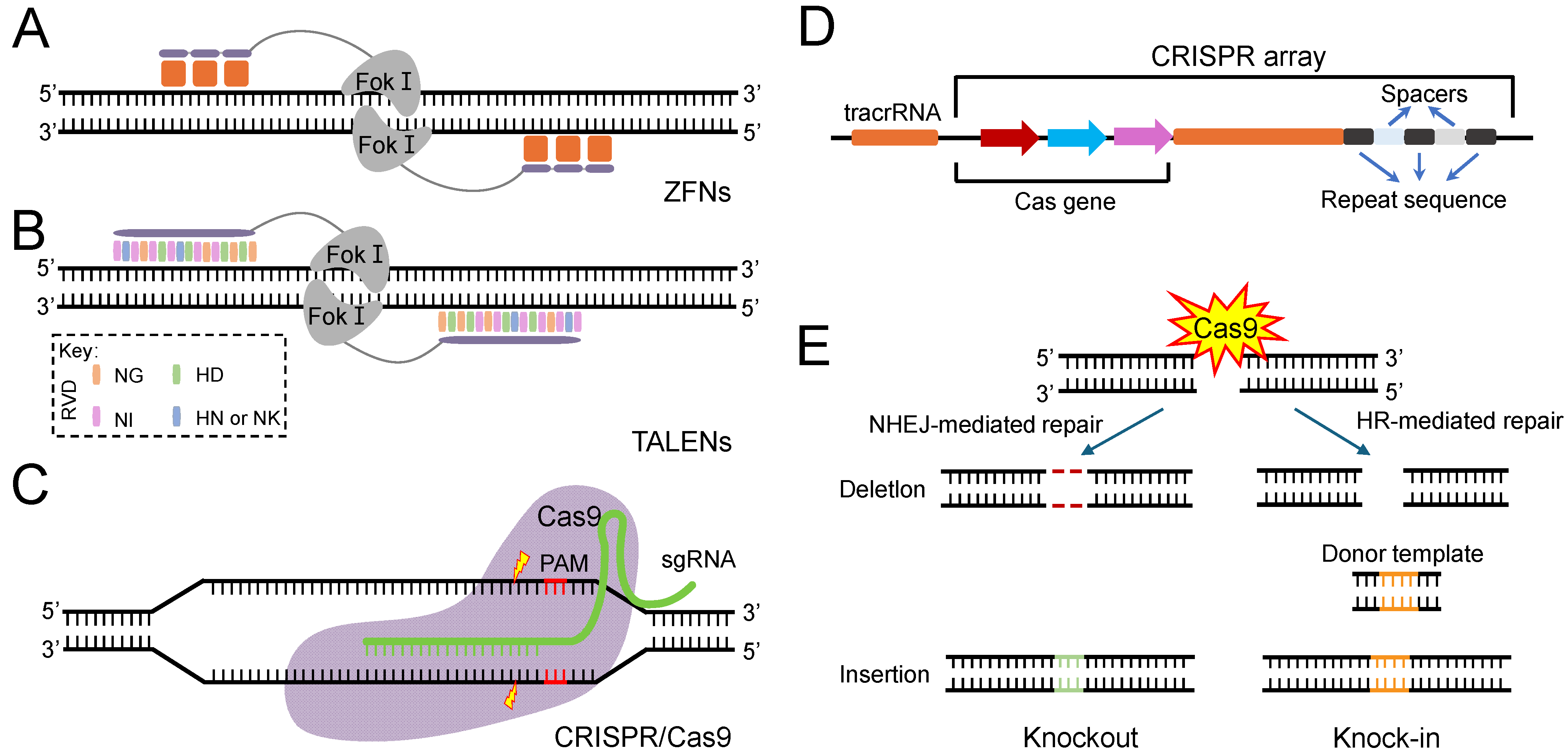
CRISPR/Cas Technology in Insect Insecticide Resistance
Simple Summary
Abstract
1. Introduction
2. Applications of CRISPR/Cas9 in Insecticide Resistance
2.1. Diptera
2.1.1. Drosophila melanogaster
2.1.2. Aedes aegypti, Culex quinquefasciatus, and Anopheles gambiae
2.2. Lepidoptera
2.2.1. Helicoverpa armigera, Helicoverpa zea, and Pectinophora gossypiella
2.2.2. Plutella xylostella, Trichoplusia ni, and Tuta absoluta
2.2.3. Spodoptera frugiperda, Spodoptera exigua, and Spodoptera litura
2.2.4. Ostrinia furnacalis and Chilo suppressalis
2.3. Hemiptera
2.3.1. Bemisia tabaci
2.3.2. Nilaparvata lugens
2.3.3. Myzus persicae
3. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Telleria, E.L.; Martins-da-Silva, A.; Tempone, A.J.; Traub-Csekö, Y.M. Leishmania, microbiota and sand fly immunity. Parasitology 2018, 145, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of Begomoviruses and Other Whitefly-Borne Viruses: Dependence on the Vector Species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Y.; Cheng, J.; Wang, Y.; Lan, C.; Shen, Y. Response of the mosquito immune system and symbiotic bacteria to pathogen infection. Parasites Vectors 2024, 17, 69. [Google Scholar] [CrossRef]
- Norris, E.J.; Coats, J.R. Current and Future Repellent Technologies: The Potential of Spatial Repellents and Their Place in Mosquito-Borne Disease Control. Int. J. Environ. Res. Public Health 2017, 14, 124. [Google Scholar] [CrossRef]
- Alyokhin, A.; Chen, Y. Adaptation to toxic hosts as a factor in the evolution of insecticide resistance. Curr. Opin. Insect Sci. 2017, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Neglected Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Jayapal, P.K.; Joshi, R.; Sathasivam, R.; Nguyen, B.V.; Faqeerzada, M.A.; Park, S.U.; Sandanam, D.; Cho, B.K. Non-destructive measurement of total phenolic compounds in Arabidopsis under various stress conditions. Front. Plant Sci. 2022, 13, 982247. [Google Scholar] [CrossRef]
- Zhang, B.; Li, C.; Luan, Y.; Lu, Y.; Hu, H.; Liu, Y.; Lu, K.; Zhang, G.; Dai, F.; Tong, X. The Role of Chitooligosaccharidolytic β-N-Acetylglucosamindase in the Molting and Wing Development of the Silkworm Bombyx mori. Int. J. Mol. Sci. 2022, 23, 3850. [Google Scholar] [CrossRef]
- Burc, E.; Girard-Tercieux, C.; Metz, M.; Cazaux, E.; Baur, J.; Koppik, M.; Rêgo, A.; Hart, A.F.; Berger, D. Life-history adaptation under climate warming magnifies the agricultural footprint of a cosmopolitan insect pest. Nat. Commun. 2025, 16, 827. [Google Scholar] [CrossRef]
- Nie, D.; Li, J.; Xie, Q.; Ai, L.; Zhu, C.; Wu, Y.; Gui, Q.; Zhang, L.; Tan, W. Nanoparticles: A Potential and Effective Method to Control Insect-Borne Diseases. Bioinorg. Chem. Appl. 2023, 2023, 5898160. [Google Scholar] [CrossRef]
- Araujo, S.; Mabille, D.; Garcia, A.B.; Caljon, G. A breath of fresh air: Impact of insect-borne protozoan parasites on the respiratory system. Trends Parasitol. 2024, 40, 717–730. [Google Scholar] [CrossRef]
- Al-Osaimi, H.M.; Kanan, M.; Marghlani, L.; Al-Rowaili, B.; Albalawi, R.; Saad, A.; Alasmari, S.; Althobaiti, K.; Alhulaili, Z.; Alanzi, A.; et al. A systematic review on malaria and dengue vaccines for the effective management of these mosquito borne diseases: Improving public health. Hum. Vaccines Immunother. 2024, 20, 2337985. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, K.; Liu, Y.; Zhou, Z.; Karthi, S.; Yang, H.; Li, C. A review of physiological resistance to insecticide stress in Nilaparvata lugens. 3 Biotech 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Gnankiné, O.; Bassolé, I.H.; Chandre, F.; Glitho, I.; Akogbeto, M.; Dabiré, R.K.; Martin, T. Insecticide resistance in Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Anopheles gambiae Giles (Diptera: Culicidae) could compromise the sustainability of malaria vector control strategies in West Africa. Acta Trop. 2013, 128, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Vreysen, M.J.; Dupé, S.; Lees, R.S.; Gilles, J.R.; Gouagna, L.C.; Chhem, R. Current status and future challenges for controlling malaria with the sterile insect technique: Technical and social perspectives. Acta Trop. 2014, 132, S130–S139. [Google Scholar] [CrossRef] [PubMed]
- Gott, R.C.; Kunkel, G.R.; Zobel, E.S.; Lovett, B.R.; Hawthorne, D.J. Implicating ABC Transporters in Insecticide Resistance: Research Strategies and a Decision Framework. J. Econ. Entomol. 2017, 110, 667–677. [Google Scholar] [CrossRef]
- Reid, M.C.; McKenzie, F.E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 2016, 15, 107. [Google Scholar] [CrossRef]
- Zuo, Y.; Shi, Y.; Zhang, F.; Guan, F.; Zhang, J.; Feyereisen, R.; Fabrick, J.A.; Yang, Y.; Wu, Y. Genome mapping coupled with CRISPR gene editing reveals a P450 gene confers avermectin resistance in the beet armyworm. PLoS Genet. 2021, 17, e1009680. [Google Scholar] [CrossRef]
- Hu, B.; Huang, H.; Hu, S.; Ren, M.; Wei, Q.; Tian, X.; Esmail Abdalla Elzaki, M.; Bass, C.; Su, J.; Reddy Palli, S. Changes in both trans- and cis-regulatory elements mediate insecticide resistance in a lepidopteron pest, Spodoptera exigua. PLoS Genet. 2021, 17, e1009403. [Google Scholar] [CrossRef]
- Kaplanoglu, E.; Chapman, P.; Scott, I.M.; Donly, C. Overexpression of a cytochrome P450 and a UDP-glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Qin, J.; Yuchi, Z. Identification of a Novel Inhibitor of Cimex lectularius Acetylcholinesterase. J. Agric. Food Chem. 2024, 72, 12498–12507. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Zhou, X.; Holmes, A.M.; Ranson, H.; Small, G.; Hemingway, J.; Ng, J.D.; Chen, L.; Meehan, E.J. Crystallization of agGST1-6, a recombinant glutathione S-transferase from a DDT-resistant strain of Anopheles gambiae. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Gu, Y.; Hu, X.; Sun, Y.; Ma, L.; Li, X.; Sun, L.; Sun, J.; Qian, J.; Zhu, C. Cloning and overexpression of CYP6F1, a cytochrome P450 gene, from deltamethrin-resistant Culex pipiens pallens. Acta Biochim. Biophys. Sin. 2005, 37, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Han, Z.; Wang, Y. Mechanisms of monocrotophos resistance in cotton bollworm, Helicoverpa armigera (Hübner). Arch. Insect Biochem. Physiol. 2002, 51, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, R.; Shivakumar, M.S. Involvement of metabolic resistance and F1534C kdr mutation in the pyrethroid resistance mechanisms of Aedes aegypti in India. Acta Trop. 2015, 148, 137–141. [Google Scholar] [CrossRef]
