How Insects Balance Reproductive Output and Immune Investment
Simple Summary
Abstract
1. Introduction
2. Energy Allocation and Life History Theory
3. Reproduction
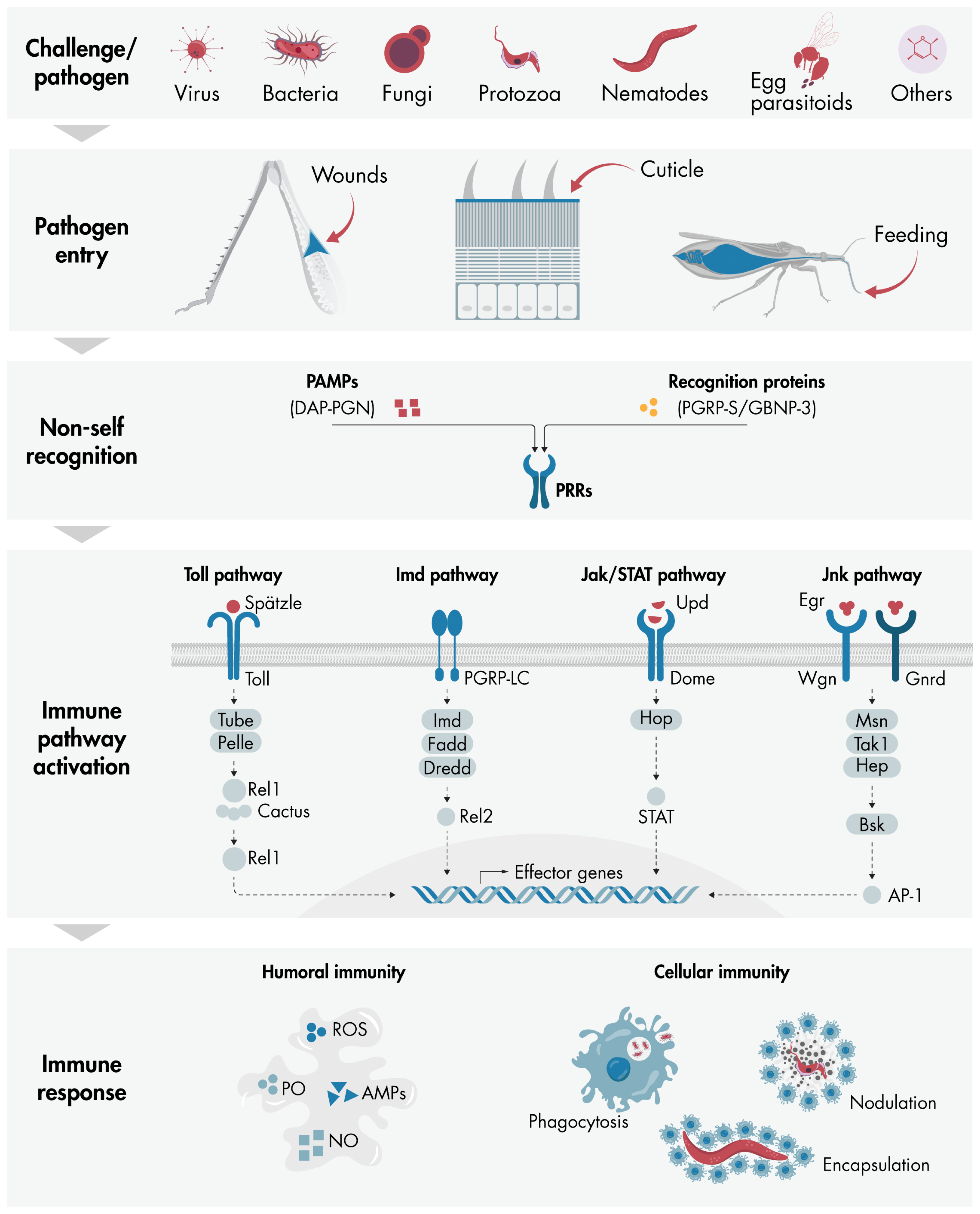
4. Immunity
5. Immune Status Affects Oogenesis, and Vice Versa
| Immune Status | Order | Species | Challenge/ Phenotype | Effect on Reproduction |
|---|---|---|---|---|
| Challenged with non-pathogenic organisms/ noninfectious elicitors | Hemiptera | Rhodnius prolixus | Aspergillus niger (fungus) | Reduced number of eggs, increased number of atretic follicles [47]. |
| Orthoptera | Acheta domesticus | Nylon filament | Reduced number and size of eggs [56]. | |
| Gryllus texensis | Wound, heat-killed (HK) Serratia marcescens (bacterium) | Reduced oviposition rate and egg protein content [57]. | ||
| Hemideina crassidens | Lipopolysaccharide (LPS) | Reduced number of eggs and egg protein content [58]. | ||
| Locusta migratoria | Micrococcus luteus (bacterium) | Reduced ovarian development, reduced vitellogenin expression [46]. | ||
| Diptera | Drosophila melanogaster | LPS, HK bacteria, Escherichia coli, M. luteus and Pectinobacterium carotovorum carotovorum (bacteria) | Reduced fecundity and number of eggs. No effect on pre-copulatory behaviors [48,49,50,51,52,72]. | |
| Anopheles gambiae | LPS, Sephadex beads | Reduced ovarian protein content, reduced number of eggs, increased number of apoptotic follicles [53,54]. | ||
| Coleoptera | Euoniticellus intermedius | LPS | Reduced number of eggs [55]. | |
| Nicrophorus Vespilloides | Wound | Reduced number of larvae [59]. | ||
| Challenged with pathogens | Hemiptera | Pyrrhocoris apterus | Steinernema carpocapsae (nematode), Isaria fumosorosea (fungus) | Reduced vitellogenin expression [80]. |
| Orthoptera | Teleogryllus oceanicus | S. marcescens | Reduced viability of stored sperm [66]. | |
| Diptera | Drosophila melanogaster | Providencia rettgeri, S. marcescens, Staphylococcus aureus, and Listeria monocytogenes (bacteria); Beauveria bassiana (fungus); Asobara tabida (parasitoid wasp) | Reduced fecundity. No effect on pre-copulatory behaviors [49,51,60,61,62,72]. | |
| Drosophila nigrospiracula | Macrocheles subbadius (acarus) | Reduced number of eggs [64]. | ||
| Aedes aegypti | Enterobacter cloacae (bacterium) | Reduced vitellogenin expression and number of eggs [26]. | ||
| Anopheles gambiae | Plasmodium yoelii nigeriensis (protozoon) | Increased number of apoptotic follicles [54]. | ||
| Anopheles stephensi | Plasmodium yoelii nigeriensis | Reduced fecundity [63]. | ||
| Armigeres subalbatus | Brugia malayi (nematode) | Reduced ovarian protein content and increased time to oviposition [65]. | ||
| Coleoptera | Tenebrio molitor | Inactivated Bacillus cereus | Reduced number of eggs [71]. | |
| Lepidoptera | Heliothis virescens | Death cells of Serratia entomophila | Reduced signal behavior by females (production of pheromones for male attraction) [70]. | |
| Hymenoptera | Apis mellifera | Sacbrood and black queen cell virus | Lower sperm viability and fewer sperm in the spermatheca [67]. | |
| Increased resistance to pathogens | Diptera | Drosophila melanogaster | Resistance to Pseudomonas aeruginosa and Providencia rettgeri (bacteria) | Reduced fecundity and egg viability [51,75,76]. |
| Aedes aegypti | Resistance to Plasmodium gallinaceum | Reduced fecundity and hatchability [77]. | ||
| Lepidoptera | Plodia interpunctella | Resistance to Plodia interpunctella granulovirus | Reduced egg viability [78]. | |
| Coleoptera | Tenebrio molitor | Increased pigmentation (equivalent to phenoloxidase activity) | No effect on fecundity [79]. |
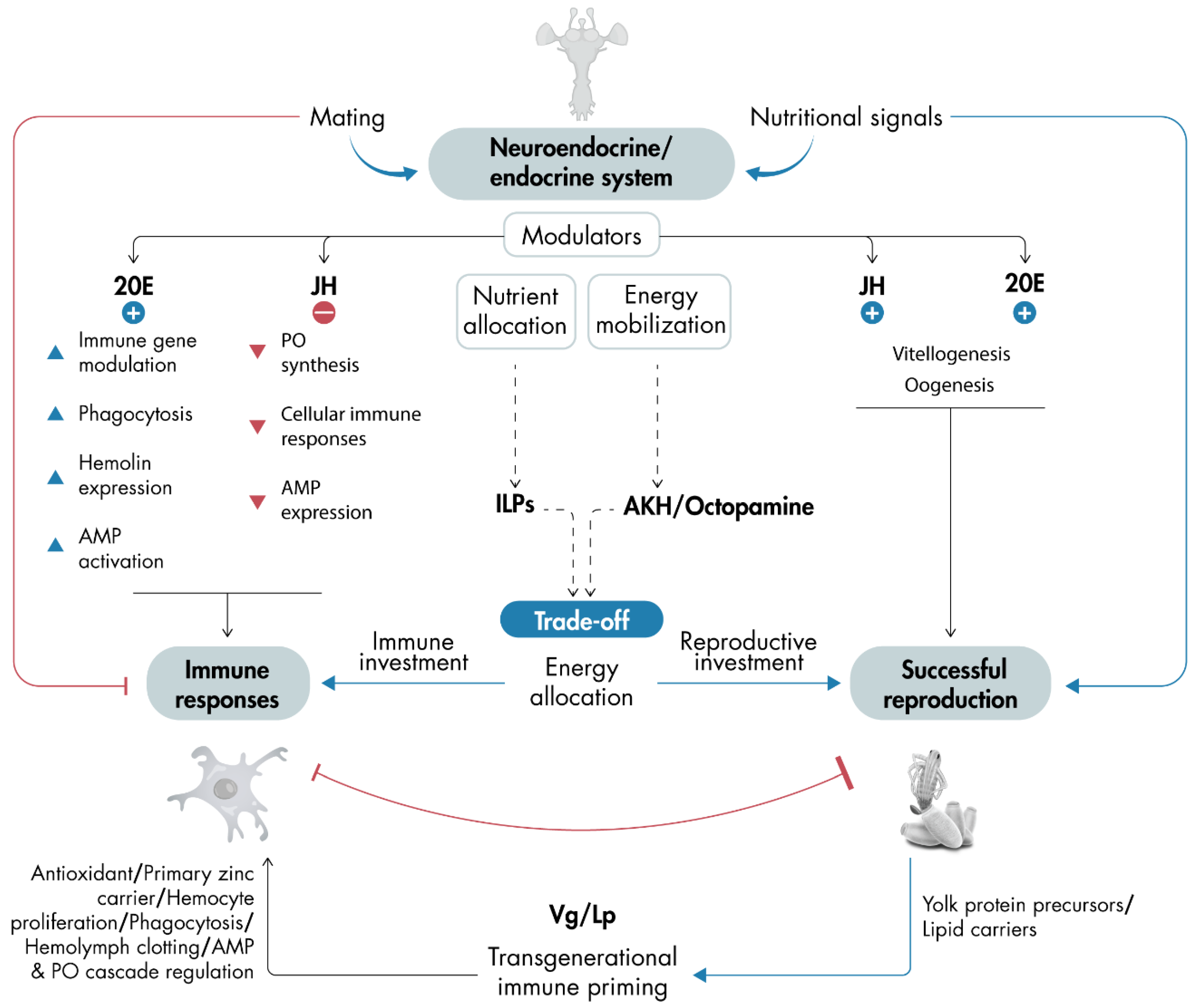
6. Neuroendocrine and Endocrine Regulators
7. Dual Role of the Yolk Protein Precursors Vitellogenin and Lipophorin
8. Conclusions and Subjects for Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 20E | 20-Hydroxyecdysone |
| AKH | Adipokinetic hormone |
| AMPs | Antimicrobial peptides |
| AP-1 | Activator protein 1 |
| ApoLp | Apolipophorin |
| bHLH-PAS | Basic Helix-Loop-Helix—Per-ARNT-Sim (bHLH-PAS) |
| Bsk | Basket |
| DAP-PGN | Diaminopimelic acid peptidoglycan |
| Dome | Domeless |
| DREDD | Death-related ced-3/nedd2-like proteinase |
| EcR | Ecdysone receptor |
| Egr | Eiger |
| FADD | Fas-associated death domain |
| FAS2 | Fatty acid synthase 2 |
| GNBP | Gram-negative binding protein |
| GPCR | G protein-coupled receptor |
