Comparative Transcriptome Analysis of the Effects of a Non-Insect Artificial Diet on the Nutritional Development of Harmonia axyridis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Fecundity
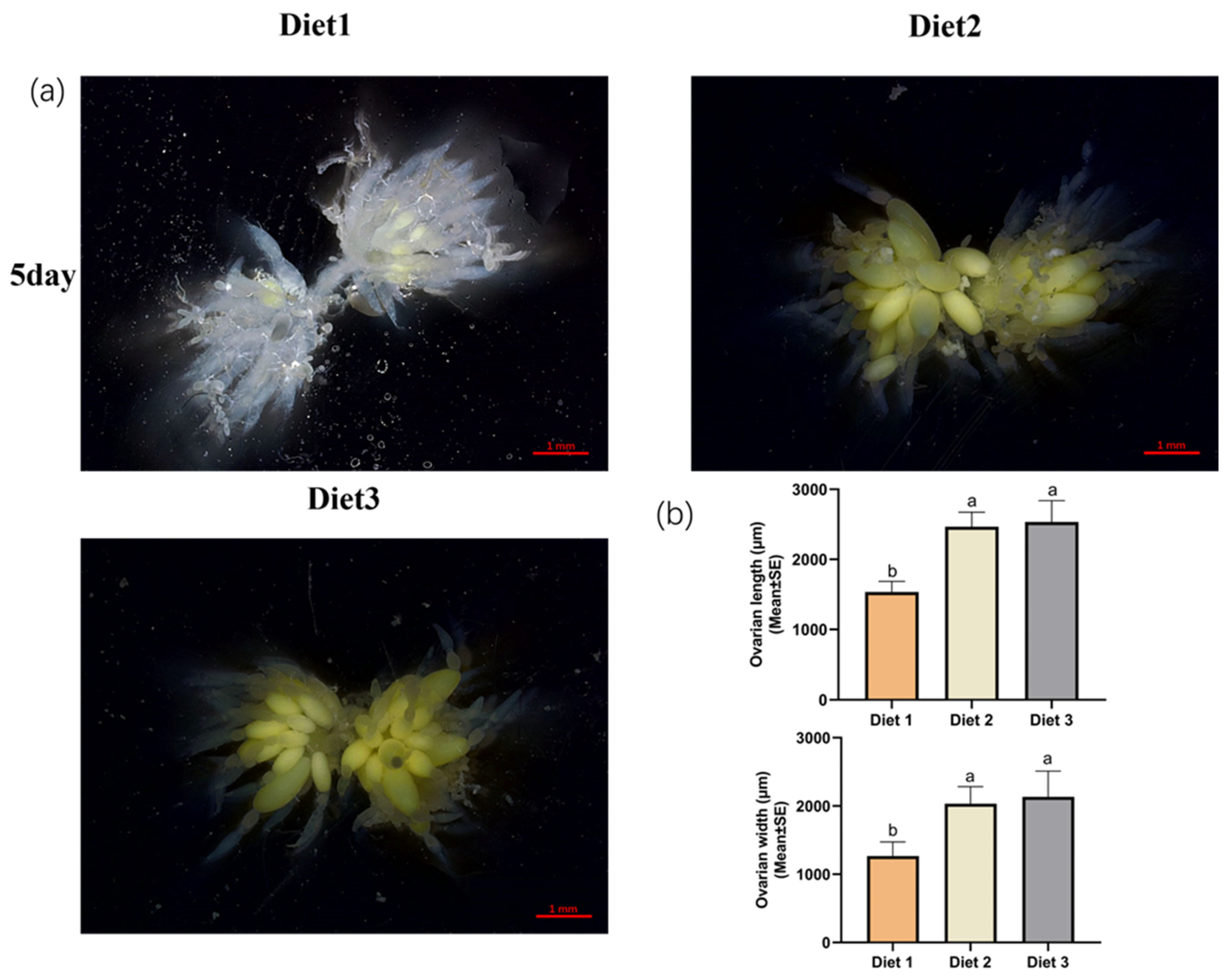
2.3. Dietary Effects on Ovarian Development
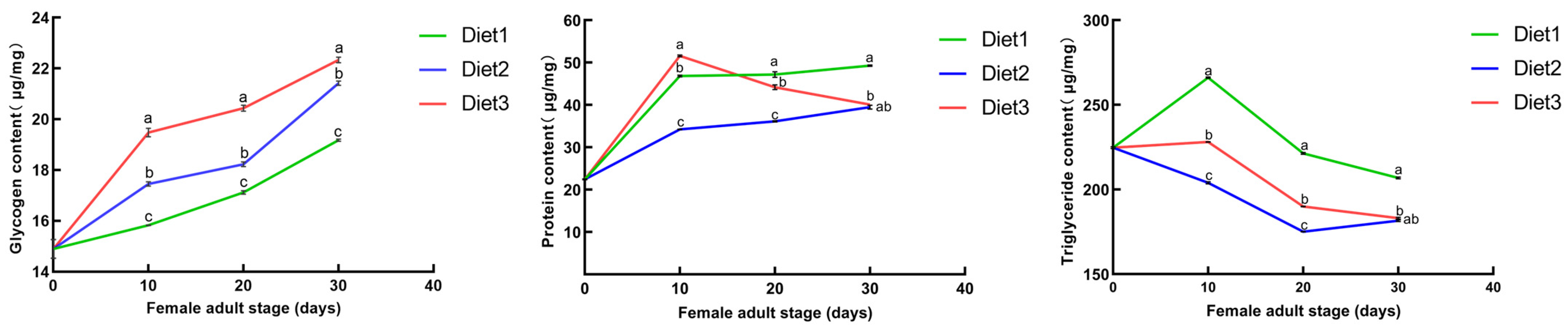
2.4. Measurement of Energy Substances in Different Artificial Diets for H. axyridis
2.5. Illumina Sequencing for Transcriptome Analysis
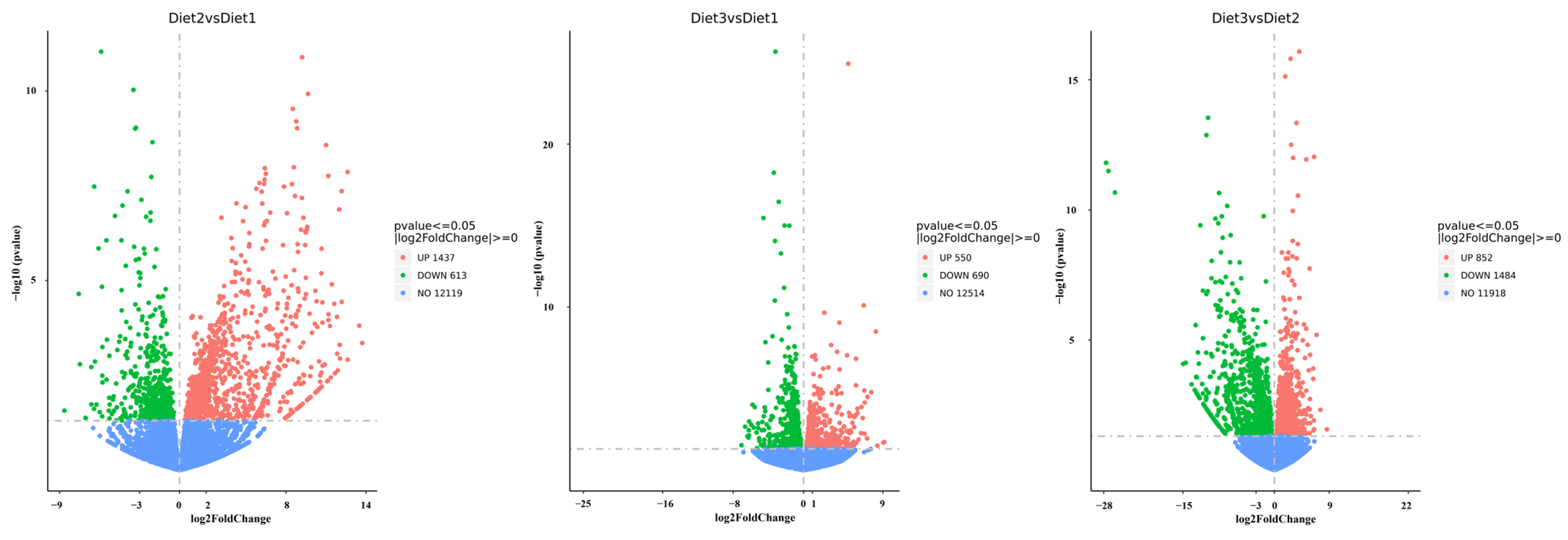
2.6. Identification of Differentially Expressed Transcripts
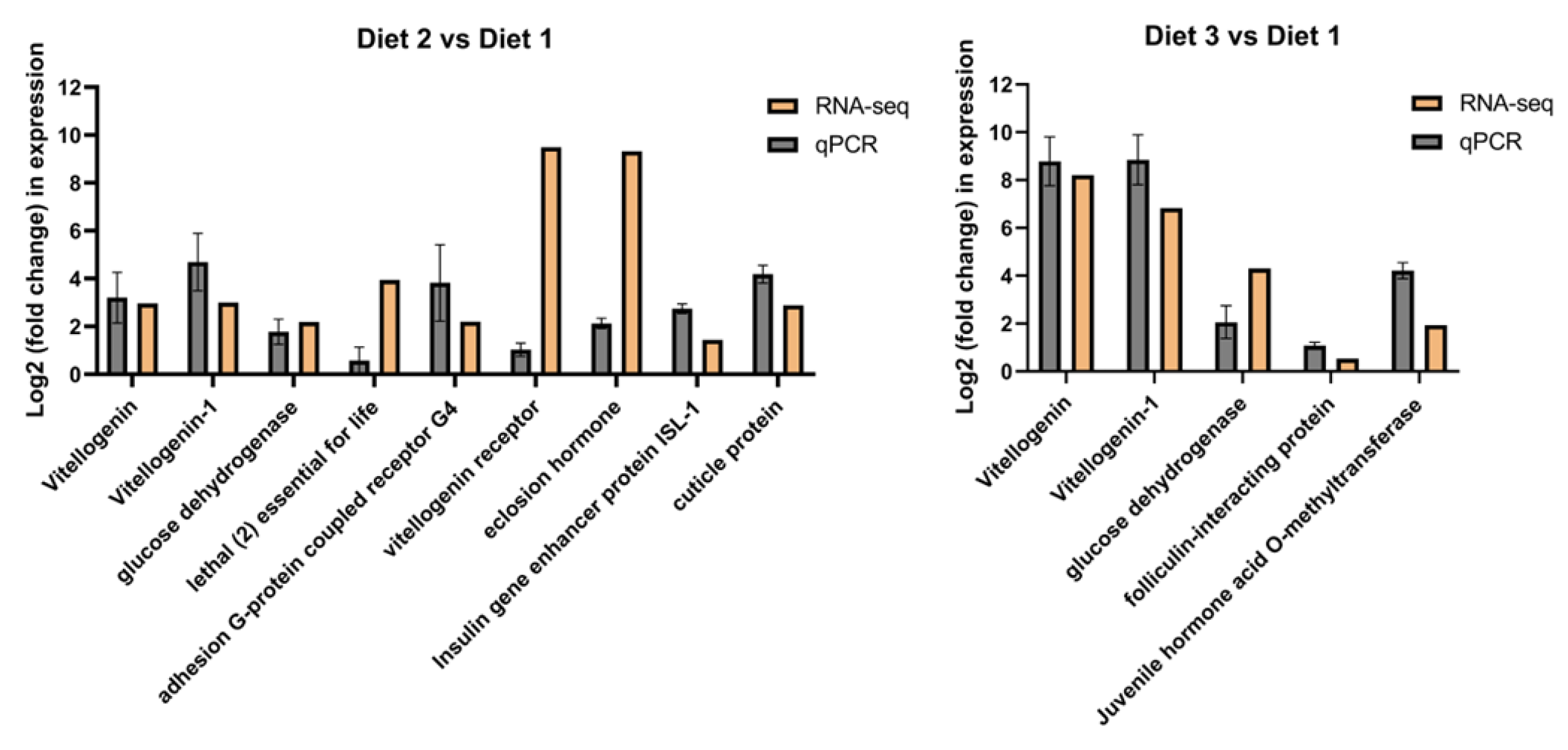
2.7. Real-Time Quantitative PCR (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of Different Artificial Diets on H. axyridis Reproduction
| Group | Total Egg | Egg Weight/mg | Hatchability/% | Pre-Oviposition/Days |
|---|---|---|---|---|
| Diet 1 | 4.25 ± 2.24 b | 1.73 ± 0.03 a | 83.33 ± 0.36 a | 19.75 ± 3.68 a |
| Diet 2 | 23.58 ± 8.73 a | 1.83 ± 0.03 a | 85.00 ± 0.24 a | 13 ± 1.17 b |
| Diet 3 | 24.65 ± 6.8 a | 1.77 ± 0.03 a | 86.67 ± 0.36 a | 11 ± 0.69 b |
3.2. Arrest of Ovarian Development After Feeding Different Artificial Diets
3.3. Effects of the Artificial Diets on Protein, Glycogen, and Triglyceride Content
3.4. Transcriptome Assembly and Differentially Expressed Genes
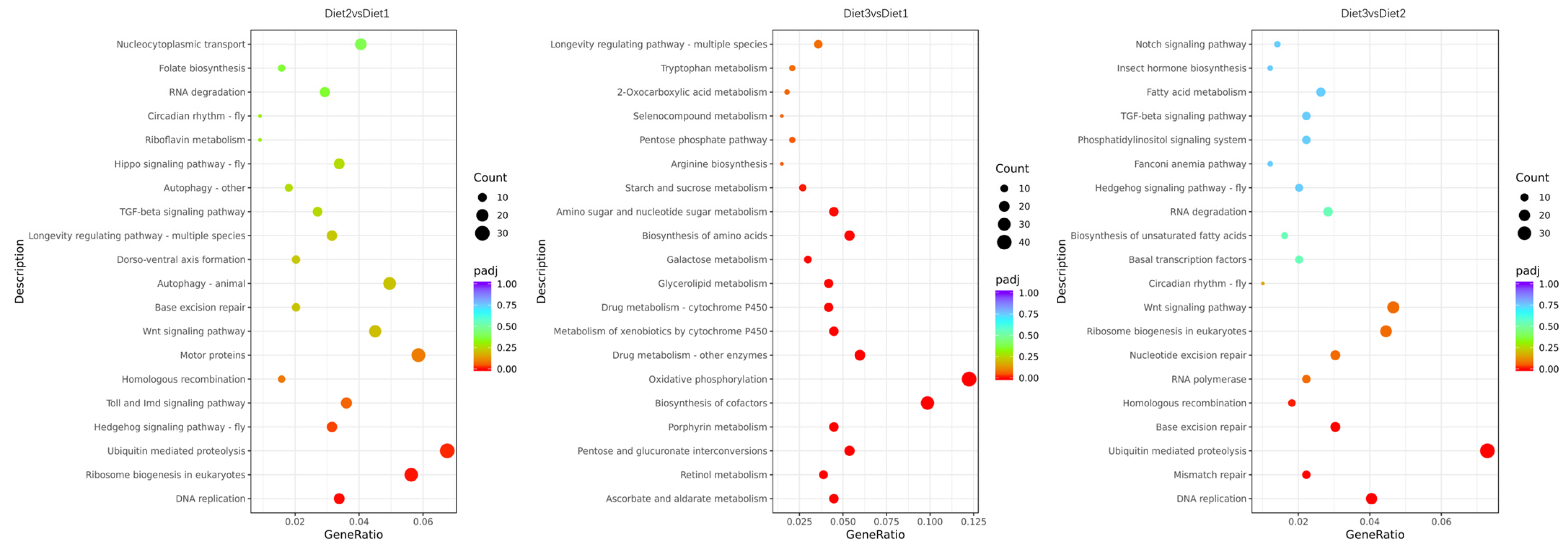
3.5. GO Functional Annotation and KEGG Enrichment Analysis of DEGs
3.6. Identification of Development-Related Genes Differentially Expressed Under Different Artificial Diets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferran, A.; Niknam, H.; Kabiri, F.; Picart, J.L.; De Herch, C.; Brun, J.; Iperti, G.; Lapchin, L. The use of Harmonia axyridis larvae (Coleoptera: Coccinellidae) against Macrosiphum rosae (Hemiptera: Sternorrhyncha: Aphididae) on rose bushes. Eur. J. Entomol. 1996, 93, 59–67. [Google Scholar]
- Yasuda, H.; Takagi, T.; Kogi, K. Effects of conspecific and heterospecific larval tracks on the oviposition behaviour of the predatory ladybird, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2000, 97, 551–553. [Google Scholar] [CrossRef]
