A New Species Amecephala micra sp. nov. (Hemiptera: Liadopsyllidae) from Mid-Cretaceous Myanmar Amber †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting
2.2. Morphology and Documentation
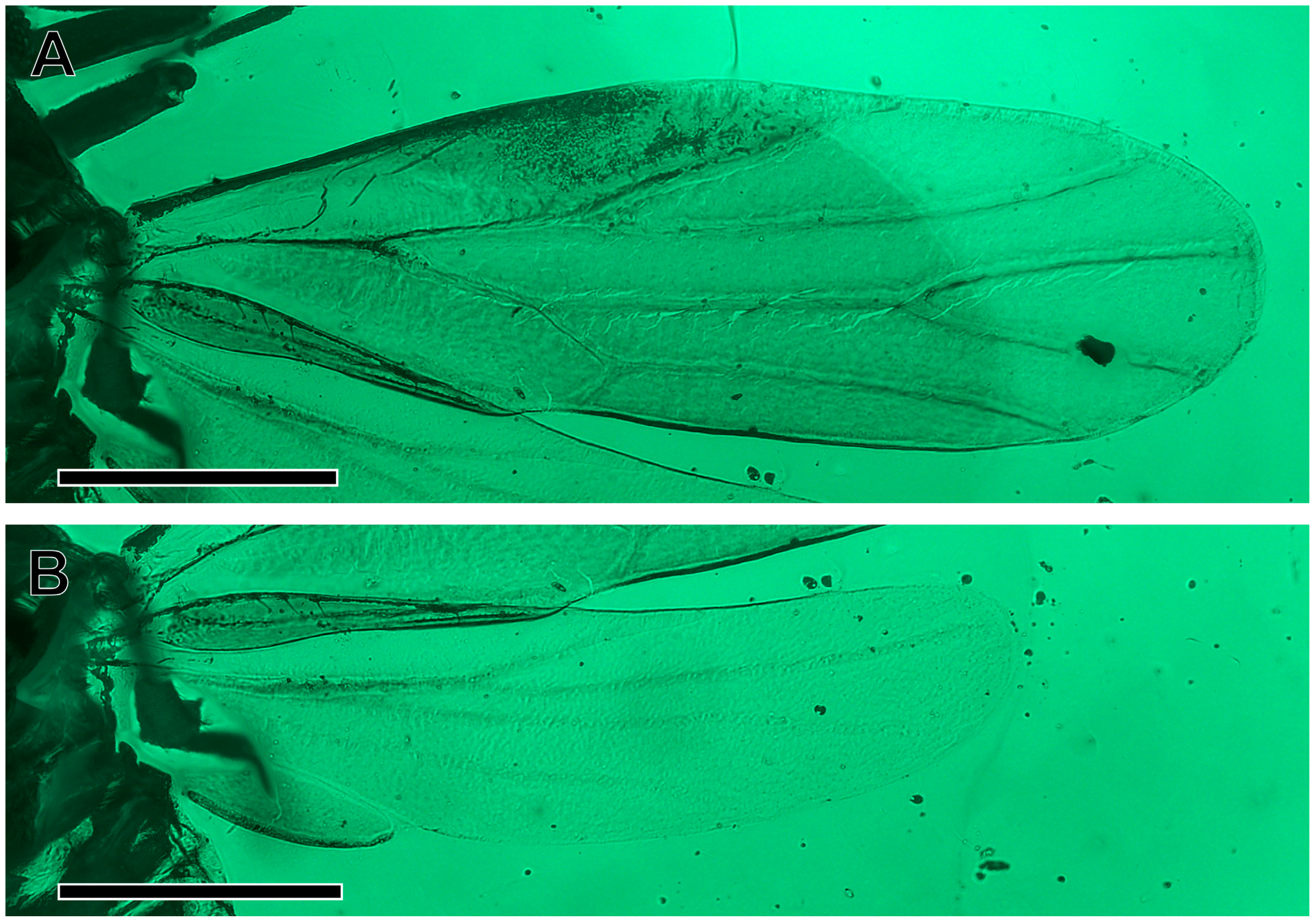
3. Results
Systematic Palaeontology





4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gullan, P.J.; Martin, J.H. Sternorrhyncha: (jumping plant-lice, whiteflies, aphids, and scale insects). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 957–967. ISBN 978-0-12-374144-8. [Google Scholar] [CrossRef]
- Hardy, N.B. The biodiversity of Sternorrhyncha: Scale insects, aphids, psyllids, and whiteflies. In Insect Biodiversity. Science and Society; Foottit, A.G., Adler, P.H., Eds.; Wiley: Chichester, UK, 2018; Volume 2, pp. 591–625. ISBN 9781118945575. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J.; Żyła, D.; Huang, D.-Y.; Müller, P. Fossils reshape the Sternorrhyncha evolutionary tree (Insecta, Hemiptera). Sci. Rep. 2020, 10, 11390. [Google Scholar] [CrossRef] [PubMed]
- Drohojowska, J.; Szwedo, J.; Müller, P.; Burckhardt, D. New fossil from mid-Cretaceous Burmese amber confirms monophyly of Liadopsyllidae (Hemiptera: Psylloidea). Sci. Rep. 2020, 10, 17607. [Google Scholar] [CrossRef]
- Ross, A.J. Burmese (Myanmar) amber checklist and bibliography 2018. Palaeoentomology 2019, 2, 22–84. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2019. Palaeoentomology 2020, 3, 103–118. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2020. Palaeoentomology 2021, 4, 57–76. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2021. Palaeoentomology 2022, 5, 27–45. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2022. Palaeoentomology 2023, 6, 22–40. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2023. Palaeoentomology 2024, 7, 148–165. [Google Scholar] [CrossRef]
- Ross, A.J. Complete checklist of Burmese (Myanmar) amber taxa 2023. Mesozoic 2024, 1, 21–57. [Google Scholar] [CrossRef]
- Laufer, B. Historical Jottings on Amber in Asia; Smithsonian Institution: Washington, DC, USA, 1907; Volume 1, pp. 211–244. [Google Scholar]
- So, J.F. Scented trails: Amber as aromatic in medieval China. J. Roy. Asiat. Soc. Ser. 3 2013, 23, 85–101. [Google Scholar] [CrossRef]