| Grnd | Grindelwald |
| Hep | Hemipterous |
| Hop | Hopscotch |
| ILPs | Insulin-like peptides |
| Imd | Immune deficiency |
| Jak-STAT | Janus kinase–signal transducer and activator of transcription |
| JBU | Jack bean urease |
| JH | Juvenile hormone |
| Jnk | c-Jun N-terminal kinase pathway |
| Lp | Lipophorin |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| Met | Methoprene-tolerant |
| MF | Methyl farnesoate |
| Msn | Misshapen |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OA | Octopamine |
| PAMPs | Pathogen-associated molecular patterns |
| PGRP-LC | Peptidoglycan recognition protein LC |
| PGRP-S | Peptidoglycan recognition protein short |
| PO | Phenoloxidase |
| PPO1 | Prophenoloxidase 1 |
| PRRs | Pattern recognition receptors |
| Ras-MAPK | Ras-mitogen-activated protein kinase |
| Rel1 | Relish transcription factor 1 |
| Rel2 | Relish transcription factor 2 |
| ROS | Reactive oxygen species |
| RSV | Rice stripe virus |
| RTK | Receptor tyrosine kinase |
| Tai | Taiman |
| TAK1 | TGF-β-Activated kinase 1 |
| TEP1 | Thioester-containing protein 1 |
| TNF | Tumor necrosis factor |
| Upds | Unpaired proteins |
| USP | Ultraspiracle |
| Vg | Vitellogenin |
| Wgn | Wengen |
| WNV | West Nile virus |
References
- Brown, J.H.; Sibly, R.M. Life-history evolution under a production constraint. Proc. Natl. Acad. Sci. USA 2006, 103, 17595–17599. [Google Scholar] [CrossRef] [PubMed]
- Smykal, V.; Raikhel, A.S. Nutritional control of insect reproduction. Curr. Opin. Insect Sci. 2015, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-Immunity Trade-Offs in Insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Nunes, C.; Sucena, É.; Koyama, T. Endocrine regulation of immunity in insects. FEBS J. 2020, 288, 3928–3947. [Google Scholar] [CrossRef]
- Leyria, J. Endocrine factors modulating vitellogenesis and oogenesis in insects: An update. Mol. Cell. Endocrinol. 2024, 587, 112211. [Google Scholar] [CrossRef]
- Oku, K.; Price, T.A.R.; Wedell, N. Does mating negatively affect female immune defenses in insects? Anim. Biol. 2019, 69, 117–136. [Google Scholar] [CrossRef]
- Nanfack-Minkeu, F.; Sirot, L.K. Effects of Mating on Gene Expression in Female Insects: Unifying the Field. Insects 2022, 13, 69. [Google Scholar] [CrossRef]
- Castillo, J.; Brown, M.R.; Strand, M.R. Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 2011, 7, e1002274. [Google Scholar] [CrossRef]
- Martinson, E.O.; Chen, K.; Valzania, L.; Brown, M.R.; Strand, M.R. Insulin-like peptide 3 stimulates hemocytes to proliferate in anautogenous and facultatively autogenous mosquitoes. J. Exp. Biol. 2022, 225, jeb243460. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Hemolymph Lipoproteins: Role in Insect Reproduction. In Hemolymph Proteins and Functional Peptides: Recent Advances in Insects and Other Arthropods; Bentham Science Publisher: Sharjah, United Arab Emirates, 2012; pp. 3–19. [Google Scholar]
- Vilcinskas, A. Mechanisms of transgenerational immune priming in insects. Dev. Comp. Immunol. 2021, 124, 104205. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Jaime, V.; Broderick, N.A.; Maya-Maldonado, K. Metal ions in insect reproduction: A crosstalk between reproductive physiology and immunity. Curr. Opin. Insect Sci. 2022, 52, 100924. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J. Social immunity and the evolution of group living in insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140102. [Google Scholar] [CrossRef]
- Kozłowski, J.; Wiegert, R.G. Optimal allocation of energy to growth and reproduction. Theor. Popul. Biol. 1986, 29, 16–37. [Google Scholar] [CrossRef]
- David, G.; Giffard, B.; Van Halder, I.; Piou, D.; Jactel, H. Energy allocation during the maturation of adults in a long-lived insect: Implications for dispersal and reproduction. Bull. Entomol. Res. 2015, 105, 629–636. [Google Scholar] [CrossRef]
- Charnov, E.L. Oxford Series in Ecology and Evolution: Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology; Oxford Academic: Oxford, UK, 1993. [Google Scholar]
- Charnov, E.L.; Turner, T.F.; Winemiller, K.O. Reproductive constraints and the evolution of life histories with indeterminate growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9460–9464. [Google Scholar] [CrossRef]
- Rose, M.R.; Charlesworth, B. Genetics of life history in Drosophila melanogaster. I. Sib analysis of adult females. Genetics 1981, 97, 173–186. [Google Scholar] [CrossRef]
