanoids Are Crucial Mediators of Insect Cellular Immunity
| Species | Life stage | Immune elicitor | Reference |
|---|---|---|---|
| Lepidoptera | |||
| Manduca sexta | larvae | Serratia marcescens | [22] |
| Beauveria bassiana | [63] | ||
| Metarhizium anisopliae | [58] | ||
| Agrotis ipsilon | larvae | S. marcescens | [77] |
| P. unipuncta | larvae | S. marcescens | [77] |
| G. mellonella | larvae | glass beads | [30] |
| Bombyx mori | larvae | S. marcescens | [71] |
| Colias eurytheme | larvae | S. marcescens | [70] |
| Spodoptera exigua | larvae | Xenorhabdus nematophila | [44] |
| BAWNPV | [69] | ||
| S. frugiperda | larvae | SfNPV | [69] |
| Ostrinia nubilalis | larvae | S. marcescens | [74] |
| Galleria mellonella | larvae | Virus | [55] |
| Pieris brassicae | larvae | B. bassiana | [72] |
| Lymantria dispar | larvae | LdMNPV | [68] |
| Helicoverpa zea | larvae | HzSNPV | [69] |
| Coleoptera | |||
| Zophobas attraus | larvae | S. marcesens | [64] |
| Lipopolysaccharide | [54] | ||
| Tribolium castaneum | larvae | E. coli | [51] |
| Diptera | |||
| D. melanogaster | larvae | L. boulardi eggs | [56] |
| Neobellieria bullata | larvae | laminarin | [60] |
| Anopheles albimanus | adult | Micrococcus luteus | [61] |
| Klebsiella pneumonia | [61] | ||
| Chryusomya megacephala | larvae | Ureaplasma urealyticum | [76] |
| Hymenoptera | |||
| Apis mellifera | adult | S. marcescens | [67] |
| Pimpla turionellae | adult | Herpes virus | [59] |
| Orthoptera | |||
| Gryllus assimilis | adult | S. marcesens | [65] |
| G. firmus | adult | X. nematophila | [66] |
| P. americana | adult | S. marcesens | [73] |
| L. migratoria | adult | laminarin | [62] |
| Homoptera | |||
| M. septendecim | adult | S. marcesens | [75] |
| M. cassini | adult | S.marcesens | [75] |
| Dactylopius coccus | adult | laminarin | [57] |
| Hemiptera | |||
| R. prolixus | larvae | T. rangeli | [47] |
5. Eicosanoids Influence Specific Cellular Actions
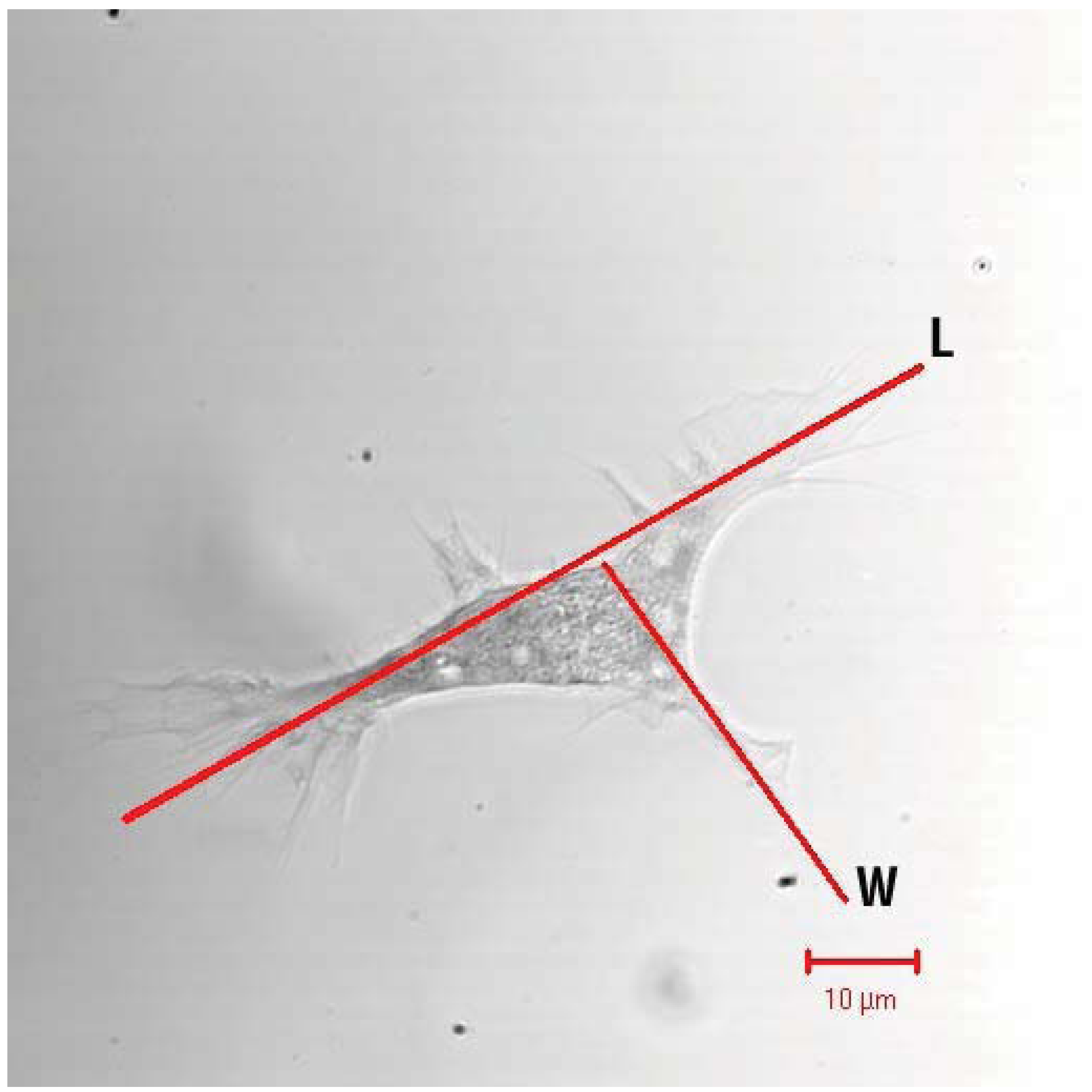
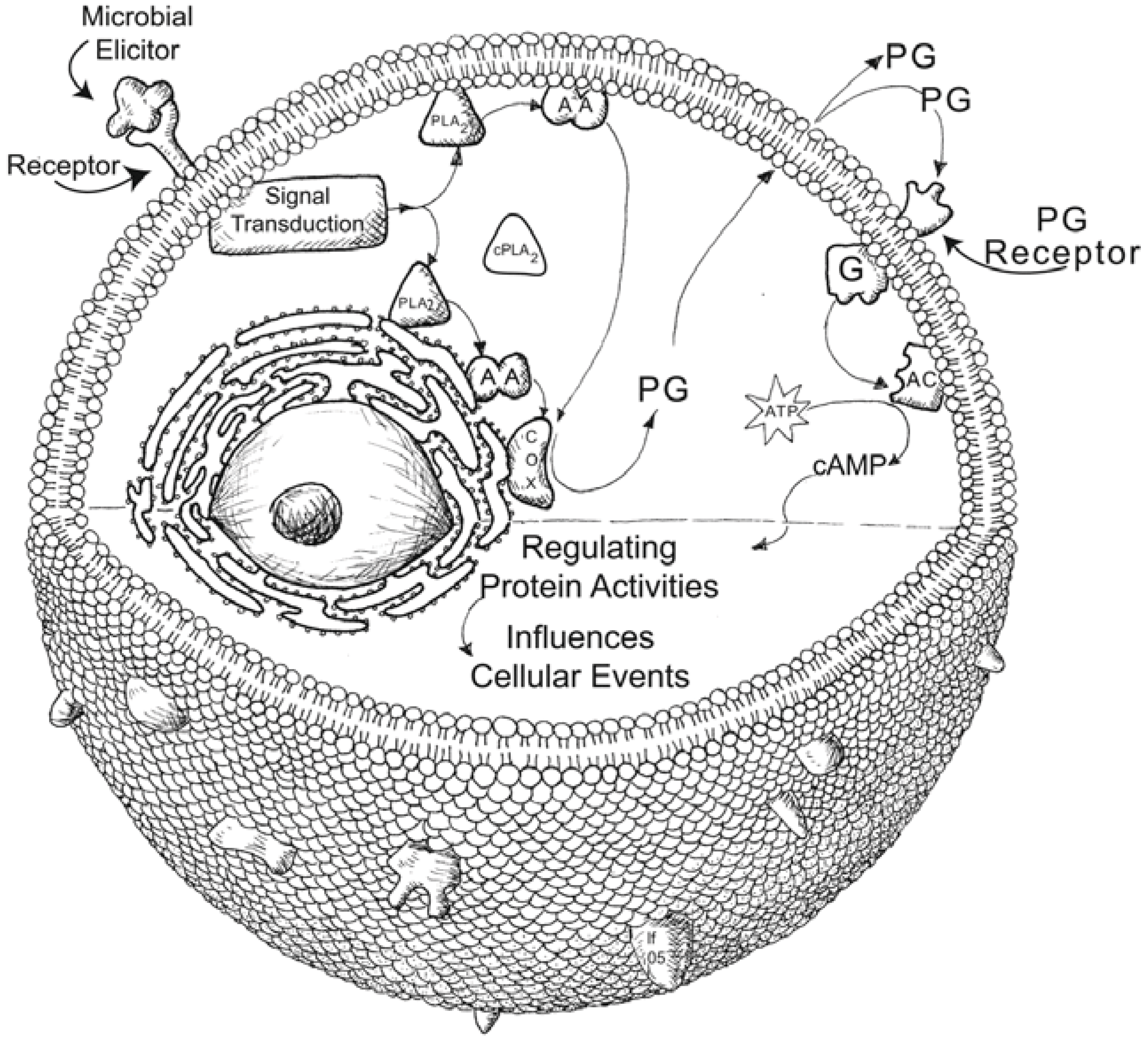
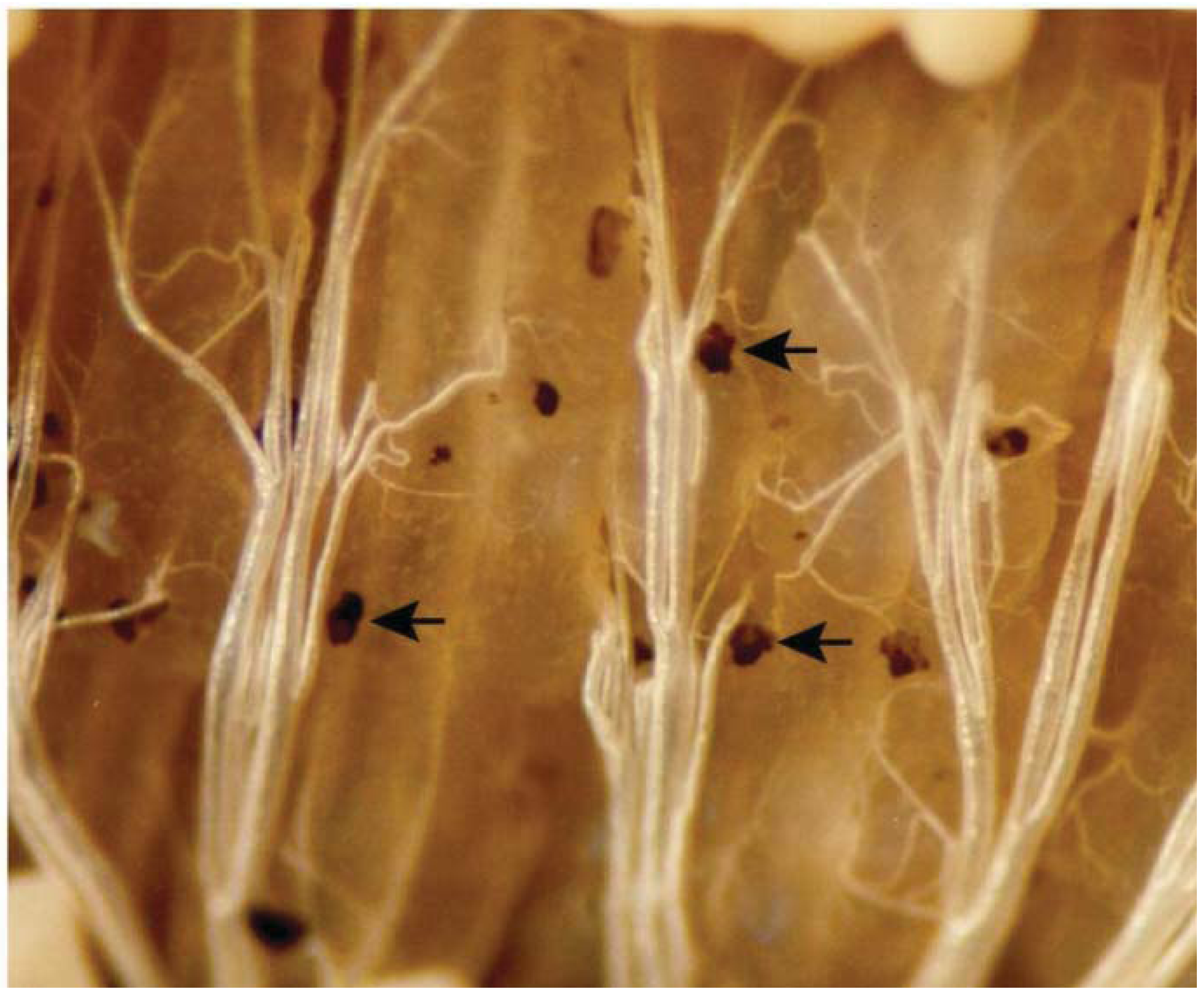
6. Eicosanoids Influence Humoral Immune Reactions
7. Invaders Target Eicosanoid Biosynthesis at the PLA2 Step to Suppress Host Immunity
8. Improving Biological Control Technologies: Targeting Insect Eicosanoid Signaling
9. Conclusions
Acknowledgments
