A Novel Leaf-Derived Trapping Material Is More Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bed Bugs
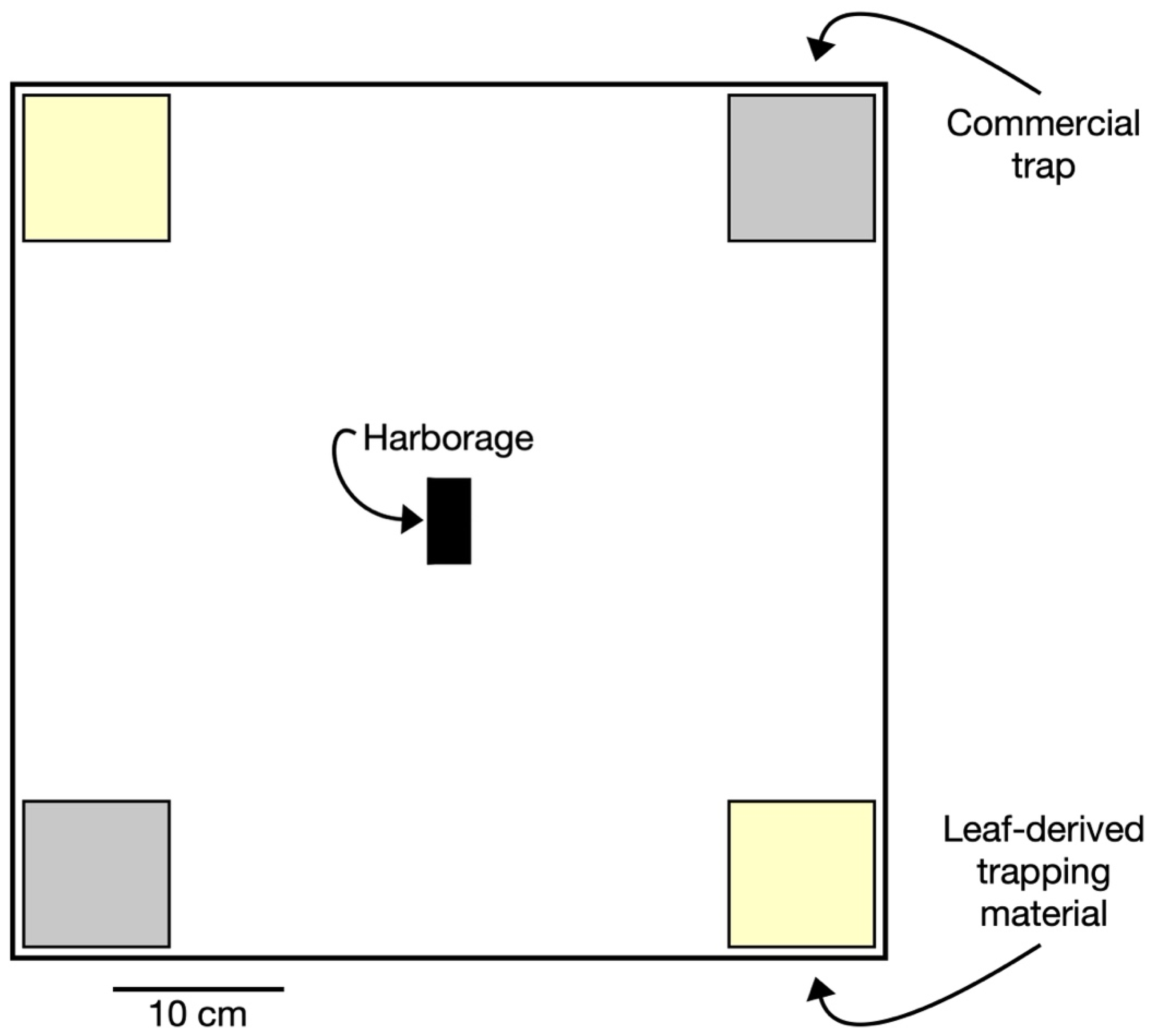
2.2. Experimental Arenas
2.3. Commercial Bed Bug Traps
2.4. Experimental Monitoring Surface
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
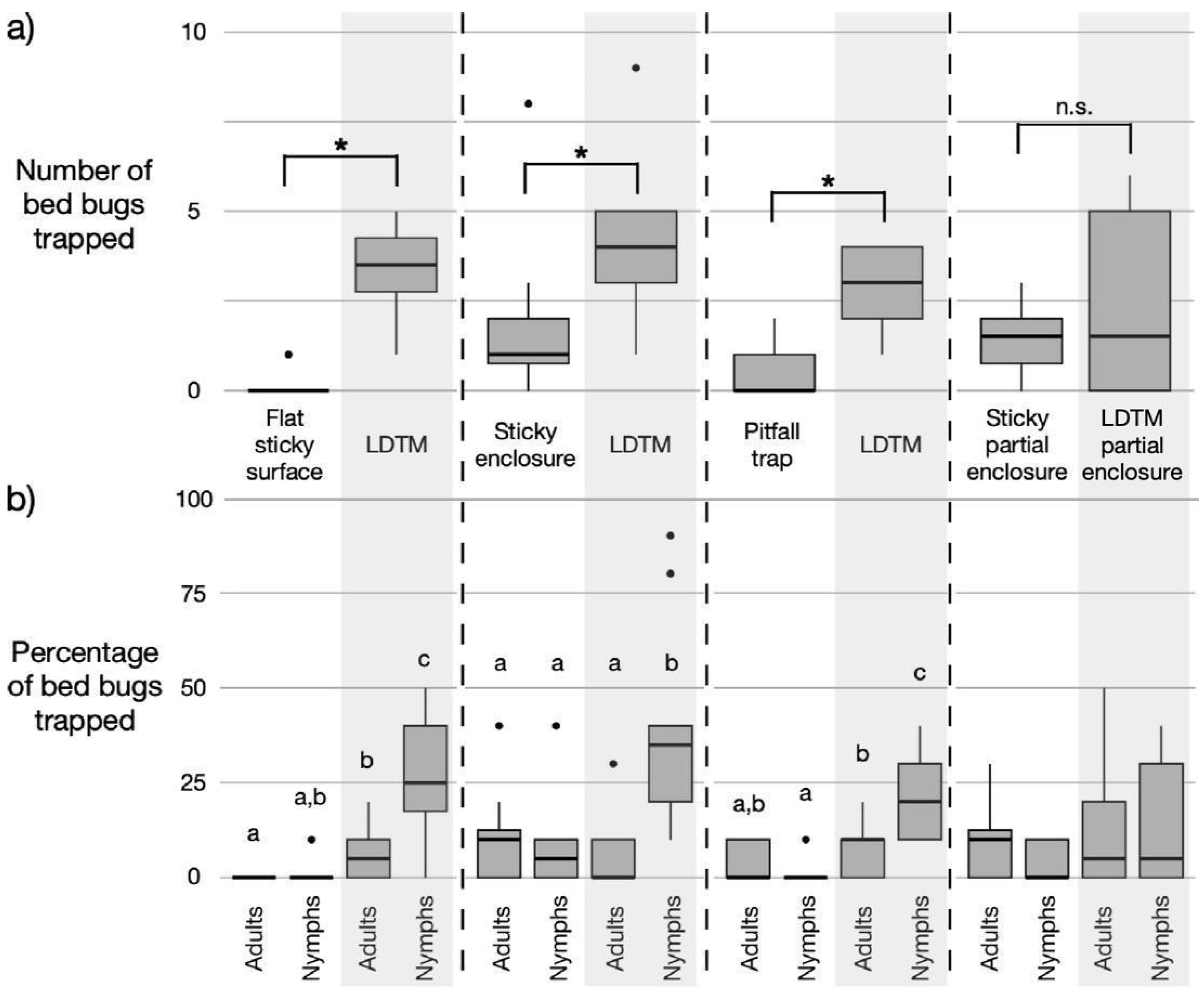
3.1. LDTM Trap Performance Compared to Selected Commercial Traps
3.2. Trapping Efficiency of LDTM and Commercial Traps for Adult and Nymphal Bed Bugs
3.3. Effect of Bed Bug Life Stage on LDTM Trap Status
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usinger, R.L. Monograph of Cimicidae (Hemiptera-Heteroptera); Entomological Society of America: Annapolis, MD, USA, 1966; Volume 7, ISBN 9780977620920. [Google Scholar]
- Koganemaru, R.; Miller, D.M. The Bed Bug Problem: Past, Present, and Future Control Methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Doggett, S.L.; Miller, D.M.; Lee, C.-Y. (Eds.) Advances in the Biology and Management of Modern Bed Bugs; John Wiley & Sons: Nashville, TN, USA, 2018; Volume 1, ISBN 9781119171522. [Google Scholar]
- Wang, C.; Cooper, R. [Bed Bug Supplement] Detection Tools and Techniques. Available online: https://www.pctonline.com/article/pct0811-bed-bug-monitor-detection-tools-techniques/ (accessed on 26 June 2024).