- Hill, K.; Hurtado, A.M. Ache Life History, 1st ed.; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Partridge, L.; Harvey, P.H. The ecological context of life history evolution. Science 1988, 241, 1449–1455. [Google Scholar] [CrossRef]
- Leyria, J.; Guarneri, A.A.; Lorenzo, M.G.; Nouzova, M.; Noriega, F.G.; Benrabaa, S.A.M.; Fernandez-Lima, F.; Valadares Tose, L.; Orchard, I.; Lange, A.B. Effects of mating on female reproductive physiology in the insect model, Rhodnius prolixus, a vector of the causative parasite of Chagas disease. PLoS Negl. Trop. Dis. 2023, 17, e0011640. [Google Scholar] [CrossRef]
- De Loof, A. Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J. Insect Physiol. 2011, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tang, Y.; Jin, Y.; Xu, J.; Zhao, H.; Zhou, M.; Tang, B.; Wang, S. Fatty acid synthase 2 knockdown alters the energy allocation strategy between immunity and reproduction during infection by Micrococcus luteus in Locusta migratoria. Pestic. Biochem. Physiol. 2024, 205, 106127. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Chang, M.; Wang, X.; Shi, Z.; Raikhel, A.S.; Zou, Z. Ecdysone signaling mediates the trade-off between immunity and reproduction via suppression of amyloids in the mosquito Aedes aegypti. PLoS Pathog. 2022, 18, e1010837. [Google Scholar] [CrossRef]
- Hudson, A.L.; Moatt, J.P.; Vale, P.F. Terminal investment strategies following infection are dependent on diet. J. Evol. Biol. 2020, 33, 309–317. [Google Scholar] [CrossRef]
- Duffield, K.R.; Hampton, K.J.; Houslay, T.M.; Hunt, J.; Rapkin, J.; Sakaluk, S.K.; Sadd, B.M. Age-dependent variation in the terminal investment threshold in male crickets. Evolution 2018, 72, 578–589. [Google Scholar] [CrossRef]
- Reyes-Ramírez, A.; Rocha-Ortega, M.; Córdoba-Aguilar, A. Dietary macronutrient balance and fungal infection as drivers of spermatophore quality in the mealworm beetle. Curr. Res. Insect Sci. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Olzer, R.; Ehrlich, R.L.; Heinen-Kay, J.L.; Tanner, J.; Zuk, M. Reproductive behavior. In Insect Behavior: From Mechanisms to Ecological and Evolutionary Consequences; Oxford University Press: Oxford, UK, 2018; ISBN 9780198797500. [Google Scholar]
- Klowden, M.J.; Palli, S.R. Chapter 4: Reproductive Systems; Academic Press; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Leyria, J.; Fruttero, L.L.; Canavoso, L.E. Lipids in Insect Reproduction: Where, How, and Why. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2024; pp. 1–32. [Google Scholar] [CrossRef]
- Gondim, K.C.; Majerowicz, D. Lipophorin: The Lipid Shuttle. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–18. [Google Scholar]
- Orchard, I.; Lange, A.B. The neuroendocrine and endocrine systems in insect—Historical perspective and overview. Mol. Cell. Endocrinol. 2024, 580, 112108. [Google Scholar] [CrossRef]
- Kordaczuk, J. Insect immunity—Adaptive mechanisms and survival strategies. Postep. Biochem. 2024, 70, 382–399. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Mello, C.B.; Castro, H.C.; Dyson, P.; Figueiredo, M. Immune Reactions of Vector Insects to Parasites and Pathogens. Microorganisms 2024, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila Innate Immunity Involves Multiple Signaling Pathways and Coordinated Communication Between Different Tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, J.; El Moussawi, L.; Osta, M.A. The Melanization Response in Insect Immunity. Adv. Insect Phys. 2017, 52, 83–109. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Paglione, P.A.; Leyria, J.; Canavoso, L.E.; Fruttero, L.L. JBU affects reproduction in the Chagas disease vector Rhodnius prolixus: Implications for lipid dynamics. In Proceedings of the LX Annual Meeting of the Argentine Society for Biochemistry and Molecular Biology Research (SAIB), Córdoba, Argentina, 5–8 November 2024; p. 49. [Google Scholar]
- Eleftherianos, I.; Zhang, W.; Heryanto, C.; Mohamed, A.; Contreras, G.; Tettamanti, G.; Wink, M.; Bassal, T. Diversity of insect antimicrobial peptides and proteins—A functional perspective: A review. Int. J. Biol. Macromol. 2021, 191, 277–287. [Google Scholar] [CrossRef]
