Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Record
2.2. Sources and Filtering of Environment Variables
2.3. MaxEnt Model Construction and Validation
2.4. Habitat Classification and Centroid Method
3. Results and Analysis
3.1. Model Prediction Evaluation and Selection of Environmental Factors
3.2. Distribution and Patterns of Suitable Habitats for L. chinensis
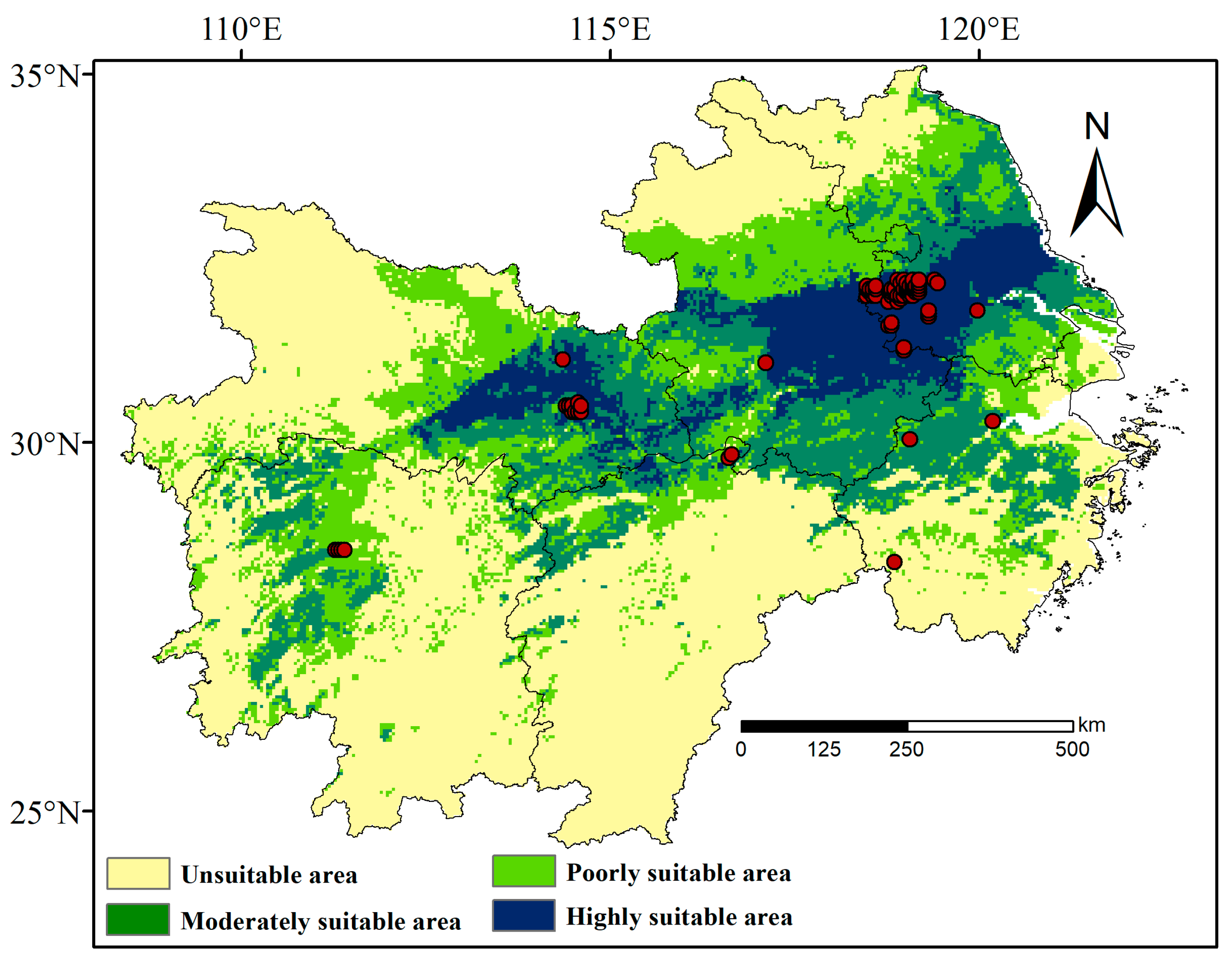
3.2.1. Current Distribution of Suitable Habitats
3.2.2. Future Predictions of Suitable Habitats
3.3. Centroid Migration of Suitable Habitats for L. chinensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Chen, X.; Wang, J.; Wu, S.; Zhang, H.; Xing, L.; You, P. The complete mitochondrial genomes of three populations in Luehdorfia chinensis (Leech, 1893) (Lepidoptera: Papilionidae), with a phylogenetic analysis of Papilionidae. Orient. Insects 2019, 53, 320–339. [Google Scholar]
- Fang, L.; Zhang, Y.; Gao, K.; Ding, C.; Zhang, Y. Butterfly communities along the Heihe River Basin in Shaanxi Province, a biodiversity conservation priority area in China. J. Insect Conserv. 2019, 23, 873–883. [Google Scholar] [CrossRef]
- Nishida, R. Oviposition stimulant of a zeryntiine swallowtail butterfly, Luehdorfia japonica. Phytochemistry 1994, 36, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-C.; Ma, G.-Q.; Xu, D.-Y.; Hu, C.; Ye, G.-Y. The habitat, biology and conservation status of Luehdorfia chinensis (Lepidoptera: Papilionidae). Biodivers. Sci. 1998, 6, 105. [Google Scholar]
- Ueda, S.; Nakasuji, C.; Nakahama, N.; Hirai, N.; Ishii, M. Development of microsatellite markers for the endangered butterfly Luehdorfia japonica Leech, 1889 (Lepidoptera: Papilionidae). Entomol. Sci. 2024, 27, 7. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, G.-F.; Pang, H.-C.; Hong, F. The mitochondrial genome of the Chinese special butterfly Luehdorfia chinensis Leech (Lepidoptera: Papilionidae). Mitochondrial DNA 2013, 24, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Soto, V.; Melgoza-Castillo, A.; Villarreal-Guerrero, F.; Wehenkel, C.; Pinedo-Alvarez, C. Modeling the Potential Distribution of Picea chihuahuana Martinez, an Endangered Species at the Sierra Madre Occidental, Mexico. Forests 2015, 6, 692–707. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the Distribution of the Invasive Species Leptocybe invasa: Combining MaxEnt and Geodetector Models. Insects 2021, 12, 92. [Google Scholar] [CrossRef]
