Developmental Regulation of Corazonin, Eclosion Hormone, and Bursicon Messages and RNAi Suppression of Corazonin in Adult, Female American Dog Ticks, Dermacentor variabilis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ticks
2.2. Synganglion Collection and Sample Preparation
2.3. Bioinformatics
2.4. Quantitative PCR (qPCR) and Analysis
2.5. RNA Interference (RNAi)
2.6. Statistics
3. Results
3.1. Expression of Corazonin at Different Stages
3.2. Expression of Eclosion Hormone at Different Developmental Stages
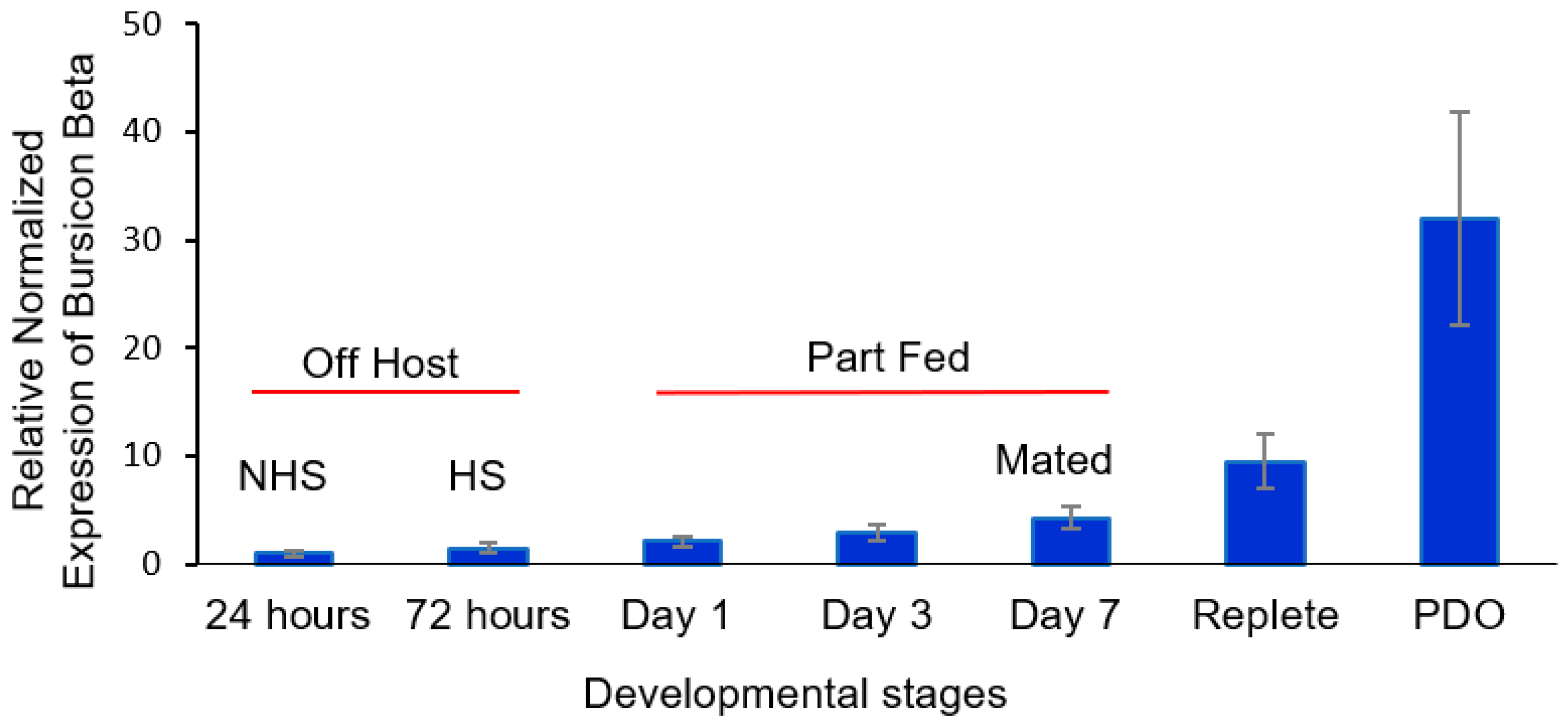
3.3. Expression of α and β Bursicon
3.4. Corazonin Message Suppression by RNAi and Effect on Egg Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sonenshine, D.E.; Roe, R.M. Overview: Ticks, people, and animals. In Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; Volume 1, pp. 3–16. [Google Scholar]
- Lees, K.; Bowman, A.S. Tick neurobiology: Recent advances and the post-genomic era. Invertebr. Neurosci. 2007, 7, 183–198. [Google Scholar] [CrossRef]
- Guerrero, F.; Pérez de León, A.; Rodriguez-Vivas, R.; Jonsson, N.; Miller, R.; Andreotti, R.; Sonenshine, D.; Roe, R. Acaricide research and development, resistance and resistance monitoring. In Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 353–381. [Google Scholar]
- George, J.E.; Pound, J.M.; Davey, R.B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129, S353–S366. [Google Scholar] [CrossRef] [PubMed]
- Roe, R.M.; Donahue, K.; Khalil, S.; Bissinger, B.W.; Zhu, J.; Sonenshine, D.E. Hormonal regulation of metamorphosis and reproduction in ticks. In Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; Volume 1, p. 416. [Google Scholar]
- Ogihara, M.H.; Taylor, D. Female reproductive system. In Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; Volume 1. [Google Scholar]
- Neese, P.A.; Sonenshine, D.E.; Kallapur, V.L.; Apperson, C.S.; Roe, R.M. Absence of insect juvenile hormones in the American dog tick, Dermacentor variabilis (Say) (Acari:Ixodidae), and in Ornithodoros parkeri Cooley (Acari:Argasidae). J. Insect Physiol. 2000, 46, 477–490. [Google Scholar] [CrossRef]
- Klowden, M.J.; Palli, S.R. Nervous Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 527–605. [Google Scholar]
- Clark, A.C.; del Campo, M.L.; Ewer, J. Neuroendocrine control of larval ecdysis behavior in Drosophila: Complex regulation by partially redundant neuropeptides. J. Neurosci. 2004, 24, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989, 250, 231–234. [Google Scholar] [CrossRef]
- Veenstra, J.A. Presence of corazonin in three insect species, and isolation and identification of [His7]corazonin from Schistocerca americana. Peptides 1991, 12, 1285–1289. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hua, Y.J.; Roller, L.; Tanaka, S. Corazonin reduces the spinning rate in the silkworm, Bombyx mori. J. Insect Physiol. 2002, 48, 707–714. [Google Scholar] [CrossRef]