- Church, S.H.; De Medeiros, B.A.S.; Donoughe, S.; Márquez Reyes, N.L.; Extavour, C.G. Repeated loss of variation in insect ovary morphology highlights the role of development in life-history evolution. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210150. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Xia, Z.; Xie, G.; Tang, B.; Wang, S. Transcriptome analysis of the molecular mechanism underlying immunity- and reproduction trade-off in Locusta migratoria infected by Micrococcus luteus. PLoS ONE 2019, 14, e0211605. [Google Scholar] [CrossRef]
- Medeiros, M.N.; Ramos, I.B.; Oliveira, D.M.P.; da Silva, R.C.B.; Gomes, F.M.; Medeiros, L.N.; Kurtenbach, E.; Chiarini, L.B.; Masuda, H.; de Souza, W.; et al. Microscopic and molecular characterization of ovarian follicle atresia in Rhodnius prolixus Stahl under immune challenge. J. Insect Physiol. 2011, 57, 945–953. [Google Scholar] [CrossRef]
- Nystrand, M.; Dowling, D.K. Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J. Evol. Biol. 2014, 27, 876–888. [Google Scholar] [CrossRef]
- Bashir-Tanoli, S.; Tinsley, M.C. Immune response costs are associated with changes in resource acquisition and not resource reallocation. Funct. Ecol. 2014, 28, 1011–1019. [Google Scholar] [CrossRef]
- Zerofsky, M.; Harel, E.; Silverman, N.; Tatar, M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 2005, 4, 103–108. [Google Scholar] [CrossRef] [PubMed]
- McKean, K.A.; Yourth, C.P.; Lazzaro, B.P.; Clark, A.G. The evolutionary costs of immunological maintenance and deployment. BMC Evol. Biol. 2008, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.L.; Charroux, B.; Chaduli, D.; Viallat-Lieutaud, A.; Royet, J. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. Elife 2017, 6, e21937. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Baggott, S.L.; Maingon, R.; Hurd, H. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 2002, 97, 371–377. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Hurd, H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 2006, 8, 308–315. [Google Scholar] [CrossRef]
- Reaney, L.T.; Knell, R.J. Immune activation but not male quality affects female current reproductive investment in a dung beetle. Behav. Ecol. 2010, 21, 1367–1372. [Google Scholar] [CrossRef]
- Bascuñán-García, A.P.; Lara, C.; Córdoba-Aguilar, A. Immune investment impairs growth, female reproduction and survival in the house cricket, Acheta domesticus. J. Insect Physiol. 2010, 56, 204–211. [Google Scholar] [CrossRef]
- Stahlschmidt, Z.R.; Rollinson, N.; Acker, M.; Adamo, S.A. Are all eggs created equal? Food availability and the fitness trade-off between reproduction and immunity. Funct. Ecol. 2013, 27, 800–806. [Google Scholar] [CrossRef]
- Kelly, C.D. Reproductive and physiological costs of repeated immune challenges in female Wellington tree weta (Orthoptera: Anostostomatidae). Biol. J. Linn. Soc. 2011, 104, 38–46. [Google Scholar] [CrossRef]
- Reavey, C.E.; Warnock, N.D.; Vogel, H.; Cotter, S.C. Trade-offs between personal immunity and reproduction in the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 2014, 25, 415–423. [Google Scholar] [CrossRef]
- Howick, V.M.; Lazzaro, B.P. Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol. Biol. 2014, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Flores, H.A.; Lorigan, J.G.; Yourth, C.P. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathog. 2008, 4, e1000025. [Google Scholar] [CrossRef]
- Fellowes, M.D.E.; Kraaijeveld, A.R.; Godfray, H.C.J. The relative fitness of Drosophila melanogaster (Diptera, Drosophilidae) that have successfully defended themselves against the parasitoid Asobara tabida (Hymenoptera, Braconidae). J. Evol. Biol. 1999, 12, 123–128. [Google Scholar] [CrossRef]
- Hogg, J.C.; Hurd, H. Plasmodium yoelii nigeriensis: The effect of high and low intensity of infection upon the egg production and bloodmeal size of Anopheles stephensi during three gonotrophic cycles. Parasitology 1995, 111, 555–562. [Google Scholar] [CrossRef]
- Polak, M. Ectoparasitic effects on host survival and reproduction: The Drosophila-Macrocheles association. Ecology 1996, 77, 1379–1389. [Google Scholar] [CrossRef]
- Ferdig, M.T.; Beerntsen, B.T.; Spray, F.J.; Li, J.; Christensen, B.M. Reproductive costs associated with resistance in a mosquito-filarial worm system. Am. J. Trop. Med. Hyg. 1993, 49, 756–762. [Google Scholar] [CrossRef]
- Mcnamara, K.B.; van Lieshout, E.; Simmons, L.W. Females suffer a reduction in the viability of stored sperm following an immune challenge. J. Evol. Biol. 2014, 27, 133–140. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Pettis, J.S.; Foster, L.J.; Tarpy, D.R. Trade-offs between sperm viability and immune protein expression in honey bee queens (Apis mellifera). Commun. Biol. 2021, 4, 48. [Google Scholar] [CrossRef]