- Ouyang, W.; Qiu, H.; Chen, Z.; Wu, Y.; Li, J. Changes in the Potential Habitat Distribution of Typical Fire-Resistant Forest Species under Climate Change in the Subtropical Regions of China. Forests 2023, 14, 1897. [Google Scholar] [CrossRef]
- Cao, C.; Tao, J. Predicting the Areas of Suitable Distribution for Zelkova serrata in China under Climate Change. Sustainability 2021, 13, 1493. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Zhao, L.; Cui, S. Potentially Suitable Geographical Area for Monochamous alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects 2023, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Taylor, I.E.; Frey, J.K. Predicting the distribution of a rare chipmunk (Neotamias quadrivittatus oscuraensis): Comparing MaxEnt and occupancy models. J. Mammal. 2020, 101, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, D. Climate change and anthropogenic activities shrink the range and dispersal of an endangered primate in Sichuan Province, China. Environ. Sci. Pollut. Res. 2023, 30, 122921–122933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.-T.; Gao, Y.; Yang, L.; Yu, H. Prediction of global potential suitable habitats of Nicotiana alata Link et Otto based on MaxEnt model. Sci. Rep. 2023, 13, 4851. [Google Scholar] [CrossRef]
- Guo, W.; Wang, B.; Jiao, X.; Huang, L.; Yang, H.; Yu, L.; Wang, H. Synergistic Evolution and Attribution Analysis of Water-Sediment in the Middle and Lower Reaches of the Yangtze River. J. Hydrol. Reg. Stud. 2024, 51, 101626. [Google Scholar]
- Mahatara, D.; Acharya, A.; Dhakal, B.; Sharma, D.; Ulak, S.; Paudel, P. Maxent modelling for habitat suitability of vulnerable tree Dalbergia latifolia in Nepal. Silva Fenn. 2021, 55, 17. [Google Scholar] [CrossRef]
- Li, S.Y.; Miao, L.-J.; Jiang, Z.-H.; Wang, G.-J.; Gnyawali, K.R.; Zhang, J.; Zhang, H.; Fang, K.; He, Y.; Li, C. Projected drought conditions in Northwest China with CMIP6 models under combined SSPs and RCPs for 2015-2099. Adv. Clim. Change Res. 2020, 11, 210–217. [Google Scholar] [CrossRef]