References
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Löwy, I. On guinea pigs, dogs and men: Anaphylaxis and the study of biological individuality, 1902–1939. Stud. Hist. Philos. Biol. Biomed. Sci. 2003, 34, 399–423. [Google Scholar] [CrossRef]
- Chernysh, S.; Cociancich, S.; Briand, J.-P.; Hetru, C.; Bulet, P. The inducible antibacterial peptides of the hemipteran insect Palmena prasinas: Identification of a unique family of proline rich peptides and of a novel insect defensin. J. Insect Physiol. 1996, 42, 81–89. [Google Scholar] [CrossRef]
- Rolff, J.; Siva-Jothy, M.T. Invertebrate ecological immunity. Science 2003, 301, 472–475. [Google Scholar] [CrossRef]
- Tunaz, H.; Stanley, D. An immunological axis of biocontrol: Infections in field-trapped insects. Naturwissenschaften 2009, 96, 1115–1119. [Google Scholar] [CrossRef]
- Haine, E.R.; Moret, Y.; Siva-Jothy, M.T.; Rolff, J. Antimicrobial defense and persistent infection in insects. Science 2008, 322, 1257–1259. [Google Scholar] [CrossRef]
- Boman, H.G.; Steiner, H. Humoral immunity in Cecropia pupae. Curr. Top. Microbiol. Immunol. 1981, 94–95, 75–91. [Google Scholar]
- Bulet, P.; Stöklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Peptide Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J.A. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- An, S.; Dong, S.; Wang, Q.; Li, S.; Gilbert, L.I.; Stanley, D.; Song, Q. Insect neuropeptide bursicon homodimers induce innate immune and stress genes during molting by activating the NF-κB transcription factor Relish. PLoS One 2012, in press. [Google Scholar]
- von Euler, U.S. On the specific vasodilating and plain muscle stimulating substances from accessory genital glands in men and certain animals (prostaglandin and vesiglandin). J. Physiol. (Lond.) 1936, 88, 213–234. [Google Scholar]
- Bergström, S.; Ryhage, R.; Samuelsson, B.; Sjovall, J. The structure of prostaglandin E, F1 and F2. Acta Chem. Scand. 1962, 16, 501–502. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar]
- Corey, E.J.; Albright, J.O.; Barton, A.E.; Hashimoto, S. Chemical and enzymic synthesis of 5-HPETE, a key biological precursor of slow-reacting substance of anaphylaxis (SRS) and 5-HETE. J. Am. Chem. Soc. 1980, 102, 1435–1436. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phosphoipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef]