- Brown, P.M.J.; Thomas, C.E.; Lombaert, E.; Jeffries, D.L.; Estoup, A.; Lawson Handley, L.J. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): Distribution, dispersal and routes of invasion. BioControl 2011, 56, 623–641. [Google Scholar] [CrossRef]
- Dang, G.R.; Zhang, Y.; Chen, H.Y.; Zhang, L.S.; Wang, M.Q.; Liu, C.X. Effect of Different Artificial Diets on the Development and Fecundity of the Green Lacewing Chrysopa pallens (Rambur). Sci. Agric. Sin. 2012, 45, 4818–4825. [Google Scholar] [CrossRef]
- Niijima, K.; Takahashi, H. Nutritional studies of an aphidophagous coccinellid, Harmonia axyridis: II. Significance of Minrals for Larval Growth. Appl. Entomol. Zool. 1980, 12, 325–329. [Google Scholar] [CrossRef]
- Niijima, K.; Takahashi, H. Nutritional studies of an aphidophagous coccinellid, Harmonia axyridis. IV. Effects on reproduction of a chemically defined diet and some fractions extracted from drone honeybee brood. Bull. Fac. Agric. Tamagawa Univ. 1980, 20, 47–55. [Google Scholar]
- Zhang, S.; Mao, J.; Zeng, F. Effects of an artificial diet with non-insect ingredient on biological characteristics of Harmonia axyridis (Pallas). Chin. J. Biol. Control 2015, 31, 35–40. [Google Scholar]
- Morales-Ramos, J.A.; Rojas, M.G.; Coudron, T.A. Artificial diet development for entomophagous arthropods. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Elsevier: New York, NY, USA, 2014; pp. 203–240. [Google Scholar]
- Seko, T.; Abe, J.; Miura, K. Effect of supplementary food containing Artemia salina on the development and survival of flightless Harmonia axyridis in greenhouses. BioControl 2019, 64, 333–341. [Google Scholar] [CrossRef]
- Ricupero, M.; Dai, C.; Siscaro, G.; Russo, A.; Biondi, A.; Zappalà, L. Potential diet regimens for laboratory rearing of the harlequin ladybird. BioControl 2020, 65, 583–592. [Google Scholar] [CrossRef]
- Specty, O.; Febvay, G.; Grenier, S.; Delobel, B.; Piotte, C.; Pageaux, J.; Ferran, A.; Guillaud, J. Nutritional plasticity of the predatory lady beetle Harmonia axyridis (Coleoptera: Coccinellidae): Comparison between natural and substitution prey. Arch. Insect Biochem. Physiol. 2003, 52, 81–91. [Google Scholar] [CrossRef]