- Kogo, B.K.; Kumar, L.; Koech, R.; Kariyawasam, C.S. Modelling Climate Suitability for Rainfed Maize Cultivation in Kenya Using a Maximum Entropy (MaxENT) Approach. Agronomy 2019, 9, 727. [Google Scholar] [CrossRef]
- Zhu, G.P.; Yuan, X.J.; Fan, J.Y.; Wang, M.L. Effects of model parameters in MaxEnt modeling of ecological niche and geographic distribution: Case study of the brown marmorated stink bug, Halyomorpha haly. J. Biosaf. 2018, 27, 46–51. [Google Scholar]
- Venne, S.; Currie, D.J. Can habitat suitability estimated from MaxEnt predict colonizations and extinctions ? Divers. Distrib. 2021, 27, 873–886. [Google Scholar] [CrossRef]
- Zhu, G.; Qiao, H. Effect of the Maxent model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189. [Google Scholar] [CrossRef]
- Cobos, M.E.; Townsend Peterson, A.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Osorio-Olvera, L.; Jiménez-García, D. An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecol. Inform. 2019, 53, 100983. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, C.; Xie, Y.; Jiang, X. Nature reserves and reforestation expend the potential habitats for endangered plants: A model study in Cangshan, China. J. Nat. Conserv. 2024, 77, 126533. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Wu, S. MaxEnt Modeling to Estimate the Impact of Climate Factors on Distribution of Pinus densiflora. Forests 2022, 13, 402. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Yan, M.; Liu, B.W.; Tiamiyu, B.B.; Zhang, Y.; Ning, W.Y.; Si, J.Y.; Dong, N.C.; Lv, X.L. Modeling the Present and Future Geographical Distribution Potential of Dipteronia dyeriana, a Critically Endangered Species from China. Diversity 2024, 16, 545. [Google Scholar] [CrossRef]
- Hending, D.; McCabe, G.; Cotton, S.; Holderied, M. Conservation Biogeography of the Dwarf Lemurs (Cheirogaleus) of Madagascar, Investigated via Ecological Niche Modelling. Int. J. Primatol. 2023, 44, 960–983. [Google Scholar] [CrossRef]
- Atsawawaranunt, K.; Whibley, A.; Cain, K.E.; Major, R.E.; Santure, A.W. Projecting the current and potential future distribution of New Zealand’s invasive sturnids. Biol. Invasions 2024, 26, 1345–1366. [Google Scholar] [CrossRef]