- Kodrík, D. Adipokinetic hormone functions that are not associated with insect flight. Physiol. Entomol. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Fellet, M.R.; Lorenzo, M.G.; Elliot, S.L.; Carrasco, D.; Guarneri, A.A. Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS ONE 2014, 9, e105255. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Staudacher, H.; Schmaltz, A.; Heckel, D.G.; Groot, A.T. Sex-specific consequences of an induced immune response on reproduction in a moth. BMC Evol. Biol. 2015, 15, 282. [Google Scholar] [CrossRef] [PubMed]
- Jehan, C.; Sabarly, C.; Rigaud, T.; Moret, Y. Senescence of the immune defences and reproductive trade-offs in females of the mealworm beetle, Tenebrio molitor. Sci. Rep. 2022, 12, 19747. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Beckwith, E.J.; Burmester, C.; May, R.C.; Dionne, M.S.; Rezaval, C. Pre-copulatory reproductive behaviours are preserved in Drosophila melanogaster infected with bacteria. Proc. Biol. Sci. 2022, 289, 20220492. [Google Scholar] [CrossRef]
- Adamo, S.A.; Jensen, M.; Younger, M. Changes in lifetime immunocompetence in male and female Gryllus texensis (formerly G. integer): Trade-offs between immunity and reproduction. Anim. Behav. 2001, 62, 417–425. [Google Scholar] [CrossRef]
- Knell, R.J.; Webberley, K.M. Sexually transmitted diseases of insects: Distribution, evolution, ecology and host behaviour. Biol. Rev. Camb. Philos. Soc. 2004, 79, 557–581. [Google Scholar] [CrossRef]
- Ye, Y.H.; Chenoweth, S.F.; McGraw, E.A. Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PLoS Pathog. 2009, 5, e1000385. [Google Scholar] [CrossRef]
- Luong, L.T.; Polak, M. Costs of resistance in the Drosophila-Macrocheles system: A negative genetic correlation between ectoparasite resistance and reproduction. Evolution 2007, 61, 1391–1402. [Google Scholar] [CrossRef]
- Yan, G.; Severson, D.W.; Christensen, B.M. Costs and benefits of mosquito refractoriness to malaria parasites: Implications for genetic variability of mosquitoes and genetic control of malaria. Evolution 1997, 51, 441–450. [Google Scholar] [CrossRef]
- Boots, M.; Begon, M. Trade-offs with resistance to a granulosis virus in the Indian meal moth, examined by a laboratory evolution experiment. Funct. Ecol. 1993, 7, 528–534. [Google Scholar] [CrossRef]
- Armitage, S.A.O.; Thompson, J.J.W.; Rolff, J.; Siva-Jothy, M.T. Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 2003, 16, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Kodrík, D.; Ibrahim, E.; Gautam, U.K.; Frydrychová, R.Č.; Bednářová, A.; Krištufek, V.; Jedlička, P. Changes in vitellogenin expression caused by nematodal and fungal infections in insects. J. Exp. Biol. 2019, 222, jeb202853. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.A.; Scanes, C.G. Neuroendocrine-Immune Interactions. Poult. Sci. 1994, 73, 1049–1061. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex hormone signaling and regulation of immune function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef]
- Nair, R.R.; Verma, P.; Singh, K. Immune-endocrine crosstalk during pregnancy. Gen. Comp. Endocrinol. 2017, 242, 18–23. [Google Scholar] [CrossRef]
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod. Med. Biol. 2024, 23, e12600. [Google Scholar] [CrossRef]
- Lutton, B.; Callard, I. Evolution of reproductive-immune interactions. Integr. Comp. Biol. 2006, 46, 1060–1071. [Google Scholar] [CrossRef]
- De Loof, A.; Schoofs, L.; Huybrechts, R. The endocrine system controlling sexual reproduction in animals: Part of the evolutionary ancient but well conserved immune system? Gen. Comp. Endocrinol. 2016, 226, 56–71. [Google Scholar] [CrossRef]
- Bittova, L.; Jedlicka, P.; Dracinsky, M.; Kirubakaran, P.; Vondrasek, J.; Hanus, R.; Jindra, M. Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem. 2019, 294, 410–423. [Google Scholar] [CrossRef]
- Riddiford, L.M. Rhodnius, golden oil, and Met: A history of juvenile hormone research. Front. Cell Dev. Biol. 2020, 8, 679. [Google Scholar] [CrossRef]
- Ekoka, E.; Maharaj, S.; Nardini, L.; Dahan-Moss, Y.; Koekemoer, L.L. 20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors. Parasites Vectors 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Rizki, M.T.M. Tumor formation in relation to metamorphosis in Drosophila melanogaster. J. Morphol. 1957, 100, 459–472. [Google Scholar] [CrossRef]