- Sighinolfi, L.; Febvay, G.; Dindo, M.L.; Rey, M.; Pageaux, J.; Baronio, P.; Grenier, S. Biological and biochemical characteristics for quality control of Harmonia axyridis (Pallas) (Coleoptera, Coccinellidae) reared on a liver-based diet. Arch. Insect Biochem. Physiol. 2008, 68, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.; Zanuncio, J.C.; Serrão, J.E.; Lima, E.R.; Figueiredo, M.L.C.; Cruz, I. Suitability of different artificial diets for development and survival of stages of the predaceous ladybird beetle Eriopis connexa. Phytoparasitica 2009, 37, 115–123. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, Y.; Lu, Y.; Zhou, M.; Wang, S.; Wang, S. The Effect of Different Dietary Sugars on the Development and Fecundity of Harmonia axyridis. Front. Physiol. 2020, 11, 574851. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, L.; Lv, J.; Pu, Z.; Zhang, L.; Chen, G.; Hu, X.; Zhang, Z.; Zhang, H. Dietary Effects on Biological Parameters and Gut microbiota of Harmonia axyridis. Front. Microbiol. 2021, 12, 818787. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Zeng, J.; Zhang, L.; Zeng, F.; Mao, J.; Zhang, G. Search for nutritional fitness traits in a biological pest control agent Harmonia axyridis using comparative transcriptomics. Front. Physiol. 2019, 10, 1148. [Google Scholar] [CrossRef]
- Chung, S.L.; Ferrier, L.K.; Squires, E.J. Survey of cholesterol level of commercial eggs produced on Canadian farms. Can. J. Anim. Sci. 1991, 71, 205–209. [Google Scholar] [CrossRef]
- Jing, X.; Behmer, S.T. Insect sterol nutrition: Physiological mechanisms, ecology, and applications. Annu. Rev. Entomol. 2020, 65, 251–271. [Google Scholar] [CrossRef]
- Brookheart, R.T.; Swearingen, A.R.; Collins, C.A.; Cline, L.M.; Duncan, J.G. High-sucrose-induced maternal obesity disrupts ovarian function and decreases fertility in Drosophila melanogaster. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1255–1263. [Google Scholar] [CrossRef]
- Heier, C.; Kühnlein, R.P. Triacylglycerol Metabolism in Drosophila melanogaster. Genetics 2018, 210, 1163–1184. [Google Scholar]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory Mechanisms of Vitellogenesis in Insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef]