- Hánová, A.; Bryja, J.; de Bellocq, J.G.; Baird, S.J.E.; Cuypers, L.; Konečný, A.; Mikula, O. Historical demography and climatic niches of the Natal multimammate mouse (Mastomys natalensis) in the Zambezian region. Mamm. Biol. 2023, 103, 239–251. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, T.; Shibuya, Y.; Goto, M.; Yokoyama, J.; Kato, T.; Ando, M.; Okamoto, T.; Tsuchida, K. Expansion processes of two emblematic Luehdorfia butterflies across the Japanese archipelago. J. Biogeogr. 2023, 50, 1710–1723. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Heath, A.C.G.; Lawrence, K.E.; Ganta, R.R.; Peterson, A.T.; Pomroy, W.E. Predicting the potential distribution of Amblyomma americanum (Acari: Ixodidae) infestation in New Zealand, using maximum entropy-based ecological niche modelling. Exp. Appl. Acarol. 2020, 80, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zagortchev, L.; Ma, J.; Yan, M.; Li, J. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 2020, 20, 28. [Google Scholar] [CrossRef]
- Zhang, F.; Chu, C.H.; Xu, Q.; Fu, S.P.; Hu, J.H.; Xiao, H.B.; Liang, X.M. A new amide from Asarum forbesii Maxim. J. Asian Nat. Prod. Res. 2005, 7, 1–5. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, C.; Li, X.; Wang, Y.; Yan, Y. Analysis of the spatiotemporal trends and influencing factors of Hyphantria cunea in China. J. For. Res. 2024, 35, 100. [Google Scholar] [CrossRef]
- Lu, M.; Huang, J. Predicting negative Effects of Climate Change on Taiwan’s endemic Bumblebee Bombus formosellus. J. Insect Conserv. 2023, 27, 193–203. [Google Scholar] [CrossRef]
- Wei, J.; Li, X.; Lu, Y.; Zhao, L.; Zhang, H.; Zhao, Q. Modeling the Potential Global Distribution of Phenacoccus madeirensis Green under Various Climate Change Scenarios. Forests 2019, 10, 773. [Google Scholar] [CrossRef]

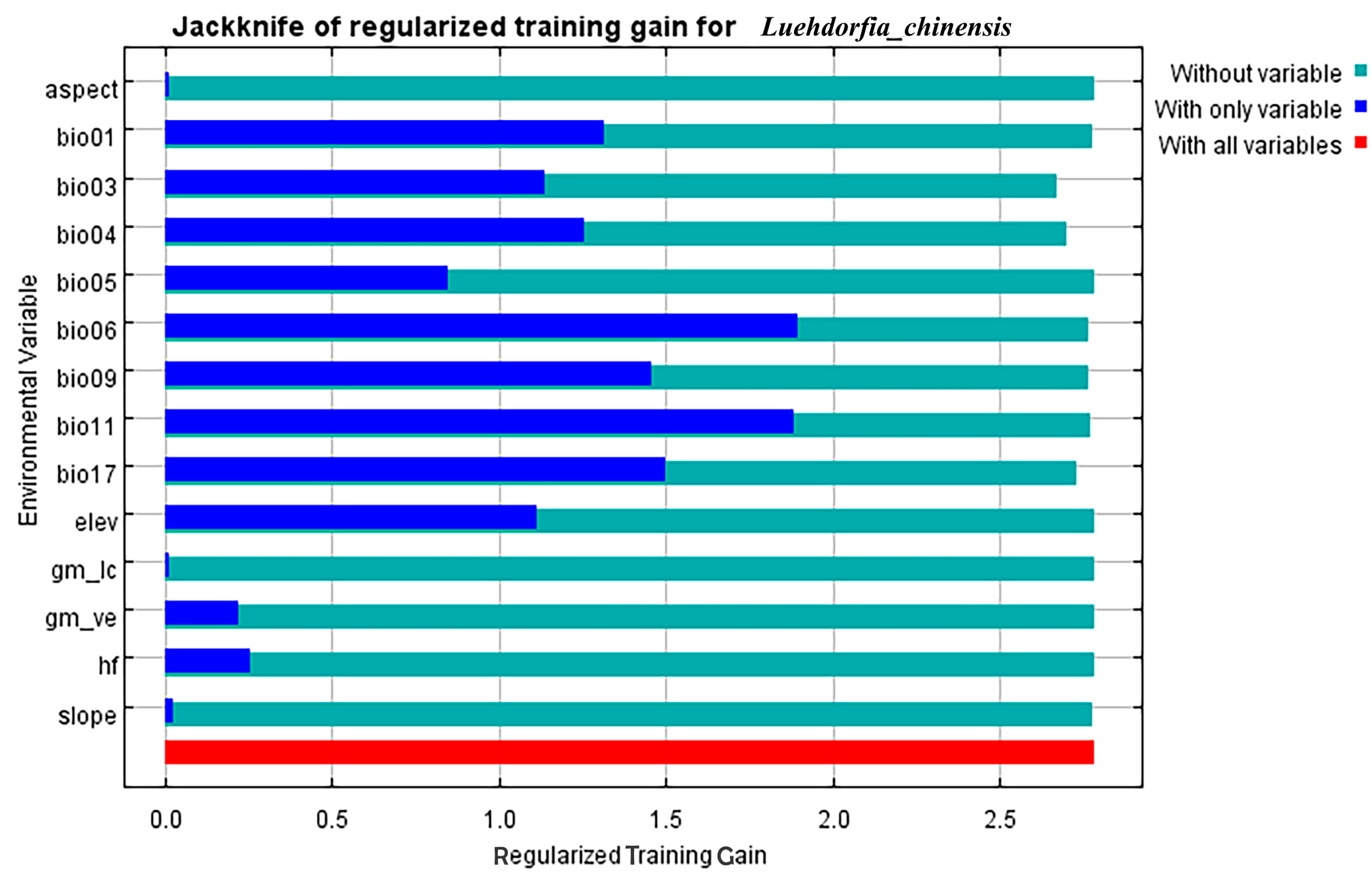


| Environment Variable | Description | Contribution Rate | Permutation Importance |
|---|---|---|---|
| bio17 | Precipitation in the driest quarter | 36.70% | 3.70% |
| bio03 | Isothermal property | 17.30% | 46.80% |