- Rizki, M.T.M. Melanotic tumor formation in Drosophila. J. Morphol. 1960, 106, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rizki, T.M. Experimental analysis of hemocyte morphology in insects. Am. Zool. 1962, 2, 247–256. [Google Scholar] [CrossRef]
- Jones, J.C.; Liu, D.P. The effects of ligaturing Galleria mellonella larvae on total haemocyte counts and on mitotic indices among haemocytes. J. Insect Physiol. 1969, 15, 1703–1708. [Google Scholar] [CrossRef]
- Hoffmann, J.A. Endocrine regulation of production and differentiation of hemocytes in an orthopteran insect: Locusta migratoria migratoroides. Gen. Comp. Endocrinol. 1970, 15, 198–219. [Google Scholar] [CrossRef]
- Pathak, J.P.N. Effect of endocrine glands on the unfixed total haemocyte counts of the bug Halys dentata. J. Insect Physiol. 1983, 29, 91–94. [Google Scholar] [CrossRef]
- Lange, A.B.; Leyria, J.; Orchard, I. The hormonal and neural control of egg production in the historically important model insect, Rhodnius prolixus: A review, with new insights in this post-genomic era. Gen. Comp. Endocrinol. 2022, 321–322, 114030. [Google Scholar] [CrossRef]
- Jones, J.C. Effects of repeated haemolymph withdrawals and of ligaturing the head on differential haemocyte counts of Rhodnius prolixus Stål. J. Insect Physiol. 1967, 13, 1351–1360. [Google Scholar] [CrossRef]
- Das, Y.T.; Gupta, A.P. Nature and precursors of juvenile hormone-induced excessive cuticular melanisation in German cockroach. Nature 1977, 268, 139–140. [Google Scholar] [CrossRef]
- Regan, J.C.; Brandão, A.S.; Leitão, A.B.; Mantas Dias, Â.R.; Sucena, É.; Jacinto, A.; Zaidman-Rémy, A. Steroid Hormone Signaling Is Essential to Regulate Innate Immune Cells and Fight Bacterial Infection in Drosophila. PLoS Pathog. 2013, 9, e1003720. [Google Scholar] [CrossRef] [PubMed]
- Sampson, C.J.; Amin, U.; Couso, J.P. Activation of Drosophila hemocyte motility by the ecdysone hormone. Biol. Open 2013, 2, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Franssens, V.; Smagghe, G.; Simonet, G.; Claeys, I.; Breugelmans, B.; De Loof, A.; Vanden Broeck, J. 20-Hydroxyecdysone and juvenile hormone regulate the laminarin-induced nodulation reaction in larvae of the flesh fly, Neobellieria bullata. Dev. Comp. Immunol. 2006, 30, 735–740. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Kwon, H.; Smith, R.C. 20-Hydroxyecdysone Primes Innate Immune Responses That Limit Bacterial and Malarial Parasite Survival in Anopheles gambiae. mSphere 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Meister, M.; Richards, G. Ecdysone and insect immunity: The maturation of the inducibility of the diptericin gene in Drosophila larvae. Insect Biochem. Mol. Biol. 1996, 26, 155–160. [Google Scholar] [CrossRef]
- Dimarcq, J.L.; Imler, J.L.; Lanot, R.; Ezekowitz, R.A.B.; Hoffmann, J.A.; Janeway, C.A.; Lagueux, M. Treatment of l(2)mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem. Mol. Biol. 1997, 27, 877–886. [Google Scholar] [CrossRef]
- Silverman, N.; Zhou, R.; Stöven, S.; Pandey, N.; Hultmark, D.; Maniatis, T. A Drosophila IκB kinase complex required for relish cleavage and antibacterial immunity. Genes Dev. 2000, 14, 2461–2471. [Google Scholar] [CrossRef]
- Roxström-Lindquist, K.; Assefaw-Redda, Y.; Rosinska, K.; Faye, I. 20-Hydroxyecdysone indirectly regulates Hemolin gene expression in Hyalophora cecropia. Insect Mol. Biol. 2005, 14, 645–652. [Google Scholar] [CrossRef]
- Chernysh, S.I.; Simonenko, N.P.; Braun, A.; Meister, M. Developmental variability of the antibacterial response in larvae and pupae of Calliphora vicina (Diptera: Calliphoridae) and Drosophila melanogaster (Diptera: Drosophilidae). Eur. J. Entomol. 1995, 92, 203–209. [Google Scholar]
- Beckstead, R.B.; Lam, G.; Thummel, C.S. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005, 6, R99. [Google Scholar] [CrossRef]