- Kania, I.; Wang, B.; Szwedo, J. Dicranoptycha Osten Sacken, 1860 (Diptera, Limoniidae) from the earliest Cenomanian Burmese amber. Cret. Res. 2015, 52, 522–530. [Google Scholar] [CrossRef]
- Thu, K.; Zaw, K. Gem deposits of Myanmar. In Myanmar: Geology, Resources and Tectonics; Barber, A.J., Zaw, K., Crow, M.J., Eds.; Geological Society: London, UK, 2017; Volume 48, pp. 497–529. ISBN 9781862399693. [Google Scholar] [CrossRef]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]
- Kazubski, M. Burmite. Can it compete with succinite? Bursztynisko/Amber Mag. 2016, 38, 45–47. [Google Scholar]
- Xing, L.D.; McKellar, R.C.; Wang, M.; Bai, M.; O’Connor, J.K.; Benton, M.J.; Zhang, J.P.; Wang, Y.; Tseng, K.W.; Lockley, M.G.; et al. Mummified precocial bird wings in mid-Cretaceous Burmese amber. Nat. Commun. 2016, 7, 12089. [Google Scholar] [CrossRef]
- Xing, L.D.; McKellar, R.C.; Xu, X.; Li, G.; Bai, M.; Persons IV, W.S.; Miyashita, T.; Benton, M.J.; Zhang, J.P.; Wolfe, A.P.; et al. A feathered dinosaur tail with primitive plumage trapped in mid-Cretaceous amber. Curr. Biol. 2016, 26, 3352–3360. [Google Scholar] [CrossRef]
- Xing, L.D.; Sames, B.; Xi, D.P.; McKellar, R.C.; Bai, M.; Wan, X.Q. A gigantic marine ostracod (Crustacea: Myodocopa) trapped in mid-Cretaceous Burmese amber. Sci. Rep. 2018, 8, 1365. [Google Scholar] [CrossRef]
- Helm, O. On a new, fossil, amber-like resin occurring in Burma. Rec. Geol. Surv. India 1892, 25, 180–181. [Google Scholar]
- Helm, O. Further note on Burmite, a new amber-like fossil resin from Upper Burma. Rec. Geol. Surv. India 1893, 26, 61–64. [Google Scholar]
- Noetling, F. On the occurrence of Burmite, a new fossil resin from Upper Burma. Rec. Geol. Surv. India 1893, 26, 31–40. [Google Scholar]
- Shi, G.H.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.C.; Lei, W.Y.; Li, Q.L.; Li, X.H. Age constraint on Burmese amber based on U–Pb dating of zircons. Cret. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Wright, C.W.; Calloman, H.J.; Howarth, K.M. Cretaceous Ammonoidea. In Treatise on Invertebrate Paleontology; Part. L, Mollusca 4 (Revised); The Geological Society of America: Boulder, CO, USA; the University of Kansas: Lawrence, KS, USA, 1996; Volume 4, pp. 1–393. ISBN 9780813731124. [Google Scholar]