- Sun, D.; Guo, Z.; Liu, Y.; Zhang, Y. Progress and Prospects of CRISPR/Cas Systems in Insects and Other Arthropods. Front. Physiol. 2017, 8, 608. [Google Scholar] [CrossRef]
- Riordan, S.M.; Heruth, D.P.; Zhang, L.; Ye, S. Application of CRISPR/Cas9 for biomedical discoveries. Cell Biosci. 2015, 5, 33. [Google Scholar] [CrossRef]
- Merlin, C.; Beaver, L.E.; Taylor, O.R.; Wolfe, S.A.; Reppert, S.M. Efficient targeted mutagenesis in the monarch butterfly using zinc-finger nucleases. Genome Res. 2013, 23, 159–168. [Google Scholar] [CrossRef]
- Jo, Y.I.; Kim, H.; Ramakrishna, S. Recent developments and clinical studies utilizing engineered zinc finger nuclease technology. Cell. Mol. Life Sci. 2015, 72, 3819–3830. [Google Scholar] [CrossRef]
- Bibikova, M.; Golic, M.; Golic, K.G.; Carroll, D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 2002, 161, 1169–1175. [Google Scholar] [CrossRef]
- Beumer, K.; Bhattacharyya, G.; Bibikova, M.; Trautman, J.K.; Carroll, D. Efficient gene targeting in Drosophila with zinc-finger nucleases. Genetics 2006, 172, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Beumer, K.J.; Morton, J.J.; Bozas, A.; Trautman, J.K. Gene targeting in Drosophila and Caenorhabditis elegans with zinc-finger nucleases. Methods Mol. Biol. 2008, 435, 63–77. [Google Scholar] [PubMed]
- Sajwan, S.; Takasu, Y.; Tamura, T.; Uchino, K.; Sezutsu, H.; Zurovec, M. Efficient disruption of endogenous Bombyx gene by TAL effector nucleases. Insect Biochem. Mol. Biol. 2013, 43, 17–23. [Google Scholar] [CrossRef]
- Mock, U.; Machowicz, R.; Hauber, I.; Horn, S.; Abramowski, P.; Berdien, B.; Hauber, J.; Fehse, B. mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res. 2015, 43, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Yu, Z.; Huang, P.; Wu, H.; Wei, C.; Zhu, N.; Shen, Y.; Chen, Y.; Zhang, B.; et al. Efficient and specific modifications of the Drosophila genome by means of an easy TALEN strategy. J. Genet. Genom. 2012, 39, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Randau, L.; Plagens, A. Exploiting CRISPR/Cas: Interference mechanisms and applications. Int. J. Mol. Sci. 2013, 14, 14518–14531. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Lander, E.S. The Heroes of CRISPR. Cell 2016, 164, 18–28. [Google Scholar] [CrossRef]
- Lai, S.; Wei, S.; Zhao, B.; Ouyang, Z.; Zhang, Q.; Fan, N.; Liu, Z.; Zhao, Y.; Yan, Q.; Zhou, X.; et al. Generation of Knock-In Pigs Carrying Oct4-tdTomato Reporter through CRISPR/Cas9-Mediated Genome Engineering. PLoS ONE 2016, 11, e0146562. [Google Scholar] [CrossRef]
- Lamb, A.M.; Walker, E.A.; Wittkopp, P.J. Tools and strategies for scarless allele replacement in Drosophila using CRISPR/Cas9. Fly 2017, 11, 53–64. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, X.; Li, Y.; Sun, Y.; Zhu, Q.; Sun, J. Highly Efficient Targeted Gene Editing in Upland Cotton Using the CRISPR/Cas9 System. Int. J. Mol. Sci. 2018, 19, 3000. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Aumann, R.A.; Häcker, I.; Schetelig, M.F. CRISPR-based genetic control strategies for insect pests. J. Integr. Agric. 2023, 22, 651–668. [Google Scholar] [CrossRef]
- Itokawa, K.; Komagata, O.; Kasai, S.; Ogawa, K.; Tomita, T. Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci. Rep. 2016, 6, 24652. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef]
- Perkin, L.C.; Adrianos, S.L.; Oppert, B. Gene Disruption Technologies Have the Potential to Transform Stored Product Insect Pest Control. Insects 2016, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Bi, H.; Xu, J.; Liu, Z.; Niu, C.; He, L.; James, A.A.; Li, K.; Huang, Y. Mutation of the seminal protease gene, serine protease 2, results in male sterility in diverse lepidopterans. Insect Biochem. Mol. Biol. 2020, 116, 103243. [Google Scholar] [CrossRef]
- Yang, J.; Schleicher, T.R.; Dong, Y.; Park, H.B.; Lan, J.; Cresswell, P.; Crawford, J.; Dimopoulos, G.; Fikrig, E. Disruption of mosGILT in Anopheles gambiae impairs ovarian development and Plasmodium infection. J. Exp. Med. 2020, 217, e20190682. [Google Scholar] [CrossRef]
- Nateghi Rostami, M. CRISPR/Cas9 gene drive technology to control transmission of vector-borne parasitic infections. Parasite Immunol. 2020, 42, e12762. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.J.; Ma, X.; Zhang, Z.J.; Wang, T.C.; Sun, F.; Hou, C.X.; Li, M.W. A palmitoyltransferase Approximated gene Bm-app regulates wing development in Bombyx mori. Insect Sci. 2020, 27, 2–13. [Google Scholar] [CrossRef]
- Denecke, S.; Fusetto, R.; Martelli, F.; Giang, A.; Battlay, P.; Fournier-Level, A.; RA, O.H.; Batterham, P. Multiple P450s and Variation in Neuronal Genes Underpins the Response to the Insecticide Imidacloprid in a Population of Drosophila melanogaster. Sci. Rep. 2017, 7, 11338. [Google Scholar] [CrossRef] [PubMed]
- Fusetto, R.; Denecke, S.; Perry, T.; O’Hair, R.A.J.; Batterham, P. Partitioning the roles of CYP6G1 and gut microbes in the metabolism of the insecticide imidacloprid in Drosophila melanogaster. Sci. Rep. 2017, 7, 11339. [Google Scholar] [CrossRef] [PubMed]
- Kaduskar, B.; Kushwah, R.B.; Auradkar, A.; Guichard, A.; Li, M.; Bennett, J.B.; Julio, A.H.; Marshall, J.M.; Montell, C.; Bier, E. Reversing insecticide resistance with allelic-drive in Drosophila melanogaster. Nat. Commun. 2022, 13, 291. [Google Scholar] [CrossRef]