- Stanley, D.W. Eicosanoids in Invertebrate Signal Transduction Systems; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Stanley, D.W. Eicosanoids. In Comprehensive Insect Molecular Science, Vol 4; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 307–339. [Google Scholar]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H. Insect Immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar]
- Steiner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar]
- Lemaitre, B.; Kromer-Metzger, E.; Michaut, L.; Nicolas, E.; Meister, M.; Georgel, P.; Reichhart, J.-M.; Hoffmann, J.A. A recessive mutation, immune deficiency (imd), define two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. USA 1995, 92, 9465–9469. [Google Scholar]
- Stanley-Samuelson, D.W.; Jensen, E.; Nickerson, K.W.; Tiebel, K.; Ogg, C.L.; Howard, R.W. Insect immune response to bacterial infection is mediated by eicosanoids. Proc. Natl. Acad. Sci. USA 1991, 88, 1064–1068. [Google Scholar]
- Miller, J.S.; Nguyen, T.; Stanley-Samuelson, D.W. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. USA 1994, 91, 12418–12422. [Google Scholar]
- Stanley-Samuelson, D.W.; Ogg, C.L. Prostaglandin biosynthesis by fat body from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 1994, 24, 481–491. [Google Scholar] [CrossRef]
- Gadelhak, G.G.; Pedibhotla, V.K.; Stanley-Samuelson, D.W. Eicosanoid biosynthesis by hemocytes from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 1995, 25, 743–749. [Google Scholar] [CrossRef]
- Uscian, J.M.; Stanley-Samuelson, D.W. Phospholipase A2 activity in the fat body of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 1993, 24, 187–201. [Google Scholar] [CrossRef]
- Schleusener, D.R.; Stanley-Samuelson, D.W. Phospholipase A2 in hemocytes of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 1996, 33, 63–74. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Garcia, E.S.; Azambuja, P. Blockades of phospholipase A2 and platelet-activating factor receptors reduce the hemocyte phagocytosis in Rhodnius prolixus: In vitro experiments. J. Insect Physiol. 2008, 54, 344–350. [Google Scholar] [CrossRef]
- Stanley, D.W. The non-venom insect phospholipases A2. Biochim. Biophys. Acta 2006, 1761, 1383–1390. [Google Scholar]