- Yamada, T.; Habara, O.; Kubo, H.; Nishimura, T. Correction: Fat body glycogen serves as a metabolic safeguard for the maintenance of sugar levels in Drosophila. Development 2018, 145, dev165910. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Festuccia, W.T.; Laplante, M. A comparative perspective on lipid storage in animals. J. Cell Sci. 2013, 126, 1541–1552. [Google Scholar] [PubMed]
- Nur Aliah, N.A.; Ab-Rahim, S.; Moore, H.E.; Heo, C.C. Juvenile hormone: Production, regulation, current application in vector control and its future applications. Trop. Biomed. 2021, 38, 254–264. [Google Scholar] [CrossRef]
- Yin, C.M.; Zou, B.X.; Jiang, M.; Li, M.F.; Qin, W.; Potter, T.L.; Stoffolano, J.G. Identification of juvenile hormone III bisepoxide (JHB3), juvenile hormone III and methyl farnesoate secreted by the corpus allatum of Phormia regina (Meigen), in vitro and function of JHB3 either applied alone or as a part of a juvenoid blend. J. Insect Physiol. 1995, 41, 473–479. [Google Scholar] [CrossRef]
- Behmer, S.T. Overturning dogma: Tolerance of insects to mixed-sterol diets is not universal. Curr. Opin. Insect Sci. 2017, 23, 89–95. [Google Scholar] [CrossRef]
- HuangFu, N.; Zhu, X.; Chang, G.; Wang, L.; Li, D.; Zhang, K.; Gao, X.; Ji, J.; Luo, J.; Cui, J. Dynamic transcriptome analysis and Methoprene-tolerant gene knockdown reveal that juvenile hormone regulates oogenesis and vitellogenin synthesis in Propylea japonica. Genomics 2021, 113, 2877–2889. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, Y.H.; Ran, H.Y.; Li, F. Effects of different hormones as dietary supplements on biological characteristics of Coccinella septempunctata L. J. Appl. Entomol. 2023, 147, 888–894. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zeng, F. Effects of an artificial diet on development, reproduction and digestive physiology of Chrysopa septempunctata. BioControl 2013, 58, 789–795. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85, Erratum in Anal. Biochem. 1987, 163, 279. [Google Scholar] [CrossRef]