- Samantsidis, G.R.; Panteleri, R.; Denecke, S.; Kounadi, S.; Christou, I.; Nauen, R.; Douris, V.; Vontas, J. ‘What I cannot create, I do not understand’: Functionally validated synergism of metabolic and target site insecticide resistance. Proc. Biol. Sci. 2020, 287, 20200838. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Garrood, W.T.; Puinean, A.M.; Eckel-Zimmer, M.; Williamson, M.S.; Davies, T.G.; Bass, C. A CRISPR/Cas9 mediated point mutation in the alpha 6 subunit of the nicotinic acetylcholine receptor confers resistance to spinosad in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2016, 73, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Chen, W.; Ghazali, R.; Yang, Y.T.; Christesen, D.; Martelli, F.; Lumb, C.; Bao Luong, H.N.; Mitchell, J.; Holien, J.K.; et al. Role of nicotinic acetylcholine receptor subunits in the mode of action of neonicotinoid, sulfoximine and spinosyn insecticides in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2021, 131, 103547. [Google Scholar] [CrossRef]
- Hernandes, N.; Qi, X.; Bhide, S.; Brown, C.; Camm, B.J.; Baxter, S.W.; Robin, C. Acetylcholine esterase of Drosophila melanogaster: A laboratory model to explore insecticide susceptibility gene drives. Pest Manag. Sci. 2024, 80, 2950–2964. [Google Scholar] [CrossRef]
- Vernon, S.W.; Goodchild, J.; Baines, R.A. The VAChTY49N mutation provides insecticide-resistance but perturbs evoked cholinergic neurotransmission in Drosophila. PLoS ONE 2018, 13, e0203852. [Google Scholar] [CrossRef] [PubMed]
- Denecke, S.; Fusetto, R.; Batterham, P. Describing the role of Drosophila melanogaster ABC transporters in insecticide biology using CRISPR-Cas9 knockouts. Insect Biochem. Mol. Biol. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, W.; Ma, S.; Chen, J.; Huang, J.; Qiao, X. Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster. Insects 2024, 15, 334. [Google Scholar] [CrossRef]
- Pacheco, S.; Gallegos, A.S.; Peláez-Aguilar, Á.E.; Sánchez, J.; Gómez, I.; Soberón, M.; Bravo, A. CRISPR-Cas9 knockout of membrane-bound alkaline phosphatase or cadherin does not confer resistance to Cry toxins in Aedes aegypti. PLoS Neglected Trop. Dis. 2024, 18, e0012256. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; López Del Amo, V.; Mameli, E.; Lee, M.; Bishop, A.L.; Perrimon, N.; Gantz, V.M. Optimized CRISPR tools and site-directed transgenesis towards gene drive development in Culex quinquefasciatus mosquitoes. Nat. Commun. 2021, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Grigoraki, L.; Cowlishaw, R.; Nolan, T.; Donnelly, M.; Lycett, G.; Ranson, H. CRISPR/Cas9 modified An. gambiae carrying kdr mutation L1014F functionally validate its contribution in insecticide resistance and combined effect with metabolic enzymes. PLoS Genet. 2021, 17, e1009556. [Google Scholar] [CrossRef]
- Williams, J.; Cowlishaw, R.; Sanou, A.; Ranson, H.; Grigoraki, L. In vivo functional validation of the V402L voltage gated sodium channel mutation in the malaria vector An. gambiae. Pest Manag. Sci. 2022, 78, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sun, D.; Kang, S.; Zhou, J.; Gong, L.; Qin, J.; Guo, L.; Zhu, L.; Bai, Y.; Luo, L.; et al. CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect Biochem. Mol. Biol. 2019, 107, 31–38. [Google Scholar] [CrossRef]
- Guo, Z.J.; Guo, L.; Qin, J.Y.; Ye, F.; Sun, D.; Wu, Q.J.; Wang, S.L.; Crickmore, N.; Zhou, X.G.; Bravo, A.; et al. A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness. Nat. Commun. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.J.; Kang, S.; Sun, D.; Gong, L.J.; Zhou, J.L.; Qin, J.Y.; Guo, L.; Zhu, L.H.; Bai, Y.; Ye, F.; et al. MAPK-dependent hormonal signaling plasticity contributes to overcoming Bacillus thuringiensis toxin action in an insect host. Nat. Commun. 2020, 11, 14. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Guo, L.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; Guo, Z.; et al. A versatile contribution of both aminopeptidases N and ABC transporters to Bt Cry1Ac toxicity in the diamondback moth. BMC Biol. 2022, 20, 33. [Google Scholar] [CrossRef]
- Sun, D.; Xu, Q.; Guo, L.; Bai, Y.; Shentu, X.; Yu, X.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; et al. The role of GPI-anchored membrane-bound alkaline phosphatase in the mode of action of Bt Cry1A toxins in the diamondback moth. Fundam. Res. 2024; In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, S.; Ma, X.; Baxter, S.W.; Vasseur, L.; Xiong, L.; Huang, Y.; Yang, G.; You, S.; You, M. Resistance to Bacillus thuringiensis Cry1Ac toxin requires mutations in two Plutella xylostella ATP-binding cassette transporter paralogs. PLoS Pathog. 2020, 16, e1008697. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, D.; Wang, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Independent and Synergistic Effects of Knocking out Two ABC Transporter Genes on Resistance to Bacillus thuringiensis Toxins Cry1Ac and Cry1Fa in Diamondback Moth. Toxins 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.Z.; Ye, F.; Wu, Q.J.; Wang, S.L.; Crickmore, N.; Zhou, X.G.; Guo, Z.J.; Zhang, Y.J. CRISPR/Cas9-based functional characterization of PxABCB1 reveals its roles in the resistance of Plutella xylostella (L.) to Cry1Ac, abamectin and emamectin benzoate. J. Integr. Agric. 2023, 22, 3090–3102. [Google Scholar] [CrossRef]
- Ye, M.; Xiong, L.; Dong, Y.; Xie, C.; Zhang, Z.; Shen, L.; Li, Z.; Yue, Z.; Jiang, P.; Yuchi, Z.; et al. The Potential Role of the Methionine Aminopeptidase Gene PxMetAP1 in a Cosmopolitan Pest for Bacillus thuringiensis Toxin Tolerance. Int. J. Mol. Sci. 2022, 23, 13005. [Google Scholar] [CrossRef]
- You, S.; Yao, S.; Chen, X.; Hou, Q.; Liu, Z.; Lei, G.; Xie, X.; Liang, Z.; Yuchi, Z.; You, M.; et al. CRISPR/Cas9-Mediated Knockout of the PxJHBP Gene Resulted in Increased Susceptibility to Bt Cry1Ac Protoxin and Reduced Lifespan and Spawning Rates in Plutella xylostella. J. Agric. Food Chem. 2024, 72, 8180–8188. [Google Scholar] [CrossRef]
- Guo, Z.J.; Guo, L.; Bai, Y.; Kang, S.; Sun, D.; Qin, J.Y.; Ye, F.; Wang, S.L.; Wu, Q.J.; Xie, W.; et al. Retrotransposon-mediated evolutionary rewiring of a pathogen response orchestrates a resistance phenotype in an insect host. Proc. Natl. Acad. Sci. USA 2023, 120, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, L.; Cheng, Z.; Dong, L.; Guo, L.; Bai, Y.; Wu, Q.; Wang, S.; Yang, X.; Xie, W.; et al. A midgut transcriptional regulatory loop favors an insect host to withstand a bacterial pathogen. Innovation 2024, 5, 100675. [Google Scholar] [CrossRef] [PubMed]