- Vaidyanathan, R.; Feldlaufer, M.F. Bed Bug Detection: Current Technologies and Future Directions. Am. J. Trop. Med. Hyg. 2013, 88, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Sutherland, A.M.; Gouge, D.H.; Spafford, H.; Nair, S.; Lewis, V.; Choe, D.-H.; Li, S.; Young, D. Pest Management Strategies for Bed Bugs (Hemiptera: Cimicidae) in Multiunit Housing: A Literature Review on Field Studies. J. Integr. Pest Manag. 2017, 8, 13. [Google Scholar] [CrossRef]
- Potter, M.F.; Haynes, K.F.; Fredericks, J. Bed Bugs across America: The 2015 Bugs without Borders Survey. PestWorld 2015, 7, 4–14. [Google Scholar]
- Cooper, R.; Wang, C. Detection and Monitoring. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 1, pp. 241–255. ISBN 9781119171522. [Google Scholar]
- Naylor, R. Bed Bug Industry Standards: Europe. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 1, pp. 217–220. ISBN 9781119171522. [Google Scholar]
- Potter, M. [Bed Bugs] The Sensitivity Spectrum: Human Reactions to Bed Bug Bites. Available online: https://www.pctonline.com/article/pct1002-bedbugs/ (accessed on 12 August 2024).
- Sutherland, A.M.; Choe, D.-H.; Lewis, V.; Young, D.; Romero, A.; Spafford, H.; Gouge, D. Survey Sheds Light on Bed Bugs in Multi-Unit Housing. Available online: https://www.pctonline.com/article/pct0915-bed-bugs-multi-unit-housing/ (accessed on 23 August 2024).
- Lee, C.-Y.; Miller, D.M.; Doggett, S.L. Chemical Control. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 1, pp. 285–310. ISBN 9781119171522. [Google Scholar]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.-Y. Insecticide Resistance and Resistance Mechanisms in Bed Bugs, Cimex Spp. (Hemiptera: Cimicidae). Parasit. Vectors 2017, 10, 318. [Google Scholar] [CrossRef]
- Wang, C.; Saltzmann, K.; Bennett, G.; Gibb, T. Comparison of Three Bed Bug Management Strategies in a Low-Income Apartment Building. Insects 2012, 3, 402–409. [Google Scholar] [CrossRef]
- Cooper, R.; Wang, C.; Singh, N. Effects of Various Interventions, Including Mass Trapping with Passive Pitfall Traps, on Low-Level Bed Bug Populations in Apartments. J. Econ. Entomol. 2016, 109, 762–769. [Google Scholar] [CrossRef]
- Pereira, R.M.; Koehler, P.G.; Pfiester, M.; Walker, W. Lethal Effects of Heat and Use of Localized Heat Treatment for Control of Bed Bug Infestations. J. Econ. Entomol. 2009, 102, 1182–1188. [Google Scholar] [CrossRef]
- Agnew, J.L.; Romero, A. Behavioral Responses of the Common Bed Bug, Cimex Lectularius, to Insecticide Dusts. Insects 2017, 8, 83. [Google Scholar] [CrossRef]
- Doggett, S.L.; Lee, C.-Y. Historical and Contemporary Control Options Against Bed Bugs, Cimex spp. Annu. Rev. Entomol. 2023, 68, 169–190. [Google Scholar] [CrossRef]
- Doggett, S. A Code of Practice for the Control of Bed Bug Infestations in Australia; Department of Medical Entomology and The Australian Environmental Pest Managers Association: Hendra, Australia, 2013; Volume 4, ISBN 9781740801423. [Google Scholar]
- Wang, C.; Gibb, T.; Bennett, G.W. Evaluation of Two Least Toxic Integrated Pest Management Programs for Managing Bed Bugs (Heteroptera: Cimicidae) with Discussion of a Bed Bug Intercepting Device. J. Med. Entomol. 2009, 46, 566–571. [Google Scholar] [PubMed]
- Wang, C.; Saltzmann, K.; Chin, E.; Bennett, G.W.; Gibb, T. Characteristics of Cimex Lectularius (Hemiptera: Cimicidae), Infestation and Dispersal in a High-Rise Apartment Building. J. Econ. Entomol. 2010, 103, 172–177. [Google Scholar] [PubMed]