- Hiruma, K.; Riddiford, L.M. Granular phenoloxidase involved in cuticular melanization in the tobacco hornworm: Regulation of its synthesis in the epidermis by juvenile hormone. Dev. Biol. 1988, 130, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Rolff, J.; Siva-Jothy, M.T. Copulation corrupts immunity: A mechanism for a cost of mating in insects. Proc. Natl. Acad. Sci. USA 2002, 99, 9916–9918. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Vainikka, A.; Kortet, R. The role of juvenile hormone in immune function and pheromone production trade-offs: A test of the immunocompetence handicap principle. Proc. R. Soc. B Biol. Sci. 2003, 270, 2257–2261. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.; Madanagopal, N. Antagonistic effect of juvenile hormone on hemocyte-spreading behavior of Spodoptera exigua in response to an insect cytokine and its putative membrane action. J. Insect Physiol. 2008, 54, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Hepat, R.; Kim, Y. JH modulates a cellular immunity of Tribolium castaneum in a Met-independent manner. J. Insect Physiol. 2014, 63, 40–47. [Google Scholar] [CrossRef]
- Flatt, T.; Tu, M.-P.; Tatar, M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 2005, 27, 999–1010. [Google Scholar] [CrossRef]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef]
- Tobe, S.S.; Clarke, N.; Stay, B.; Ruegg, R.P. Changes in cell number and activity of the corpora allata of the cockroach Diploptera punctata: A role for mating and the ovary. Can. J. Zool. 1984, 62, 2178–2182. [Google Scholar] [CrossRef]
- Lee, K.Y.; Horodyski, F.M. Effects of starvation and mating on corpora allata activity and allatotropin (Manse-AT) gene expression in Manduca sexta. Peptides 2006, 27, 567–574. [Google Scholar] [CrossRef]
- Castella, G.; Christe, P.; Chapuisat, M. Mating triggers dynamic immune regulations in wood ant queens. J. Evol. Biol. 2009, 22, 564–570. [Google Scholar] [CrossRef]
- Domanitskaya, E.V.; Liu, H.; Chen, S.; Kubli, E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS J. 2007, 274, 5659–5668. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, K.M.; Zuk, M.; Mousseau, T.A. Immune suppresion and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 2004, 58, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zipperlen, P.; Kubli, E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 2005, 15, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Hurd, H. Host fecundity reduction: A strategy for damage limitation? Trends Parasitol. 2001, 17, 363–368. [Google Scholar] [CrossRef]
- Innocenti, P.; Morrow, E.H. Immunogenic males: A genome-wide analysis of reproduction and the cost of mating in Drosophila melanogaster females. J. Evol. Biol. 2009, 22, 964–973. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P. Juvenile Hormone Suppresses Resistance to Infection in Mated Female Drosophila melanogaster. Curr. Biol. 2017, 27, 596–601. [Google Scholar] [CrossRef]
- Gao, B.; Song, X.Q.; Yu, H.; Fu, D.Y.; Xu, J.; Ye, H. Mating-Induced Differential Expression in Genes Related to Reproduction and Immunity in Spodoptera litura (Lepidoptera: Noctuidae) Female Moths. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Marchini, D.; Manetti, A.G.; Rosetto, M.; Bernini, L.F.; Telford, J.L.; Baldari, C.T.; Dallai, R. cDNA sequence and expression of the ceratotoxin gene encoding an antibacterial sex-specific peptide from the medfly Ceratitis capitata (diptera). J. Biol. Chem. 1995, 270, 6199–6204. [Google Scholar] [CrossRef]
- Dallai, R.; Baldari, C.T.; Marchini, D.; de Filippis, T.; Rosetto, M.; Manetti, A.G.O. Juvenile hormone regulates the expression of the gene encoding ceratotoxin a, an antibacterial peptide from the female reproductive accessory glands of the medfly Ceratitis capitata. J. Insect Physiol. 1997, 43, 1161–1167. [Google Scholar] [CrossRef]
- Bryant, W.B.; Michel, K. Blood feeding induces hemocyte proliferation and activation in the African malaria mosquito, Anopheles gambiae Giles. J. Exp. Biol. 2014, 217, 1238–1245. [Google Scholar] [CrossRef]
- Ahlers, L.R.H.; Trammell, C.E.; Carrell, G.F.; Mackinnon, S.; Torrevillas, B.K.; Chow, C.Y.; Luckhart, S.; Goodman, A.G. Insulin Potentiates JAK/STAT Signaling to Broadly Inhibit Flavivirus Replication in Insect Vectors. Cell Rep. 2019, 29, 1946–1960.e5. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Kanamori, Y.; Kiuchi, M.; Kamimura, M. In vitro studies of hematopoiesis in the silkworm: Cell proliferation in and hemocyte discharge from the hematopoietic organ. J. Insect Physiol. 2003, 49, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, J.; Hu, X.; Yan, X.; Chen, S.; Li, C.; Pan, G.; Chang, H.; Tian, W.; Abbas, M.N.; et al. Integrin β2 and β3: Two plasmatocyte markers deepen our understanding of the development of plasmatocytes in the silkworm Bombyx mori. Insect Sci. 2022, 29, 1659–1671. [Google Scholar] [CrossRef]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Dunphy, G.B.; Downer, R.G.H. Octopamine, a modulator of the haemocytic nodulation response of non-immune Galleria mellonella larvae. J. Insect Physiol. 1994, 40, 267–272. [Google Scholar] [CrossRef]