- Stanley, D.W.; Miller, J.S. Eicosanoid Actions in Insect Immunology. In Insect Immunology; Beckage, N.E., Ed.; Academic Press: Amsterdam, The Netherlands, 2008; pp. 49–680. [Google Scholar]
- Mandato, C.A.; Diehl-Jones, W.L.; Moore, S.J.; Downer, R.G.H. The effects of eicosanoid biosynthesis inhibitors on prophenoloxidase activation, phagocytosis and cell spreading in Galleria mellonella. J. Insect Physiol. 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Miller, J.S. Eicosanoids influence in vitro elongation of plasmatocytes from the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 2005, 59, 42–51. [Google Scholar] [CrossRef]
- Marin, D.; Dunphy, G.B.; Mandato, C.A. Cyclic AMP affects the haemocyte responses of larval Galleria mellonella to selected antigens. J. Insect Physiol. 2005, 51, 575–586. [Google Scholar] [CrossRef]
- Merchant, D.; Ertl, R.L.; Rennard, S.I.; Stanley, D.W.; Miller, J.S. Eicosanoids mediate insect hemocyte migration. J. Insect Physiol. 2008, 54, 215–221. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Oenocytoid cell lysis to release prophenoloxidase is induced by eicosanoid via protein kinase C. J. Asia-Pac. Entomol. 2009, 12, 301–305. [Google Scholar] [CrossRef]
- Shrestha, S.; Stanley, D.; Kim, Y. PGE2 induces oenocytoids cell lysis via a G protein-coupled receptor in the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2011, 57, 1568–1576. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goodman, C.; Shiheng, A.; McIntosh, A.; Song, Q. Prostaglandins A1 and E1 influence gene expression in an established insect cell line (BCIRL-HzAM1). Insect Biochem. Mol. Biol. 2008, 38, 275–284. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goodman, C.; An, S.; Song, Q. Prostaglandin A2 influences gene expression in an established insect cell line (BCIRL-HzAM1) cells. J. Insect Physiol. 2012, in press. [Google Scholar]
- Morishima, I.; Yamano, Y.; Inoue, K.; Matsuo, N. Eicosanoids mediate induction of immune genes in the fat body of the silkworm, Bombyx mori. FEBS Lett. 1997, 419, 83–86. [Google Scholar] [CrossRef]