- Wang, C.; Tsai, W.-T.; Cooper, R.; White, J. Effectiveness of Bed Bug Monitors for Detecting and Trapping Bed Bugs in Apartments. J. Econ. Entomol. 2011, 104, 274–278. [Google Scholar] [PubMed]
- Vail, K.M.; Chandler, J.G. Bed Bug (Hemiptera: Cimicidae) Detection in Low-Income, High-Rise Apartments Using Four or Fewer Passive Monitors. J. Econ. Entomol. 2017, 110, 1187–1194. [Google Scholar] [CrossRef]
- Potter, M.F. The History of Bed Bug Management—With Lessons from the Past. Am. Entomol. 2011, 57, 14–25. [Google Scholar]
- Wang, C.; Cooper, R. The Future of Bed Bug Monitoring. Pestworld, January/Feburary 2012. pp. 4–9. Available online: https://entomology.rutgers.edu/personnel/changlu-wang/docs/Wang2012Pestworld.pdf (accessed on 26 June 2024).
- Barak, A.V.; Shinkle, M.; Burkholder, W.E. Using Attractant Traps to Help Detect and Control Cockroaches. Pest Control 1977, 45, 14–16, 18–20. [Google Scholar]
- Ballard, J.B.; Gold, R.E. Laboratory and Field Evaluations of German Cockroach (Orthoptera: Blattellidae) Traps. J. Econ. Entomol. 1984, 77, 661–665. [Google Scholar]
- Kaakeh, W.; Bennett, G.W. Evaluation of Trapping and Vacuuming Compared with Low-Impact Insecticide Tactics for Managing German Cockroaches in Residences. J. Econ. Entomol. 1997, 90, 976–982. [Google Scholar] [CrossRef]
- Vetter, R.S.; Barger, D.K. An Infestation of 2,055 Brown Recluse Spiders (Araneae: Sicariidae) and No Envenomations in a Kansas Home: Implications for Bite Diagnoses in Nonendemic Areas. J. Med. Entomol. 2002, 39, 948–951. [Google Scholar]
- Wang, C.; Bennett, G.W. Comparison of Cockroach Traps and Attractants for Monitoring German Cockroaches (Dictyoptera: Blattellidae). Environ. Entomol. 2006, 35, 765–770. [Google Scholar]
- Doggett, S.L.; Dwyer, D.E.; Peñas, P.F.; Russell, R.C. Bed Bugs: Clinical Relevance and Control Options. Clin. Microbiol. Rev. 2012, 25, 164–192. [Google Scholar] [CrossRef] [PubMed]
- Crawley, S.E.; Borden, J.H. Detection and Monitoring of Bed Bugs (Hemiptera: Cimicidae): Review of the Underlying Science, Existing Products and Future Prospects. Pest Manag. Sci. 2021, 77, 5334–5346. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.H. The Action of Bean Leaves Against the Bedbug. J. Econ. Entomol. 1943, 36, 543–545. [Google Scholar] [CrossRef]
- Szyndler, M.W.; Haynes, K.F.; Potter, M.F.; Corn, R.M.; Loudon, C. Entrapment of Bed Bugs by Leaf Trichomes Inspires Microfabrication of Biomimetic Surfaces. J. R. Soc. Interface 2013, 10, 20130174. [Google Scholar] [CrossRef]
- Bustamante, J., Jr.; Panzarino, J.F.; Rupert, T.J.; Loudon, C. Forces to Pierce Cuticle of Tarsi and Material Properties Determined by Nanoindentation: The Achilles’ Heel of Bed Bugs. Biol. Open 2017, 6, 1541–1551. [Google Scholar] [CrossRef]
- Loudon, C. Surfaces Incorporating Treated Leaves for Physical Capture of Arthropods. WO2025038705, 20 February 2025. [Google Scholar]
- Rey, D.; Neuhäuser, M. Wilcoxon-Signed-Rank Test. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1658–1659. ISBN 9783642048975. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Pearson Education: Philadelphia, PA, USA, 1995; ISBN 9780130845429. [Google Scholar]
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Pearson, K.X. On the Criterion That a given System of Deviations from the Probable in the Case of a Correlated System of Variables Is Such That It Can Be Reasonably Supposed to Have Arisen from Random Sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Fisher, R.A. On the Interpretation of χ2 from Contingency Tables, and the Calculation of P. J. R. Stat. Soc. 1922, 85, 87. [Google Scholar] [CrossRef]