- Goldsworthy, G.; Chandrakant, S.; Opoku-Ware, K. Adipokinetic hormone enhances nodule formation and phenoloxidase activation in adult locusts injected with bacterial lipopolysaccharide. J. Insect Physiol. 2003, 49, 795–803. [Google Scholar] [CrossRef]
- Goldsworthy, G.J.; Opoku-Ware, K.; Mullen, L.M. Adipokinetic hormone and the immune responses of locusts to infection. Ann. N. Y. Acad. Sci. 2005, 1040, 106–113. [Google Scholar] [CrossRef]
- Kong, H.; Dong, C.; Tian, Z.; Mao, N.; Wang, C.; Cheng, Y.; Zhang, L.; Jiang, X.; Luo, L. Altered immunity in crowded Mythimna separata is mediated by octopamine and dopamine. Sci. Rep. 2018, 8, 3215. [Google Scholar] [CrossRef]
- Mullen, L.M.; Lightfoot, M.E.; Goldsworthy, G.J. Induced hyperlipaemia and immune challenge in locusts. J. Insect Physiol. 2004, 50, 409–417. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.H.; Jeong, W.H.; Lee, J.H.; Seo, S.J.; Han, Y.S.; Lee, I.H. Effects of two hemolymph proteins on humoral defense reactions in the wax moth, Galleria mellonella. Dev. Comp. Immunol. 2005, 29, 43–51. [Google Scholar] [CrossRef]
- Urbański, A.; Konopińska, N.; Lubawy, J.; Walkowiak-Nowicka, K.; Marciniak, P.; Rolff, J. A possible role of tachykinin-related peptide on an immune system activity of mealworm beetle, Tenebrio molitor L. Dev. Comp. Immunol. 2021, 120, 104065. [Google Scholar] [CrossRef] [PubMed]
- Salmela, H.; Sundström, L. Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal. 2018, 5, e1506. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Wang, S.; Li, H.; Li, L. Vitellogenin, a multivalent sensor and an antimicrobial effector. Int. J. Biochem. Cell Biol. 2011, 43, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Whitten, M.M.A.; Tew, I.F.; Lee, B.L.; Ratcliffe, N.A. A novel role for an insect apolipoprotein (apolipophorin III) in beta-1,3-glucan pattern recognition and cellular encapsulation reactions. J. Immunol. 2004, 172, 2177–2185. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Cytryńska, M. Apolipophorins and insects immune response. Invertebr. Surviv. J. 2013, 10, 58–68. [Google Scholar]
- Salmela, H.; Amdam, G.V.; Freitak, D. Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin. PLoS Pathog. 2015, 11, e1005015. [Google Scholar] [CrossRef]
- Havukainen, H.; Münch, D.; Baumann, A.; Zhong, S.; Halskau, Ø.; Krogsgaard, M.; Amdam, G.V. Vitellogenin recognizes cell damage through membrane binding and shields living cells from reactive oxygen species. J. Biol. Chem. 2013, 288, 28369–28381. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Zhao, Y.; Bai, L.; Qin, Y.; Wang, Q.; Li, W. Distinct vitellogenin domains differentially regulate immunological outcomes in invertebrates. J. Biol. Chem. 2021, 296, 100060. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Rono, M.K.; Whitten, M.M.A.; Oulad-Abdelghani, M.; Levashina, E.A.; Marois, E. The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 2010, 8, e1000434. [Google Scholar] [CrossRef]
- Huo, Y.; Yu, Y.; Chen, L.; Li, Q.; Zhang, M.; Song, Z.; Chen, X.; Fang, R.; Zhang, L. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 2018, 14, e1006909. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Stötzel, S.; Wygrecka, M.; Preissner, K.T.; Vilcinskas, A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J. Immunol. 2008, 181, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyria, J.; Fruttero, L.L.; Paglione, P.A.; Canavoso, L.E. How Insects Balance Reproductive Output and Immune Investment. Insects 2025, 16, 311. https://doi.org/10.3390/insects16030311
Leyria J, Fruttero LL, Paglione PA, Canavoso LE. How Insects Balance Reproductive Output and Immune Investment. Insects. 2025; 16(3):311. https://doi.org/10.3390/insects16030311
Chicago/Turabian StyleLeyria, Jimena, Leonardo L. Fruttero, Pedro A. Paglione, and Lilián E. Canavoso. 2025. "How Insects Balance Reproductive Output and Immune Investment" Insects 16, no. 3: 311. https://doi.org/10.3390/insects16030311
APA StyleLeyria, J., Fruttero, L. L., Paglione, P. A., & Canavoso, L. E. (2025). How Insects Balance Reproductive Output and Immune Investment. Insects, 16(3), 311. https://doi.org/10.3390/insects16030311