- Roe, J.H.; Dailey, R.E. Determination of glycogen with the anthrone reagent. Anal. Biochem. 1966, 15, 245–250. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, M. Biochemical Estimation to Detect the Metabolic Pathways of Drosophila. In Fundamental Approaches to Screen Abnormalities in Drosophila Springer Protocols Handbooks; Mishra, M., Ed.; Springer: New York, NY, USA, 2020; pp. 135–149. ISBN 978-1-4939-9755-8. [Google Scholar]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. EMBnet J. 2010, 17, 10–12. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Chen, Z.H.; Qin, J.D.; Fan, X.M.; Li, X.L. Effects of adding lipids and juvenoid into the artificial diet on feeding and reproduction of Coccinella septempunctata L. Acta Entomol. Sin. 1984, 27, 136–146. [Google Scholar] [CrossRef]
- Zhang, T.S.; Li, K.; Zhang, L.L.; Wang, B. Effects of artificial diet on the predatory performance of Propylea japonica. Entomol. Knowl. 2008, 45, 791–794. [Google Scholar] [CrossRef]
- Sacks, F.M.; Andraski, A.B. Dietary fat and carbohydrate affect the metabolism of protein-based high-density lipoprotein subspecies. Curr. Opin. Lipidol. 2022, 33, 1–15. [Google Scholar] [CrossRef]
- Chen, P.; Liu, J.; Chi, B.; Li, D.; Li, J.; Liu, Y. Effect of different diets on the growth and development of Harmonia axyridis (Pallas). J. Appl. Entomol. 2020, 144, 911–919. [Google Scholar] [CrossRef]
- Liao, S.; Amcoff, M.; Nässel, D.R. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem. Mol. Biol. 2021, 133, 103495. [Google Scholar] [CrossRef]
- Li, K.; Jia, Q.Q.; Li, S. Juvenile hormone signaling-a mini review. Insect Sci. 2019, 26, 600–606. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Zhang, Y.; Guo, X.; Hou, J.; Li, H.; Wei, J.; Li, X. Effects of pyriproxyfen on development and hormone of the aphis, Aphis craccivora (Hemiptera: Aphididae). J. Econ. Entomol. 2024, 117, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Hunter, C.S.; Murray, J.; Noble, D.; Cai, C.L.; Evans, S.M.; Stein, R.; May, C.L. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes 2009, 58, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.P. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 2010, 40, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gou, X.; Qin, Z.; Li, D.; Wang, Y.; Ma, E.; Li, S.; Zhang, J. Identification and expression of cuticular protein genes based on Locusta migratoria transcriptome. Sci. Rep. 2017, 7, 45462. [Google Scholar] [CrossRef]