- Reinhardt, K.; Voigt, D.; Gorb, S.N. Evidence for a Sexually Selected Function of the Attachment System in Bedbugs Cimex Lectularius (Heteroptera, Cimicidae). J. Exp. Biol. 2019, 222, jeb206136. [Google Scholar] [CrossRef]
- Johnson, B. The Injurious Effects of the Hooked Epidermal Hairs of French Beans (Phaseolus vulgaris L.) on Aphis Craccivora Koch. Bull. Entomol. Res. 1953, 44, 779–788. [Google Scholar] [CrossRef]
| Trap Design | Commercial Trap Product Name | Commercially Available Trap Product Image | Dimensions | Description |
|---|---|---|---|---|
| Flat sticky surface | Harris Bed Bug Detection Trap with folds removed |  | Sticky surface area: 6.7 cm × 8.5 cm | This is a modified version of the sticky tent (see the row below) with the folds removed, leaving only the sticky surface exposed. |
| Sticky enclosure | Harris Bed Bug Detection Trap |  | Trap footprint area: 10.0 cm × 8.1 cm | All four sides of the trap fold to form a tent shape, each featuring an opening for bed bugs to enter. The waxy inner surface prevents bed bugs from escaping, and the base of the trap has a sticky surface. |
| Pitfall trap | Volcano |  | Trap footprint area: 7.2 cm × 7.2 cm | The trap has a pyramidal shape with a slanted, bumpy exterior that allows bed bugs to climb up. The smooth inner surface prevents escape. This study did not use the chemical lure module. |
| Sticky partial enclosure | Catchmaster Gluee Louee |  | Sticky surface area: 13.5 cm × 7.0 cm | Two sides of the trap fold, while the remaining two sides remain open to allow insects to crawl into the trap. The interior has a sticky surface. |
| Direct Comparison | Number of Bed Bugs Trapped with Commercial Traps | Number of Bed Bugs Trapped with LDTM | Number of Bed Bugs in Harborage | Number of Bed Bugs Elsewhere in the Arena | Number of Bed Bugs “Arrested” with LDTM |
|---|---|---|---|---|---|
| Flat sticky surface vs. LDTM | 1 | 40 | 141 | 27 | 31 |
| Sticky enclosure vs. LDTM | 21 | 52 | 113 | 50 | 4 |
| Pitfall trapvs. LDTM | 6 | 34 | 145 | 27 | 28 |
| Sticky partial enclosure vs. LDTM partial enclosure | 17 | 29 | 142 | 37 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante, J., Jr.; Liu, P.; Campbell, K.; Sutherland, A.M.; Choe, D.-H.; Loudon, C. A Novel Leaf-Derived Trapping Material Is More Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices. Insects 2025, 16, 362. https://doi.org/10.3390/insects16040362
Bustamante J Jr., Liu P, Campbell K, Sutherland AM, Choe D-H, Loudon C. A Novel Leaf-Derived Trapping Material Is More Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices. Insects. 2025; 16(4):362. https://doi.org/10.3390/insects16040362
Chicago/Turabian StyleBustamante, Jorge, Jr., Patrick Liu, Kathleen Campbell, Andrew M. Sutherland, Dong-Hwan Choe, and Catherine Loudon. 2025. "A Novel Leaf-Derived Trapping Material Is More Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices" Insects 16, no. 4: 362. https://doi.org/10.3390/insects16040362
APA StyleBustamante, J., Jr., Liu, P., Campbell, K., Sutherland, A. M., Choe, D.-H., & Loudon, C. (2025). A Novel Leaf-Derived Trapping Material Is More Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices. Insects, 16(4), 362. https://doi.org/10.3390/insects16040362