- Yajima, M.; Takada, M.; Takahashi, N.; Kikuchi, H.; Ntori, S.; Oshima, Y.; Kurata, S. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A2-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (imd) pathway in insect immunity. Biochem. J. 2003, 371, 205–210. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Various eicosanoids modulate the cellular and humoral immune responses of the beet armyworm, Spodpotera exigua. Biosci. Biotechnol. Biochem. 2009, 73, 2077–2084. [Google Scholar] [CrossRef]
- Srikanth, K.; Park, J.; Stanley, D.W.; Kim, Y. Plasmatocyte-spreading peptide influences hemocyte behavior via eicosanoids. Arch. Insect Biochem. Physiol. 2011, 78, 145–160. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, L.; Zhu, J.-Y.; Li, Y.-M.; Song, Q.-S.; Stanley, D.W.; Akhtar, Z.; Ye, G.-Y. Expression of immune-response genes in lepidopteran host are suppressed by venom from an endoparasitoid, Pteromalus puparum. BMC Genomics 2010, 11. [Google Scholar] [CrossRef]
- Strand, M.R.; Pech, L.L. Immunological basis for compatibility in parasitoid-host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Stanley, D.W. The bacterium Xenorhabdus nematophila inhibits phospholipases A2 from insect, prokaryote and vertebrate sources. Naturwissenschaften 2004, 91, 371–373. [Google Scholar]
- Kim, Y.; Ji, D.; Cho, S.; Park, Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunosuppression. J. Invertbr. Pathol. 2005, 89, 258–264. [Google Scholar] [CrossRef]
- Garcia, E.S.; Machado, E.M.M.; Azambuja, P. Inhibition of hemocyte microaggregation reactions in Rhodnius prolixus larvae orally infected with Trypanosoma rangeli. Exp. Parasitol. 2004, 107, 31–38. [Google Scholar] [CrossRef]
- Oerke, C.E.; Dehne, H.W. Safeguarding production – losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesias, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate Change and U.S. Agriculture: The Impacts of Warming and Extreme Weather Events on Productivity, Plant Diseases and Pests; Center for Health and the Global Environment, Harvard Medical School: Boston, MA, USA, 2000. [Google Scholar]
- Djerassi, C.; Shi-Coleman, C.; Diekman, J. Insect control of the future: Operational and policy aspects. Science 1974, 186, 596–607. [Google Scholar]
- Shrestha, S.; Park, Y.; Stanley, D.; Kim, Y. Genes encoding phospholipases A2 mediate insect nodulation reactions to bacterial challenge. J. Insect Physiol. 2010, 56, 324–332. [Google Scholar] [CrossRef]