- Sheng, Y.; Chen, J.; Jiang, H.; Lu, Y.; Dong, Z.; Pang, L.; Zhang, J.; Wang, Y.; Chen, X.; Huang, J. The vitellogenin receptor gene contributes to mating and host-searching behaviors in parasitoid wasps. iScience 2023, 26, 106298. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef]
- Jing, Y.P.; Wen, X.; Li, L.; Zhang, S.; Zhang, C.; Zhou, S. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. USA 2021, 118, e2106908118. [Google Scholar] [CrossRef]
- Tang, J.; Yu, R.; Zhang, Y.; Xie, J.; Song, X.; Feng, F.; Gao, H.; Li, B. Molecular and functional analysis of eclosion hormone-like gene involved in post-eclosion behavior in a beetle. J. Insect Physiol. 2022, 142, 104429. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, K.O. Lethal (2) Essential for Life [l(2)efl] Gene in the Two-spotted Cricket, Gryllus bimaculatus (Orthoptera: Gryllidae). J. Life Sci. 2021, 31, 671–676. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.; Zeng, F.; Mao, J.; Liang, H.; Liu, F. Molecular Cloning of the Vitellogenin Gene and the Effects of Vitellogenin Protein Expression on the Physiology of Harmonia axyridis (Coleoptera: Coccinellidae). Sci. Rep. 2017, 7, 13926. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Kinjoh, T.; Kaneko, Y.; Itoyama, K.; Mita, K.; Hiruma, K.; Shinoda, T. Control of juvenile hormone biosynthesis in Bombyx mori: Cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem. Mol. Biol. 2007, 37, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, A.S.; Brown, M.R.; Bellés, X. Hormonal Control of Reproductive Processes. Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3, pp. 433–491. [Google Scholar]
- Ament, S.A.; Chan, Q.W.; Wheeler, M.M.; Nixon, S.E.; Johnson, S.P.; Rodriguez-Zas, S.L.; Foste, L.J.; Robinson, G.E. Mechanisms of stable lipid loss in a social insect. J. Exp. Biol. 2011, 215, 1271–1278. [Google Scholar] [CrossRef]