| bio06 | Minimum temperature in the coldest month | 12.90% | 17.80% |
| bio04 | Coefficient of seasonal variation of temperature | 11% | 7.60% |
| bio11 | Mean temperature of coldest quarter | 9.10% | 1.50% |
| elev | Altitude | 8.40% | 0.20% |
| bio09 | Mean temperature of driest quarter | 1.90% | 16.60% |
| bio05 | Max temperature of warmest month | 1.70% | 1.60% |
| bio01 | Annual mean temperature | 0.50% | 3.10% |
| aspect | Aspect | 0.20% | 0.00% |
| slope | Slope | 0.20% | 0.70% |
| gm_ve | Vegetation coverage percentage | 0.10% | 0.20% |
| Predicted Area (103 km2) | Comparison with Current Distribution (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Period | Unsuitable | Poorly Suitable | Moderately Suitable | Highly Suitable | Unsuitable | Poorly Suitable | Moderately Suitable | Highly Suitable |
| Current | 455.2 | 173.1 | 146.6 | 75.1 | —— | —— | —— | —— |
| 2050s | 396.6 | 283.4 | 143.7 | 26.4 | −12.9 | 63.7 | −2 | −64.8 |
| 2070s | 465.2 | 218.3 | 114 | 52.5 | 2.2 | 26.1 | −22.2 | −30.1 |
| 2090s | 616.8 | 174.1 | 51.1 | 8 | 35.5 | 0.6 | −65.1 | −89.3 |
| Scene | Period | Angle/° | Direction | Displacement/km |
|---|---|---|---|---|
| Present to 2050s | 47.1 | Northeast | 176.23 | |
| SSP2-4.5 | 2050s to 2070s | 235.1 | Southwest | 164.02 |
| 2070s to 2090s | 80.16 | Northeast | 61.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Liu, B.; Zhou, R.; Zhang, H.; Zhou, L.; Xie, X.; Zhuo, Z.; Xu, D. Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model. Insects 2025, 16, 396. https://doi.org/10.3390/insects16040396
Chen A, Liu B, Zhou R, Zhang H, Zhou L, Xie X, Zhuo Z, Xu D. Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model. Insects. 2025; 16(4):396. https://doi.org/10.3390/insects16040396
Chicago/Turabian StyleChen, Anqi, Biyu Liu, Rui Zhou, Hui Zhang, Lan Zhou, Xizhu Xie, Zhihang Zhuo, and Danping Xu. 2025. "Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model" Insects 16, no. 4: 396. https://doi.org/10.3390/insects16040396
APA StyleChen, A., Liu, B., Zhou, R., Zhang, H., Zhou, L., Xie, X., Zhuo, Z., & Xu, D. (2025). Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model. Insects, 16(4), 396. https://doi.org/10.3390/insects16040396