- Douris, V.; Steinbach, D.; Panteleri, R.; Livadaras, I.; Pickett, J.A.; Van Leeuwen, T.; Nauen, R.; Vontas, J. Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 14692–14697. [Google Scholar] [CrossRef]
- Guo, Z.J.; Bai, Y.; Zhang, X.Y.; Guo, L.; Zhu, L.H.; Sun, D.; Sun, K.Y.; Xu, X.D.; Yang, X.; Xie, W.; et al. RNA m6A Methylation Suppresses Insect Juvenile Hormone Degradation to Minimize Fitness Costs in Response to A Pathogenic Attack. Adv. Sci. 2024, 11, 17. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Wang, F.; Yang, Y.; Wu, S.; Wu, Y. Disruption of nicotinic acetylcholine receptor α6 mediated by CRISPR/Cas9 confers resistance to spinosyns in Plutella xylostella. Pest Manag. Sci. 2020, 76, 1618–1625. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.; Wang, K.; Song, J.; Zhu, B.; Gao, X.; Liang, P. A novel V263I mutation in the glutamate-gated chloride channel of Plutella xylostella (L.) confers a high level of resistance to abamectin. Int. J. Biol. Macromol. 2023, 230, 123389. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.; Zhu, B.; Liang, P.; Gao, X. CRISPR/Cas9-mediated D472N substitution in the Rdl1 of Plutella xylostella confers low resistance to abamectin. Pest Manag. Sci. 2023, 79, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Jiang, D.; Yang, Y.; Wu, Y. CRISPR/Cas9 mediated ryanodine receptor I4790M knockin confers unequal resistance to diamides in Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 125, 103453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yu, Z.; He, Y.; Wang, F.; Gu, Y.; Davies, T.G.; Fan, Z.; Wang, X.; Wu, Y. Key role of the ryanodine receptor I4790K mutation in mediating diamide resistance in Plutella xylostella. Insect Biochem. Mol. Biol. 2024, 168, 104107. [Google Scholar] [CrossRef]
- Huang, J.M.; Rao, C.; Wang, S.; He, L.F.; Zhao, S.Q.; Zhou, L.Q.; Zhao, Y.X.; Yang, F.X.; Gao, C.F.; Wu, S.F. Multiple target-site mutations occurring in lepidopterans confer resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2020, 121, 103367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Wang, H.; Zhao, S.; Zuo, Y.; Yang, Y.; Wu, Y. Functional validation of cadherin as a receptor of Bt toxin Cry1Ac in Helicoverpa armigera utilizing the CRISPR/Cas9 system. Insect Biochem. Mol. Biol. 2016, 76, 11–17. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yan, R.; Wu, S.; Wu, Y.; Yang, Y. Reverse genetics reveals contrary effects of two Rdl-homologous GABA receptors of Helicoverpa armigera on the toxicity of cyclodiene insecticides. Pestic. Biochem. Physiol. 2020, 170, 104699. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Zuo, Y.; Yang, Y.; Wu, Y. CRISPR-mediated gene knockout reveals nicotinic acetylcholine receptor (nAChR) subunit α6 as a target of spinosyns in Helicoverpa armigera. Pest Manag. Sci. 2020, 76, 2925–2931. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Liu, S.; Liu, L.; Tay, W.T.; Walsh, T.K.; Yang, Y.; Wu, Y. CRISPR/Cas9 mediated genome editing of Helicoverpa armigera with mutations of an ABC transporter gene HaABCA2 confers resistance to Bacillus thuringiensis Cry2A toxins. Insect Biochem. Mol. Biol. 2017, 87, 147–153. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, M.; Yang, Y.; Zhang, J.; Yang, Y.; Liu, K.; Soberón, M.; Bravo, A.; Xiao, Y.; Wu, K. Synergistic resistance of Helicoverpa armigera to Bt toxins linked to cadherin and ABC transporters mutations. Insect Biochem. Mol. Biol. 2021, 137, 103635. [Google Scholar] [CrossRef]
- Jin, M.; Peng, Y.; Peng, J.; Zhang, H.; Shan, Y.; Liu, K.; Xiao, Y. Transcriptional regulation and overexpression of GST cluster enhances pesticide resistance in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Commun. Biol. 2023, 6, 1064. [Google Scholar] [CrossRef]
- Fabrick, J.A.; LeRoy, D.M.; Mathew, L.G.; Wu, Y.; Unnithan, G.C.; Yelich, A.J.; Carrière, Y.; Li, X.; Tabashnik, B.E. CRISPR-mediated mutations in the ABC transporter gene ABCA2 confer pink bollworm resistance to Bt toxin Cry2Ab. Sci. Rep. 2021, 11, 10377. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Heu, C.C.; LeRoy, D.M.; DeGain, B.A.; Yelich, A.J.; Unnithan, G.C.; Wu, Y.; Li, X.; Carrière, Y.; Tabashnik, B.E. Knockout of ABC transporter gene ABCA2 confers resistance to Bt toxin Cry2Ab in Helicoverpa zea. Sci. Rep. 2022, 12, 16706. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, B.; Liu, H.; Zhang, H.; Liu, R.; Yu, H.; Li, D. CRISPR/Cas9-Mediated Knockout Reveals the Involvement of CYP304F1 in β-Cypermethrin and Chlorpyrifos Resistance in Spodoptera litura. J. Agric. Food Chem. 2022, 70, 11192–11200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liao, C.; Zou, L.; Jin, M.; Shan, Y.; Quan, Y.; Yao, H.; Zhang, L.; Wang, P.; Liu, Z.; et al. Retrotransposon-mediated disruption of a chitin synthase gene confers insect resistance to Bacillus thuringiensis Vip3Aa toxin. PLoS Biol. 2024, 22, e3002704. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Tang, P.; Wang, X.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of nicotinic acetylcholine receptor (nAChR) α6 subunit confers high levels of resistance to spinosyns in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2022, 187, 105191. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; Lu, W.; Yuan, J.; Yang, Y.; Oakeshott, J.; Wu, Y. Divergent amplifications of CYP9A cytochrome P450 genes provide two noctuid pests with differential protection against xenobiotics. Proc. Natl. Acad. Sci. USA 2023, 120, e2308685120. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Song, X.; Ma, X.; Cotto-Rivera, R.O.; Kain, W.; Chu, H.; Chen, Y.R.; Fei, Z.; Wang, P. Mutation of ABC transporter ABCA2 confers resistance to Bt toxin Cry2Ab in Trichoplusia ni. Insect Biochem. Mol. Biol. 2019, 112, 103209. [Google Scholar] [CrossRef]
- Ma, X.; Shao, E.; Chen, W.; Cotto-Rivera, R.O.; Yang, X.; Kain, W.; Fei, Z.; Wang, P. Bt Cry1Ac resistance in Trichoplusia ni is conferred by multi-gene mutations. Insect Biochem. Mol. Biol. 2022, 140, 103678. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef]