- Price, D.R.G.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Abraham, E.G.; Cha, S.-J.; Jacobs-Lorena, M. Toward the genetic control of insect vectors: An overview. Entomol. Res. 2007, 37, 213–220. [Google Scholar] [CrossRef]
- Bedick, J.C.; Pardy, R.L.; Howard, R.W.; Stanley, D.W. Insect cellular reactions to the lipopolysaccharide component of the bacterium Serratia marcescens are mediated by eicosanoids. J. Insect Physiol. 2000, 46, 1481–1487. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Tunaz, H.; Stanley, D.; Büyükgüzel, K. Eicosanoids mediate Galleria mellonella cellular response to viral infection. J. Insect Physiol. 2007, 53, 99–105. [Google Scholar] [CrossRef]
- Carton, Y.; Frey, F.; Stanley, D.W.; Vass, E.; Nappi, A.J. Dexamethasone inhibition of the cellular immune response of Drosophila melanogaster against a parasitoid. J. Parasitol. 2002, 88, 405–407. [Google Scholar]
- de le Cruz Hernández-Hernández, F.; García-Gil de Muñoz, F.; Rojas-Martínez, A.; Hernández-Martínez, S.; Lanz-Mendoza, H. Carminic acid dye from the homopteran Dactylopius coccus hemolymph is consumed during treatment with different microbial elicitors. Arch. Insect Biochem. Physiol. 2003, 54, 37–45. [Google Scholar] [CrossRef]
- Dean, P.; Gadsden, J.C.; Richards, E.H.; Edwards, J.P.; Charnley, A.K.; Reynolds, S.E. Modulation by eicosanoid biosynthesis inhibitors of immune responses by the insect Manduca sexta to the pathogenic fungus. Metarhizium anisopoliae. J. Invertbr. Pathol. 2002, 79, 93–101. [Google Scholar] [CrossRef]
- Durmuş, Y.; Büyükgüzel, E.; Terzi, B.; Tunaz, H.; Stanley, D.; Büyükgüzel, K. Eicosanoids mediate melantoic nodulation reactions to viral infection in larvae of the parasitic wasp. Pimpla turioininellae. J. Insect Physiol. 2008, 54, 17–24. [Google Scholar]
- Franssens, V.; Simonet, G.; Bronckaers, A.; Claeys, I.; de Loof, A.; Vanden Broeck, J. Eicosanoids mediate the Laminarin-induced nodulation response in larvae of the flesh fly, Neobellieria bullata. Arch. Insect Biochem. Physiol. 2005, 59, 32–41. [Google Scholar] [CrossRef]
- Garcίa Gil de Muñoz, F.L.; Martίnez-Barnetche, J.; Lanz-Mendoza, H.; Rodrίguez, M.H.; Hernández-Hernández, F.C. Prostaglandin E2 modulates the expression of antimicrobial peptides in the fat body and midgut of Anopheles albimanus. Arch. Insect Biochem. Physiol. 2008, 68, 14–25. [Google Scholar] [CrossRef]