- Gospocic, J.; Shields, E.J.; Glastad, K.M.; Lin, Y.; Penick, C.A.; Yan, H.; Mikheyev, A.S.; Linksvayer, T.A.; Garcia, B.A.; Berger, S.L.; et al. The neuropeptide corazonin controls social behavior and caste identity in ants. Cell 2017, 170, 748–759.e712. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Spalovská-Valachová, I.; Cho, K.-H.; Zitnanova, I.; Park, Y.; Adams, M.E.; Zitnan, D. Corazonin receptor signaling in ecdysis initiation. Proc. Natl. Acad. Sci. USA 2004, 101, 6704–6709. [Google Scholar] [CrossRef]
- Kataoka, H.; Troetschler, R.G.; Kramer, S.J.; Cesarin, B.J.; Schooley, D.A. Isolation and primary structure of the eclosion hormone of the tobacco hornworm, Manduca sexta. Biochem. Biophy. Res. Commun. 1987, 146, 746–750. [Google Scholar] [CrossRef]
- Kingan, T.G.; Gray, W.; Žitňan, D.; Adams, M.E. Regulation of ecdysis-triggering hormone release by eclosion hormone. J. Exp. Biol. 1997, 200, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Žitňan, D.; Žitňanová, I.; Spalovská, I.; Takáć, P.; Park, Y.; Adams, M.E. Conservation of ecdysis-triggering hormone signalling in insects. J. Exp. Biol. 2003, 206, 1275–1289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roller, L.; Zitnanová, I.; Dai, L.; Simo, L.; Park, Y.; Satake, H.; Tanaka, Y.; Adams, M.E.; Zitnan, D. Ecdysis triggering hormone signaling in arthropods. Peptides 2010, 31, 429–441. [Google Scholar] [CrossRef]
- Song, Q. Bursicon, a neuropeptide hormone that controls cuticletanning and wing expansion. In Insect Endocrinology; Academic Press: Cambridge, MA, USA, 2012; pp. 93–105. [Google Scholar] [CrossRef]
- Srivastava, B.B.L.; Hopkins, T.L. Bursicon release and activity in haemolymph during metamorphosis of the cockroach, Leucophaea maderae. J. Insect Physiol. 1975, 21, 1985–1993. [Google Scholar] [CrossRef]
- Li, R.; Weng, J.; Wang, X.; Meng, Q.; Wang, Y.; Sun, J. Bursicon homodimers induce innate immune by activating the expression of anti-microbial peptide genes in the shrimp Neocaridina heteropoda. Fish Shellfish Immunol. 2019, 84, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.V.; Khalil, S.M.S.; Ross, E.; Grozinger, C.M.; Sonenshine, D.E.; Michael Roe, R. Neuropeptide signaling sequences identified by pyrosequencing of the American dog tick synganglion transcriptome during blood feeding and reproduction. Insect Biochem. Mol. Biol. 2010, 40, 79–90. [Google Scholar] [CrossRef]
- Waldman, J.; Xavier, M.A.; Vieira, L.R.; Logullo, R.; Braz, G.R.C.; Tirloni, L.; Ribeiro, J.M.C.; Veenstra, J.A.; da Silva Vaz, I., Jr. Neuropeptides in Rhipicephalus microplus and other hard ticks. Ticks Tick-Borne Dis. 2022, 13, 101910. [Google Scholar] [CrossRef]