- Zuo, Y.; Huang, J.; Wang, J.; Feng, Y.; Han, T.; Wu, Y.; Yang, Y. Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol. Biol. 2018, 27, 36–45. [Google Scholar] [CrossRef]
- Zuo, Y.; Xue, Y.; Lu, W.; Ma, H.; Chen, M.; Wu, Y.; Yang, Y.; Hu, Z. Functional validation of nicotinic acetylcholine receptor (nAChR) α6 as a target of spinosyns in Spodoptera exigua utilizing the CRISPR/Cas9 system. Pest Manag. Sci. 2020, 76, 2415–2422. [Google Scholar] [CrossRef]
- Zuo, Y.; Xue, Y.; Wang, Z.; Ren, X.; Aioub, A.A.; Wu, Y.; Yang, Y.; Hu, Z. Knockin of the G275E mutation of the nicotinic acetylcholine receptor (nAChR) α6 confers high levels of resistance to spinosyns in Spodoptera exigua. Insect Sci. 2022, 29, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Pei, Y.; Wu, W.; Hu, Z.; Zuo, Y. The knockout of the nicotinic acetylcholine receptor subunit gene α1 (nAChR α1) through CRISPR/Cas9 technology exposes its involvement in the resistance of Spodoptera exigua to insecticides. Pestic. Biochem. Physiol. 2023, 196, 105616. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Huang, J.; Jin, W.; Yang, Y.; Wu, Y. CRISPR-Mediated Knockout of the ABCC2 Gene in Ostrinia furnacalis Confers High-Level Resistance to the Bacillus thuringiensis Cry1Fa Toxin. Toxins 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Lin, Y.; Wang, X.; Jing, D.; Wang, Z.; He, K.; Bai, S.; Zhang, Y.; Zhang, T. Knockout of ABC Transporter ABCG4 Gene Confers Resistance to Cry1 Proteins in Ostrinia furnacalis. Toxins 2022, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, H.; He, L.; Liu, C.; Ge, W.; Ni, H.; Gao, C.; Wu, S. Double ryanodine receptor mutations confer higher diamide resistance in rice stem borer, Chilo suppressalis. Pest Manag. Sci. 2021, 77, 4971–4979. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Liu, C.; Hu, W.; Liu, J.; Zheng, L.; Gao, M.; Guo, F.; Qiao, S.; Liu, J.; et al. Risk assessment, fitness cost, cross-resistance, and mechanism of tetraniliprole resistance in the rice stem borer, Chilo suppressalis. Insect Sci. 2024, 31, 835–846. [Google Scholar] [CrossRef]
- Samantsidis, G.R.; O’Reilly, A.O.; Douris, V.; Vontas, J. Functional validation of target-site resistance mutations against sodium channel blocker insecticides (SCBIs) via molecular modeling and genome engineering in Drosophila. Insect Biochem. Mol. Biol. 2019, 104, 73–81. [Google Scholar] [CrossRef]
- Douris, V.; Papapostolou, K.M.; Ilias, A.; Roditakis, E.; Kounadi, S.; Riga, M.; Nauen, R.; Vontas, J. Investigation of the contribution of RyR target-site mutations in diamide resistance by CRISPR/Cas9 genome modification in Drosophila. Insect Biochem. Mol. Biol. 2017, 87, 127–135. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, J.; Yang, B.; Zhang, Y.; Liu, Z. Importance of CYP6ER1 Was Different among Neonicotinoids in Their Susceptibility in Nilaparvata lugens. J. Agric. Food Chem. 2023, 71, 4163–4171. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Ye, W.; Peng, Y.; Zhu, K.; Gao, C. CRISPR/Cas9-mediated knockout of NlCYP6CS1 gene reveals its role in detoxification of insecticides in Nilaparvata lugens (Hemiptera: Delphacidae). Pest Manag. Sci. 2023, 79, 2239–2246. [Google Scholar] [CrossRef]
- Lueke, B.; Douris, V.; Hopkinson, J.E.; Maiwald, F.; Hertlein, G.; Papapostolou, K.M.; Bielza, P.; Tsagkarakou, A.; Van Leeuwen, T.; Bass, C.; et al. Identification and functional characterization of a novel acetyl-CoA carboxylase mutation associated with ketoenol resistance in Bemisia tabaci. Pestic. Biochem. Physiol. 2020, 166, 104583. [Google Scholar] [CrossRef] [PubMed]
- Homem, R.A.; Buttery, B.; Richardson, E.; Tan, Y.; Field, L.M.; Williamson, M.S.; Davies, T.G.E. Evolutionary trade-offs of insecticide resistance—The fitness costs associated with target-site mutations in the nAChR of Drosophila melanogaster. Mol. Ecol. 2020, 29, 2661–2675. [Google Scholar] [CrossRef]
- Bassett, A.R.; Liu, J. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genom. 2014, 41, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; de Oliveira, J.L.; Abrantes, D.C.; Rogério, C.B.; Bueno, C.; Miranda, V.R.; Monteiro, R.A.; Fraceto, L.F. Recent Developments in Nanotechnology for Detection and Control of Aedes aegypti-Borne Diseases. Front. Bioeng. Biotechnol. 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.K.; Bilsborrow, J.B.; Schoen, R.T. Chronic Chikungunya Arthritis and Rheumatoid Arthritis: What They Have in Common. Am. J. Med. 2020, 133, e91–e97. [Google Scholar] [CrossRef]
- Okumu, F.O.; Moore, S.J. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: A review of possible outcomes and an outline of suggestions for the future. Malar. J. 2011, 10, 208. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Lan, J.; Zhang, L.; Chen, H.; Jiang, L.; Yu, H.; Liu, N.; Liao, C.; Han, Q. MDR49 coding for both P-glycoprotein and TMOF transporter functions in ivermectin resistance, trypsin activity inhibition, and fertility in the yellow fever mosquito, Aedes aegypti. Pestic. Biochem. Physiol. 2024, 201, 105899. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Z.; Chen, Z.; Zhang, L.; Zhao, J.; Guan, Q.; Liao, C.; Liu, N.; Han, Q. Identification of the Aedes aegypti nAChR gene family and molecular target of spinosad. Pest Manag. Sci. 2021, 77, 1633–1641. [Google Scholar] [CrossRef]
- Severson, D.W.; Behura, S.K. Mosquito genomics: Progress and challenges. Annu. Rev. Entomol. 2012, 57, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Viveiros-Rosa, S.G.; Regis, E.G.; Santos, W.C. Vector competence of Culex mosquitoes (Diptera: Culicidae) in Zika virus transmission: An integrative review. Rev. Panam. Salud. Publica. 2020, 44, e7. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Wang, L.; Liu, S.; Wu, S.; Yang, Y.; Feyereisen, R.; Wu, Y. CYP6AE gene cluster knockout in Helicoverpa armigera reveals role in detoxification of phytochemicals and insecticides. Nat. Commun. 2018, 9, 4820. [Google Scholar] [CrossRef] [PubMed]