- Goldsworthy, G.; Mullen, L.; Opuku-Ware, K.; Chandrakant, S. Interactions between the endocrine and immune systems in locusts. Physiol. Entomol. 2003, 28, 54–61. [Google Scholar] [CrossRef]
- Lord, J.C.; Anderson, S.; Stanley, D.W. Eicosanoids mediate Manduca sexta cellular response to the fungal pathogen Beauveria bassiana: A role for the lipoxygenases pathway. Arch. Insect Biochem. Physiol. 2002, 51, 46–54. [Google Scholar] [CrossRef]
- Miller, J.S.; Howard, R.W.; Nguyen, T.; Nguyen, A.; Rosario, R.M.T.; Stanley-Samuelson, D.W. Eicosanoids mediate nodulation responses to bacterial infections in larvae of the tenebrionid beetle, Zophobas atratus. J. Insect Physiol. 1996, 42, 3–12. [Google Scholar] [CrossRef]
- Miller, J.S.; Howard, R.W.; Rana, R.L.; Tunaz, H.; Stanley, D.W. Eicosanoids mediate nodulation reactions to bacterial infections in adults of the cricket, Gryllus assimulis. J. Insect Physiol. 1999, 45, 75–83. [Google Scholar] [CrossRef]
- Park, Y.; Stanley, D. The entomopathogenic bacterium, Xenorhabdus nematophila, impairs insect immunity by inhibition of eicosanoid biosynthesis in adult crickets, Gryllus firmus. Biol. Control 2006, 38, 247–253. [Google Scholar] [CrossRef]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef]
- Stanley, D.W.; Shapiro, M. Eicosanoid biosynthesis inhibitors increase susceptibility of Lymantria dispar to nucleopolyhedrovirus LdMNPV. J. Invertebr. Pathol. 2007, 95, 119–124. [Google Scholar] [CrossRef]
- Stanley, D.W.; Shapiro, M. Eicosanoids mediate insect susceptibility to nucleopolyhedroviruses. J. Invertebr. Pathol. 2009, 102, 245–249. [Google Scholar] [CrossRef]
- Stanley, D.W.; Hoback, W.W.; Bedick, J.C.; Tunaz, H.; Rana, R.L.; Nor Aliza, A.R.; Miller, J.S. Eicosanoids mediate nodulation reactions to bacterial infections in larvae of the butterfly, Colias eurytheme. Comp. Biochem. Physiol. Part C 1999, 123, 217–223. [Google Scholar]
- Stanley-Samuelson, D.W.; Pedibhotla, V.K.; Rana, R.L.; Nor Aliza, A.R.; Hoback, W.W.; Miller, J.S. Eicosanoids mediate nodulation responses to bacterial infections in larvae of the silkmoth, Bombyx mori. Comp. Biochem. Physiol. 1997, 118A, 93–100. [Google Scholar]
- Tunaz, H. Eicosanoid biosynthesis inhibitors influence mortality of Pieris brassicae larvae co-injected with fungal conidia. Arch. Insect Biochem. Physiol. 2006, 63, 93–100. [Google Scholar] [CrossRef]