- Malik, N.; Ferreira, B.I.; Hollstein, P.E.; Pablo, E.H.; Stephanie, D.C.; Elijah, T.; Sammy, W.N.; Jingting, Y.; Rebecca, G.; Kristina, H.; et al. Induction of lysosomal and mitochondrial biogenesis by AMPK phosphorylation of FNIP1. Science 2023, 380, eabj5559. [Google Scholar] [CrossRef]
- Davies, B.; Baumann, C.; Kirchhoff, C.; Ivell, R.; Nubbemeyer, R.; Habenicht, U.F.; Theuring, F.; Gottwald, U. Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol. Cell. Biol. 2004, 24, 8642–8648. [Google Scholar] [CrossRef]

| Ingridients | Diet 1 | Diet 2 | Diet 3 |
|---|---|---|---|
| Fresh pig liver | 35% | 35% | 35% |
| Whole egg | 30% | - | - |
| Egg white | - | 30% | 30% |
| Brown sugar | 14.9% | 14.9% | 14.9% |
| Pork | 9.9% | 9.9% | 9.9% |
| Yeast extract | 5% | 5% | 5% |
| Vitamin C | 0.2% | 0.2% | 0.2% |
| Linseed oil | 5% | 5% | 5% |
| Juvenile hormone III | - | - | 10% |
| Sample | Library | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate | Q30 | GC pct |
|---|---|---|---|---|---|---|---|---|
| Diet1-1 | FRAS230248041-1r | 43092126 | 6.46G | 42099192 | 6.31G | 0.02 | 94.17 | 39.36 |
| Diet1-2 | FRAS230248044-1r | 42727980 | 6.41G | 41807246 | 6.27G | 0.03 | 93.28 | 38.19 |
| Diet1-3 | FRAS230248050-1r | 43068620 | 6.46G | 42138400 | 6.32G | 0.03 | 93.95 | 39.28 |
| Diet2-1 | FRAS230248053-1r | 43065702 | 6.46G | 41918970 | 6.29G | 0.02 | 94.45 | 39.26 |
| Diet2-2 | FRAS230248056-1r | 45325406 | 6.8G | 44428656 | 6.66G | 0.03 | 93.75 | 39.02 |
| Diet2-3 | FRAS230248059-1r | 43014486 | 6.45G | 42094054 | 6.31G | 0.02 | 94.17 | 38.06 |
| Diet3-1 | FRAS230248068-1r | 44711108 | 6.71G | 44049024 | 6.61G | 0.03 | 93.95 | 39.21 |
| Diet3-2 | FRAS230248071-1r | 44141014 | 6.62G | 43360852 | 6.5G | 0.02 | 94.29 | 38.69 |
| Diet3-3 | FRAS230248074-1r | 45319418 | 6.8G | 44010148 | 6.6G | 0.03 | 93.74 | 38.52 |
| Gene ID | Homologous Function in Nr | Primer (5′-3′) | Expression Level | Fragment Size (bp) | Tm (°C) |
|---|---|---|---|---|---|
| 123686364 | Vitellogenin-1 | GATGGATACGAGTACCCACT CTTTGCTTAGCCAAGACG | up | 149 | 58 |
| 123686370 | Vitellogenin | AAGAGTCGCGCACAGAAGAA TGCAATGGGACTTGCAAACG | up | 199 | 59 |