- Gc, G.; Parajuli, K.; Gautam, I.; Banjara, M.R.; Ghimire, P.; Rijal, K.R. Efficacy of Native Bacillus thuringiensis against Mosquito Vector. J. Nepal Health Res. Counc. 2024, 21, 479–485. [Google Scholar] [CrossRef]
- Dos Santos, C.A.M.; do Nascimento, J.; Gonçalves, K.C.; Smaniotto, G.; Zechin, L.D.; Ferreira, M.D.; Polanczyk, R.A. Compatibility of Bt biopesticides and adjuvants for Spodoptera frugiperda control. Sci. Rep. 2021, 11, 8. [Google Scholar] [CrossRef]
- Kang, R.J.; Li, R.; Mjengi, J.; Abbas, Z.; Song, Y.H.; Zhang, L. A tiny sample rapid visual detection technology for imidacloprid resistance in Aphis gossypii by CRISPR/Cas12a. Sci. Total. Environ. 2024, 951, 10. [Google Scholar] [CrossRef]
- Jin, M.; Shan, Y.; Peng, Y.; Wang, W.; Zhang, H.; Liu, K.; Heckel, D.G.; Wu, K.; Tabashnik, B.E.; Xiao, Y. Downregulation of a transcription factor associated with resistance to Bt toxin Vip3Aa in the invasive fall armyworm. Proc. Natl. Acad. Sci. USA 2023, 120, e2306932120. [Google Scholar] [CrossRef]
- Jin, W.; Zhai, Y.; Yang, Y.; Wu, Y.; Wang, X. Cadherin Protein Is Involved in the Action of Bacillus thuringiensis Cry1Ac Toxin in Ostrinia furnacalis. Toxins 2021, 13, 658. [Google Scholar] [CrossRef]
- Pacheco, I.D.; Walling, L.L.; Atkinson, P.W. Gene Editing and Genetic Control of Hemipteran Pests: Progress, Challenges and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 900785. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Naqvi, R.Z.; Siddiqui, H.A.; Amin, I.; Mansoor, S. Current knowledge and implementations of Bemisia tabaci genomic technologies for sustainable control. J. Pest Sci. 2023, 96, 427–440. [Google Scholar] [CrossRef]
- Heu, C.C.; McCullough, F.M.; Luan, J.B.; Rasgon, J.L. CRISPR-Cas9-Based Genome Editing in the Silverleaf Whitefly (Bemisia tabaci). Cris. J. 2020, 3, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Qin, X.W.; Liu, J.; Liu, Q.; Zhang, R.J. Sublethal Effects of VirtakoTM on Life Table Parameters and Wing Formation of the Brown Planthopper (Homoptera: Delphacidae). J. Entomol. Sci. 2014, 49, 21–29. [Google Scholar]
- Zhang, F.; Zhang, Y.C.; Yu, Z.T.; Zeng, B.; Sun, H.; Xie, Y.Q.; Zhu, K.Y.; Gao, C.F. The G932C mutation of chitin synthase 1 gene (CHS1) mediates buprofezin resistance as confirmed by CRISPR/Cas9-mediated knock-in approach in the brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2024, 202, 105953. [Google Scholar] [CrossRef]
- Nozaki, T.; Kobayashi, Y.; Shigenobu, S. First record of the cedar bark aphid, Cinara cedri cedri Mimeur, 1936 (Hemiptera: Aphidoidea) in Japan, and identification of infecting Wolbachia strains. BioInvasions Rec. 2022, 11, 900–911. [Google Scholar] [CrossRef]
- Atencia-Pineda, M.C.; García-Leal, J.; Diaz-Ortiz, D.; Pareja-Loaiza, P.; Pacheco-Lugo, L.; Hoyos-López, R.; Calderón-Rangel, A.; Fragozo-Castilla, P.; Gutiérrez-Rodríguez, S.M.; Flores, A.E.; et al. Susceptibility to organophosphate insecticides in Aedes aegypti (Diptera: Culicidae) from northern Colombia and associated resistance mechanisms. Parasites Vectors 2025, 18, 7. [Google Scholar] [CrossRef]
- Dos Santos Hage, R.; Bohm, B.C.; Casagrande, C.P.; Silva, S.C.M.; Soares, A.T.; Lima, J.V.; Bruhn, N.C.P.; Bruhn, F.R.P. Spatiotemporal expansion of dengue in Brazilian Amazon between 2001 and 2021. Sci. Rep. 2025, 15, 1032. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.A.; Smidler, A.L.; Pai, J.J.; Chow, M.L.; Chen, S.L.; Mondal, A.; Sánchez, C.H.M.; Antoshechkin, I.; Marshall, J.M.; Akbari, O.S. Eliminating malaria vectors with precision-guided sterile males. Proc. Natl. Acad. Sci. USA 2024, 121, 12. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Sanchez, C.H.M.; Wu, S.L.; Marsha, J.M.; Akbari, O.S. Transforming insect population control with precision guided sterile males with demonstration in flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef]
- Shirai, Y.; Piulachs, M.D.; Belles, X.; Daimon, T. DIPA-CRISPR is a simple and accessible method for insect gene editing. Cell Rep. Methods 2022, 2, 100215. [Google Scholar] [CrossRef]
- Zhang, X.; Singh, A.; Soriano Martinez, K.; Ferree, P.M. Direct Parental (DIPA) CRISPR in the jewel wasp, Nasonia vitripennis. G3 Genes Genomes Genet. 2024, 14, JKAE095. [Google Scholar] [CrossRef]
- Matsuda, N.; Takahashi, M.; Shirai, Y.; Hinomoto, N.; Daimon, T. Direct parental CRISPR gene editing in the predatory bug Orius strigicollis, a biocontrol agent against small arthropods. Pest Manag. Sci. 2024, 80, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Tanaka, A.; Kunieda, T. Single-step generation of homozygous knockout/knock-in individuals in an extremotolerant parthenogenetic tardigrade using DIPA-CRISPR. PLoS Genet. 2024, 20, e1011298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, L.; Zhao, Y.; Ma, Y.; Long, G.; Guo, H.; Liu, X.; Hull, J.; Dewer, Y.; Yang, C.; et al. Efficient DIPA-CRISPR-mediated knockout of an eye pigment gene in the white-backed planthopper, Sogatella furcifera. Insect Sci. 2023, 31, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F.; Church, G.M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 2014, 3, e03401. [Google Scholar] [CrossRef]
- Gouda, M.N.R.; Jeevan, H.; Shashank, H.G. CRISPR/Cas9: A cutting-edge solution for combatting the fall armyworm, podoptera frugiperda. Mol. Biol. Rep. 2024, 51, 13. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, Y.; Lee, S.H. Recent advances in genome engineering by CRISPR technology. BMB Rep. 2024, 57, 12–18. [Google Scholar] [CrossRef]
- Zhao, F.; Ding, X.; Liu, Z.; Yan, X.; Chen, Y.; Jiang, Y.; Chen, S.; Wang, Y.; Kang, T.; Xie, C.; et al. Application of CRISPR/Cas9-based genome editing in ecotoxicology. Environ. Pollut. 2023, 336, 14. [Google Scholar] [CrossRef]
- Singh, S.; Rahangdale, S.; Pandita, S.; Saxena, G.; Upadhyay, S.K.; Mishra, G.; Verma, P.C. CRISPR/Cas9 for Insect Pests Management: A Comprehensive Review of Advances and Applications. Agriculture 2022, 12, 20. [Google Scholar] [CrossRef]
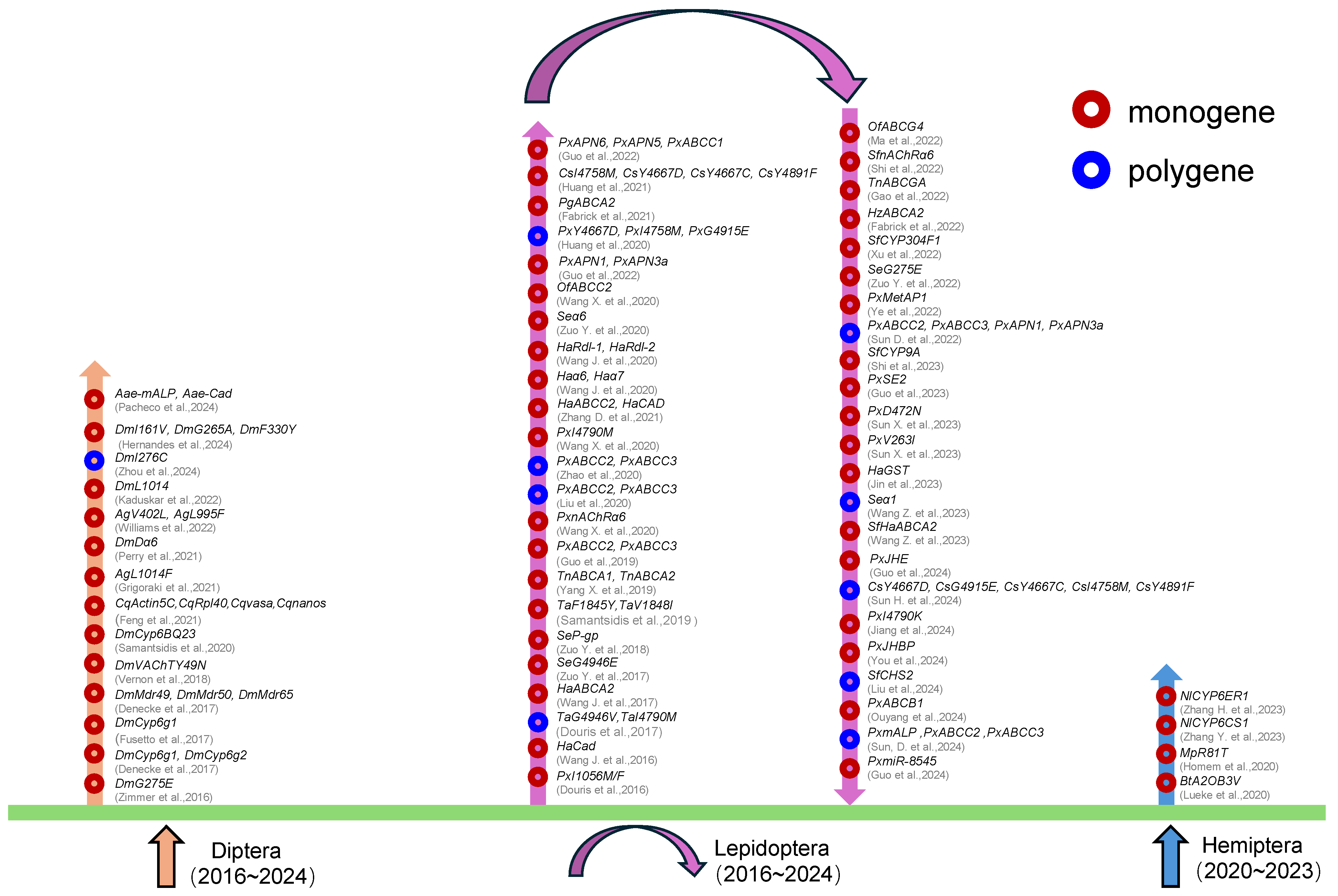
| Classification | Species | Genes | Mutation | Summary | References | |