- Tunaz, H.; Stanley, D.W. Eicosanoids mediate nodulation reactions to bacterial infections in adults of the American cockroach, Periplaneta americana (L.). Proc. Entomol. Soc. Ontario 1999, 130, 97–108. [Google Scholar]
- Tunaz, H.; Isikber, A.A.; Er, M.K. The role of eicosanoids on nodulation reactions to bacterium Serratia marcescens in larvae of Ostrinia nublialis. Turk. J. Agric. Forest. 2003, 27, 269–275. [Google Scholar]
- Tunaz, H.; Bedick, J.C.; Miller, J.S.; Hoback, W.W.; Rana, R.L.; Stanley, D.W. Eicosanoids mediate nodulation reactions to bacterial infections in adults of two 17-year periodical cicadas, Magicicada septendecim and M. cassini. J. Insect Physiol. 1999, 45, 923–931. [Google Scholar] [CrossRef]
- Zhao, F.; Stanley, D.; Wang, Y.; Zhu, F.; Lei, C.-L. Eicosanoids mediate nodulation reactions to a mollicute bacterium in larvae of the blowfly, Chrysomya megacephala. J. Insect Physiol. 2009, 55, 192–196. [Google Scholar] [CrossRef]
- Jurenka, R.A.; Miller, J.S.; Pedibhotla, V.K.; Rana, R.L.; Stanley-Samuelson, D.W. Eicosanoids mediate microaggregation and nodulation responses to bacterial infections in black cutworms, Agrotus ipsilon, and true armyworms, Pseudaletia unipuncta. J. Insect Physiol. 1997, 43, 125–133. [Google Scholar] [CrossRef]
- Stanley, D.W. Method for Modulating Eicosanoid Mediated Immune Responses in Arthropods. US Patent 6,099,834, 8 August 2000. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stanley, D.; Haas, E.; Miller, J. Eicosanoids: Exploiting Insect Immunity to Improve Biological Control Programs. Insects 2012, 3, 492-510. https://doi.org/10.3390/insects3020492
Stanley D, Haas E, Miller J. Eicosanoids: Exploiting Insect Immunity to Improve Biological Control Programs. Insects. 2012; 3(2):492-510. https://doi.org/10.3390/insects3020492
Chicago/Turabian StyleStanley, David, Eric Haas, and Jon Miller. 2012. "Eicosanoids: Exploiting Insect Immunity to Improve Biological Control Programs" Insects 3, no. 2: 492-510. https://doi.org/10.3390/insects3020492
APA StyleStanley, D., Haas, E., & Miller, J. (2012). Eicosanoids: Exploiting Insect Immunity to Improve Biological Control Programs. Insects, 3(2), 492-510. https://doi.org/10.3390/insects3020492