- Egekwu, N.; Sonenshine, D.; Garman, H.; Barshis, D.; Cox, N.; Bissinger, B.; Zhu, J.; Roe, R.M. Comparison of synganglion neuropeptides, neuropeptide receptors and neurotransmitter receptors and their gene expression in response to feeding in Ixodes scapularis (Ixodidae) vs. Ornithodoros turicata (Argasidae). Insect Mol. Biol. 2016, 25, 72–92. [Google Scholar] [CrossRef]
- Daniel, S. Biology of Ticks; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Bissinger, B.W.; Donohue, K.V.; Khalil, S.M.S.; Grozinger, C.M.; Sonenshine, D.E.; Zhu, J.; Roe, R.M. Synganglion transcriptome and developmental global gene expression in adult females of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Insect Mol. Biol. 2011, 20, 465–491. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.V.; Khalil, S.M.; Ross, E.; Mitchell, R.D.; Roe, R.M.; Sonenshine, D.E. Male engorgement factor: Role in stimulating engorgement to repletion in the ixodid tick, Dermacentor variabilis. J. Insect Physiol. 2009, 55, 909–918. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Bissinger, B.W.; Egekwu, N.; Donohue, K.V.; Khalil, S.M.; Roe, R.M. First transcriptome of the testis-vas deferens-male accessory gland and proteome of the spermatophore from Dermacentor variabilis (Acari: Ixodidae). PLoS ONE 2011, 6, e24711. [Google Scholar]
- Sonenshine, D.E.; Silverstein, R.M.; West, J.R. Occurrence of sex attractant pheromone, 2,6-dichlorophenol, in relation to age and feeding in American dog tick, Dermacentor variabilis (say) (Acari:Ixodidae). J. Chem. Ecol. 1984, 10, 95–100. [Google Scholar] [CrossRef]
- Lees, A.D. The role of cuticle growth in the feeding process of ticks. Proc. Zool. Soc. Lond. 1952, 121, 759–772. [Google Scholar] [CrossRef]
- Hackman, R.H.; Filshie, B.K. The Tick Cuticle. In Physiology of Ticks; Pergamon Press: Oxford, UK, 1982; pp. 1–42. [Google Scholar] [CrossRef]
- Hua, Y.-J.; Ishibashi, J.; Saito, H.; Tawfik, A.I.; Sakakibara, M.; Tanaka, Y.; Derua, R.; Waelkens, E.; Baggerman, G.; De Loof, A.; et al. Identification of [Arg7] corazonin in the silkworm, Bombyx mori and the cricket, Gryllus bimaculatus, as a factor inducing dark color in an albino strain of the locust, Locusta migratoria. J. Insect Physiol. 2000, 46, 853–860. [Google Scholar] [CrossRef]
- Veenstra, J.A. Isolation and structure of the Drosophila corazonin gene. Biochem. Biophys. Res. Commun. 1994, 204, 292–296. [Google Scholar] [CrossRef]
- Tayler, T.D.; Pacheco, D.A.; Hergarden, A.C.; Murthy, M.; Anderson, D.J. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 20697–20702. [Google Scholar] [PubMed]
- Tang, J.; Yu, R.; Zhang, Y.; Xie, J.; Song, X.; Feng, F.; Gao, H.; Li, B. Molecular and functional analysis of eclosion hormone-like gene involved in post-eclosion behavior in a beetle. J. Insect Physiol. 2022, 142, 104429. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Dong, S.; Wang, Q.; Li, S.; Gilbert, L.I.; Stanley, D.; Song, Q. Insect neuropeptide bursicon homodimers induce innate immune and stress genes during molting by activating the NF-κB transcription factor Relish. PLoS ONE 2012, 7, e34510. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, N.; Kobayashi, Y.; Izumikawa, K.; Sakamoto, T. Transcriptomic analysis of the kuruma prawn Marsupenaeus japonicus reveals possible peripheral regulation of the ovary. Front. Endocrinol. 2020, 11, 541. [Google Scholar] [CrossRef]