|---|---|---|---|---|---|---|
| Diptera | Drosophila melanogaster | Cyp6g1, Cyp6g2 | Knockout | monogenic | Only high expression of Cyp6g1 contributes to imidacloprid resistance, while overexpression of Cyp6g2 can metabolize imidacloprid and produce resistance. | Denecke + 2017 [51] |
| Cyp6g1 | Knockout | monogenic | The Cyp6g1 gene is associated with the oxidative metabolism of insecticides but does not directly control the production of nitro-reducing metabolites. | Fusetto + 2017 [52] | ||
| L1014 | Knock-in | monogenic | The CRISPR-based allele drive replaces the resistant kdr mutation with a susceptible wild-type counterpart. | Kaduskar + 2022 [53] | ||
| Cyp6BQ23 | Knockout | monogenic | Reduced pyrethroid affinity at the target site, delaying saturation while simultaneously extending the duration of P450-driven detoxification | Samantsidis + 2020 [54] | ||
| G275E | Knock-in | monogenic | The G275E gene was associated with spinosad resistance using the CRISPR/Cas9 system. | Zimmer + 2016 [55] | ||
| Dα6 | Knockout | monogenic | The mechanism of nAChR’s response to three different chemical classes of insecticides was explored by CRISPR. | Perry + 2021 [56] | ||
| I161V, G265A, F330Y | Knockout | monogenic | Insecticide susceptibility gene drives could be useful tools to control pest insects; however, problems with particularities of target loci and GDR will need to be overcome for them to be effective. | Hernandes + 2024 [57] | ||
| VAChT Y49N | Knock-in | monogenic | The GAL4/UAS system was combined with CRISPR technology to study the effect of VAChTY49N on CASPP resistance. | Vernon + 2018 [58] | ||
| Mdr49, Mdr50, Mdr65 | Knockout | monogenic | Demonstrated the role of ABC transporter in insecticide tolerance of D. melanogaster | Denecke + 2017 [59] | ||
| I276C | Knockout | polygenic | The intersubunit amino acids within the transmembrane M1 and M3 domains do form binding sites that are critical for the interaction of m-diamide and isoxazoline insecticides. | Zhou + 2024 [60] | ||
| Aedes aegypti | Aae-mALP, Aae-Cad | Knockout | monogenic | In addition to Aae-Cad and Aae-mALP, other midgut membrane proteins are also involved in the Cry toxin receptor mode of Aspergillus aegypti. | Pacheco + 2024 [61] | |
| Culex quinquefasciatus | Actin5C, Rpl40, vasa, nanos | Knockout | monogenic | The gRNA scaffold variant improved the transgene efficiency of Culex mosquitoes. | Feng + 2021 [62] | |
| Anopheles gambiae | L1014F | Knock-in | monogenic | Mosquitoes carrying the L1014F allele show an adaptive disadvantage in the homozygous state. | Grigoraki + 2021 [63] | |
| V402L, L995F | Knock-in | monogenic | In some cases, the lower fitness costs associated with this kdr mutation may have a selective advantage over classical kdr. | Williams + 2022 [64] | ||
| Lepidoptera | Plutella xylostella | ABCC2, ABCC3 | Knockout | monogenic | Two knockout strains were successfully constructed using the new CRISPR/Cas9 genome engineering system. | Guo+ 2019 [65] |
| APN6, APN5, ABCC1 | Knockout | polygenic | Reveals how P. xylostella adjusts MAPK phosphorylation in response to toxins and alters FTZ-F1 transcription factor binding to regulate the expression of Bt receptors or non-receptor paralogues | Guo + 2022 [66] | ||
| Lepidoptera | Plutella xylostella | APN1, APN3a | Knockout | monogenic | Evidence that the MAPK cascade response can be activated by enhanced upstream hormone signaling to counter Bt virulence in the diamondback moth. | Guo + 2022 [67] |
| ABCC2, ABCC3 APN1, APN3a | Knockout | polygenic | Bt toxins have multiple modes of action that can compensate for the loss of a single receptor. | Sun D. + 2022 [68] | ||
| PxmALP, PxABCC2, PxABCC3 | Knockout | polygenic | Reveals functional redundancy between ABC transporter proteins and PxmALP | Sun D. + 2024 [69] | ||
| PxABCC2, PxABCC3 | Knockout | polygenic | PxABCC2 and PxABCC3 are redundant or complementary. | Liu + 2020 [70] | ||
| PxABCC2, PxABCC3 | Knockout | polygenic | The value of using single-gene knockout and multi-gene knockout is emphasized. | Zhao + 2020 [71] | ||
| PxABCB1 | Knockout | monogenic | PxABCB1 protects insects from avermectin insecticides; on the other hand, it promotes the toxic effects of Bt Cry1Ac toxin. | Ouyang + 2024 [72] | ||
| PxMetAP1 | Knockout | monogenic | Revealed the important role of the MetAP gene in DBMBt tolerance | Ye + 2022 [73] | ||
| PxJHBP | Knockout | monogenic | PxJHBP is a key gene in resistance to Cry1Ac and regulation of female reproduction. | You + 2024 [74] | ||
| SE2 | Knockout | monogenic | Adoption of high-efficiency double sgRNA strategy | Guo + 2023 [75] | ||
| miR-8545 | Knockout | monogenic | Increased expression of microRNA (miR-8545) inhibits the newly discovered molting steroid degrading enzyme (PxGLD) | Guo + 2024 [76] | ||
| I1056M/F | Knockout | monogenic | With the D. Melanogaster model, scientists were able to quickly observe phenotypic changes after gene editing, thus verifying the effect of the mutation. | Douris et al. + 2016 [77] | ||
| PxJHE | Knockout | monogenic | CRISPR/Cas9-induced m6A site-specific mutation PxJHE induces fitness costs | Guo + 2024 [78] | ||
| nAChRα6 | Knockout | monogenic | Endogenous functional studies demonstrated the causal relationship of Pxα6 truncated mutations. | Wang + 2020 [79] | ||
| V263I | Knockout | monogenic | The function of V263I mutation in PxGluCl was verified for the first time | Sun + 2023 [80] | ||
| D472N | Knockout | monogenic | The homozygous D472N mutation in Rdl1 confers a low level of resistance to avermectin in P. xylostella | Sun X. + 2023 [81] | ||
| I4790M | Knock-in | monogenic | The functional role of PxRyR’s I4790M mutation in diamide resistance was confirmed for the first time. | Wang X + 2020 [82] | ||
| Lepidoptera | Plutella xylostella | I4790K | Knock-in | monogenic | The I4790K mutation reduces insecticide binding to receptors | Jiang + 2024 [83] |
| Y4667D, I4758M, G4915E | Knock-in | monogenic | Multiple mutations in RyR confer resistance to diamides in clostridium-inhibiting bacteria. | Huang + 2020 [84] | ||