| 123674747 | Glucose dehydrogenase | ACCCTTCGTGATGGTCTG CCTGTAGCCTGATAAGTTTC | up | 137 | 59 |
| 123677096 | Adhesion G-protein coupled receptor G4 | AAGTGTAAGTTAGTAGGGTGTT TAGGAATAAGTGCGAAAC | up | 172 | 59 |
| 123689048 | Vitellogenin receptor | AATCACCCAGAACGTCACCC CCGTCAGGCTGGACAGATTT | up | 186 | 58 |
| 123676458 | Eclosion hormone | TTGAAGCCTATAAGACTGG CAAGAATGGTGCAACTGA | up | 185 | 57 |
| 123678499 | Folliculin-interacting protein | GGCTACCTGTTTGCCTCG GGATGTCCTACCGCACCA | up | 111 | 58 |
| 123678119 | Lethal (2) essential for life | ATTTCCAGGCAGTTTGTT GGGATGGTTTTATGATCAATACTCT | up | 154 | 59 |
| 123672779 | Cuticle protein | CGCGTTCTCCTACAAAGTGG CGCTCCGACATTACTCCTCT | up | 226 | 58 |
| 123688747 | Insulin gene enhancer protein ISL-1 | ATGGGGCGATGCAAGGTATA GCCGTGCATCTGGTTAACAA | up | 191 | 59 |
| 123684308 | Juvenile hormone acid O-methyltransferase | TCAGGAGATGGACACACTCTG ACTCTGCTTGACAACCCAGT | up | 240 | 58 |
| 123674045 | β-actin | ACCCATCTACGAAGGTTATGC CGGTGGTGGTGAAAGAGTAA | up | 122 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yu, Y.; Li, J.; Zheng, L.; Chen, S.; Mao, J. Comparative Transcriptome Analysis of the Effects of a Non-Insect Artificial Diet on the Nutritional Development of Harmonia axyridis. Insects 2025, 16, 380. https://doi.org/10.3390/insects16040380
Zhang T, Yu Y, Li J, Zheng L, Chen S, Mao J. Comparative Transcriptome Analysis of the Effects of a Non-Insect Artificial Diet on the Nutritional Development of Harmonia axyridis. Insects. 2025; 16(4):380. https://doi.org/10.3390/insects16040380
Chicago/Turabian StyleZhang, Tingting, Yinchen Yu, Jianyu Li, Li Zheng, Shiwei Chen, and Jianjun Mao. 2025. "Comparative Transcriptome Analysis of the Effects of a Non-Insect Artificial Diet on the Nutritional Development of Harmonia axyridis" Insects 16, no. 4: 380. https://doi.org/10.3390/insects16040380
APA StyleZhang, T., Yu, Y., Li, J., Zheng, L., Chen, S., & Mao, J. (2025). Comparative Transcriptome Analysis of the Effects of a Non-Insect Artificial Diet on the Nutritional Development of Harmonia axyridis. Insects, 16(4), 380. https://doi.org/10.3390/insects16040380