- Sathapondecha, P.; Panyim, S.; Udomkit, A. A novel function of bursicon in stimulation of vitellogenin expression in black tiger shrimp, Penaeus monodon. Aquaculture 2015, 446, 80–87. [Google Scholar]
- Zhou, Y.; Nagata, S. Bursicon; Elsevier: Amsterdam, The Netherlands, 2021; pp. 743–745. [Google Scholar]
- Truman, J.W.; Taghert, P.H.; Copenhaver, P.F.; Tublitz, N.J.; Schwartz, L.M. Eclosion hormone may control all ecdyses in insects. Nature 1981, 291, 70–71. [Google Scholar] [CrossRef]
- Davis, M.M.; O’Keefe, S.L.; Primrose, D.A.; Hodgetts, R.B. A neuropeptide hormone cascade controls the precise onset of post-eclosion cuticular tanning in Drosophila melanogaster. Development 2007, 134, 4395–4404. [Google Scholar] [CrossRef]






| Gene of Interest | Primers | Sequence | Product Size (Base Pairs) |
|---|---|---|---|
| Corazonin | Forward | GAGACCAGAACTAGCAGACAAACG | 59 |
| Reverse | CAGCAGGCGACCCTTACG | ||
| α Bursicon | Forward | GCATGTGCTGCCAGGAGAT | 90 |
| Reverse | TGACCAGCTTGCGGAACTT | ||
| β Bursicon | Forward | ACGAAATTTCCAAATCGCATCT | 80 |
| Reverse | GTTGAGTCTGCCCCAGTATCG | ||
| Eclosion Hormone | Forward | TGCGCCGAGGAGTGTGT | 54 |
| Reverse | GATGTCGCAGTCAGGTTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhammi, A.; Bissinger, B.; Ponnusamy, L.; Sonenshine, D.E.; Roe, R.M. Developmental Regulation of Corazonin, Eclosion Hormone, and Bursicon Messages and RNAi Suppression of Corazonin in Adult, Female American Dog Ticks, Dermacentor variabilis. Insects 2025, 16, 343. https://doi.org/10.3390/insects16040343
Dhammi A, Bissinger B, Ponnusamy L, Sonenshine DE, Roe RM. Developmental Regulation of Corazonin, Eclosion Hormone, and Bursicon Messages and RNAi Suppression of Corazonin in Adult, Female American Dog Ticks, Dermacentor variabilis. Insects. 2025; 16(4):343. https://doi.org/10.3390/insects16040343
Chicago/Turabian StyleDhammi, Anirudh, Brooke Bissinger, Loganathan Ponnusamy, Daniel E. Sonenshine, and R. Michael Roe. 2025. "Developmental Regulation of Corazonin, Eclosion Hormone, and Bursicon Messages and RNAi Suppression of Corazonin in Adult, Female American Dog Ticks, Dermacentor variabilis" Insects 16, no. 4: 343. https://doi.org/10.3390/insects16040343
APA StyleDhammi, A., Bissinger, B., Ponnusamy, L., Sonenshine, D. E., & Roe, R. M. (2025). Developmental Regulation of Corazonin, Eclosion Hormone, and Bursicon Messages and RNAi Suppression of Corazonin in Adult, Female American Dog Ticks, Dermacentor variabilis. Insects, 16(4), 343. https://doi.org/10.3390/insects16040343