| Helicoverpa armigera | HaCad | Knockout | monogenic | HaCad provides strong reverse genetic evidence as a functional receptor of Cry1Ac. | Wang + 2016 [85] | |
| HaRdl-1, HaRdl-2 | Knockout | monogenic | HaRdl-1 and HaRdl-2 are important determinants of H. armigera sensitivity to three cyclodiene insecticides. | Wang J. + 2020 [86] | ||
| Haα6, Haα7 | Knockout | monogenic | Variation in nAChRα6 was associated with high resistance of pests to spinosyns. | Wang J. + 2020 [87] | ||
| HaABCA2 | Knockout | monogenic | The midgut brush marginal membrane vesicles of knockout populations lost their ability to bind to Cry2Ab, but retained their ability to bind to Cry1Ac. | Wang J. + 2017 [88] | ||
| HaABCC2, HaCAD | Knockout | monogenic | The synergistic effect of CAD and ABCC2/ABCC3 significantly enhanced the resistance of H. armigera to Cry1Ac. | Zhang D. + 2021 [89] | ||
| GST | Knockout | polygenic | The complex changes in GST cluster expression enhanced resistance of field populations to the highly efficient pyrethroid. | Jin + 2023 [90] | ||
| Pectinophora gossypiella | PgABCA2 | Knockout | monogenic | Demonstrated that destructive mutations lead to actual resistance to Cry2Ab and are associated with field resistance | Fabrick + 2021 [91] | |
| Helicoverpa zea | HzABCA2 | Knockout | monogenic | The mutation of the HzABCA2 gene is a key factor leading to the development of resistance to Cry2Ab in Helicoverpa zea. | Fabrick + 2022 [92] | |
| Spodoptera frugiperda | CYP304F1 | Knockout | monogenic | CYP304F1 plays an important role in resistance to β-cypermethrin and chlorpyrifos. | Xu + 2022 [93] | |
| SfCHS2 | Knockout | polygenic | Identified for the first time that the LTR retrotransposon Yaoer plays a pivotal role in the resistance mechanism against Vip3Aa | Liu + 2024 [94] | ||
| nAChRα6 | Knockout | polygenic | The team used CRISPR to knockout Sfα6 in S. frugiperda, studying its role in spinosyn susceptibility. | Shi + 2022 [95] | ||
| CYP9A | Knockout | polygenic | CYP9A gene cluster knockout in S.exigua and S. frugiperda | Shi + 2023 [96] | ||
| Trichoplusia ni | ABCA1, ABCA2 | Knockout | monogenic | ABCA2 is critical to the toxicity of Cry2Ab in T. ni. | Yang X. + 2019 [97] | |
| ABCC2 | Knock-in | monogenic | Investigated the association between ABCC2 and Trichoplusia ni resistance | Ma X. + 2022 [98] | ||
| Spodoptera exigua | G4946E | Knock-in | monogenic | The G4946E mutation in the RyR gene was shown to confer a high level of resistance to diamide insecticides. | Zuo Y. + 2017 [99] | |
| SeP-gp | Knockout | monogenic | Overexpression of SeP-gp may lead to abamectin and EB resistance in S.exigua. | Zuo Y. + 2018 [100] | ||
| Seα6 | Knockout | monogenic | Proved the functional role of Seα6 in the treatment of spinosad and spinetoram | Zuo Y + 2020 [101] | ||
| G275E | Knock-in | monogenic | Verified the role of G275E mutation in S. exiguan AChRα6 in resistance to spinosyn | Zuo Y. + 2022 [102] | ||
| Seα1 | Knockout | monogenic | Seα1 knockout results in the loss of functional transmembrane (TM)3 and TM4 elements. | Wang Z. + 2023 [103] | ||
| Ostrinia furnacalis | ABCC2 | Knockout | monogenic | The OfABCC2 protein may function as a receptor for Cry1Fa, enhancing its association with Cry1Fa toxin’s mode of action. | Wang X. + 2020 [104] | |
| ABCG4 | Knockout | monogenic | The mutant exhibited enhanced Cry1 toxin resistance, impacting larval development and population. | Gao + 2022 [105] | ||
| Lepidoptera | Chilo suppressalis | I4758M, Y4667D, Y4667C, Y4891F | Knock-in | monogenic | Revealed the role of RyR mutation in diamide resistance and how the mutation affects the binding affinity of different diamides | Huang + 2021 [106] |
| Y4667D, G4915E, Y4667C, I4758M, Y4891F | Knockout | polygenic | The I4758M and Y4667C double mutations have higher tetraniliprole resistance than the single Y4667C mutation. | Sun H. + 2024 [107] | ||
| Tuta absoluta | F1845Y, V1848I | Knockout | monogenic | The V1848I and F1845Y mutations may have formed too large a side chain to affect metaflumizone binding. | Samantsidis + 2019 [108] | |
| G4946V, I4790M | Knock-in | monogenic | Confirmed the role of RyR mutations in diamide resistance and revealed how mutations affect the binding affinity of different diamides | Douris et al. + 2017 [109] | ||
| Hemiptera | Nilaparvata lugens | CYP6ER1 | Knockout | monogenic | CYP6ER1 activity is related to the structure of an insecticide. | Zhang H. + 2023 [110] |
| NlCYP6CS1 | Knockout | monogenic | Nl6CS1KO is similar to the wild type in development and longevity, but there are differences in survival, reproduction, and body weight. | Zhang Y. + 2023 [111] | ||
| Bemisia tabaci | A2083V | Knockout | monogenic | B. tabaci is highly resistant to ketoenol insecticides, which is not related to metabolic resistance but is caused by A2083V mutation in the CT domain of ACC. | Lueke + 2020 [112] | |
| Hemiptera | Myzus persicae | R81T | Knockout | monogenic | Introduction of the R81T mutation in the black-bellied fly maggot using CRISPR/Cas9 results in enhanced resistance to neonicotinoid insecticides but reduced fitness. | Homem + 2020 [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Wang, M.; Zeng, J.; Sun, H.; Wei, X.; Jiang, H.; Shentu, X.; Sun, D. CRISPR/Cas Technology in Insect Insecticide Resistance. Insects 2025, 16, 345. https://doi.org/10.3390/insects16040345
Xu Q, Wang M, Zeng J, Sun H, Wei X, Jiang H, Shentu X, Sun D. CRISPR/Cas Technology in Insect Insecticide Resistance. Insects. 2025; 16(4):345. https://doi.org/10.3390/insects16040345
Chicago/Turabian StyleXu, Qiuchen, Mingyun Wang, Jiahui Zeng, Hangzhen Sun, Xiaoqi Wei, Hui Jiang, Xuping Shentu, and Dan Sun. 2025. "CRISPR/Cas Technology in Insect Insecticide Resistance" Insects 16, no. 4: 345. https://doi.org/10.3390/insects16040345
APA StyleXu, Q., Wang, M., Zeng, J., Sun, H., Wei, X., Jiang, H., Shentu, X., & Sun, D. (2025). CRISPR/Cas Technology in Insect Insecticide Resistance. Insects, 16(4), 345. https://doi.org/10.3390/insects16040345