- Ross, A.J.; Mellish, C.; York, P.; Crighton, B. Chapter 12. Burmese amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Penney, D., Ed.; Siri Scientific Press: Manchester, UK, 2010; pp. 208–235. ISBN 9780955863646. [Google Scholar]
- Smith, R.D.A.; Ross, A.J. Amberground pholadid bivalve borings and inclusions in Burmese amber: Implications for proximity of resin-producing forests to brackish waters, and the age of the amber. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 107, 239–247. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P.; Bashkuev, A.S.; Kopylov, D.S.; Lukashevich, E.D.; Ponomarenko, A.G.; Popov, Y.A.; Rasnitsyn, D.A.; Ryzhkova, O.V.; Sidorchuk, E.A.; Sukatsheva, I.D. Sequence and scale of changes in the terrestrial biota during the Cretaceous (based on materials from fossil resins). Cret. Res. 2016, 61, 234–255. [Google Scholar] [CrossRef]
- Zheng, D.R.; Chang, S.C.; Perrichot, V.; Dutta, S.; Rudra, A.; Mu, L.; Kelly, R.S.; Li, S.; Zhang, Q.; Zhang, Q.Q.; et al. A Late Cretaceous amber biota from central Myanmar. Nat. Commun. 2018, 9, 3170. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Kelly, R.; Mu, L.; Ross, A.J.; Kennedy, J.; Broly, P.; Xia, F.Y.; Zhang, H.C.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Natl. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef]
- Song, Z.S.; Xu, G.H.; Liang, A.P.; Szwedo, J.; Bourgoin, T. Still greater disparity in basal planthopper lineage: A new planthopper family Yetkhatidae (Hemiptera, Fulgoromorpha, Fulgoroidea) from mid-Cretaceous Myanmar amber. Cretac. Res. 2019, 101, 47–60. [Google Scholar] [CrossRef]
- Zheng, D.R.; Ramezani, J.; Ross, A.J.; Zhang, H.C.; Jarzembowski, E.A.; Zhuo, D.; Fang, Y.; Wang, H.; Zhang, Q.; Zhang, Q.Q.; et al. High-precision age constraint for Cretaceous amber in Myanmar. In Abstract Book, Proceedings of the 9th International Conference on Fossil Insects, Arthropods and Amber, Xi’an, China, 18–25 April 2024; Szwedo, J., Cai, C.Y., Xuan, Q., Eds.; Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences: Nanjing, China, 2024; p. 116. [Google Scholar]
- Liu, S.I. Gem notes. Organic materials: Burmese amber from Khamti, Sagaing Region. J. Gemmol. 2018, 36, 107–110. [Google Scholar]
- Nyunt, T.T.; Tay, T.S.; Krishnaswamy, M.; Loke, H.Y.; Cho, C.; Naing, B.B.K.; Wai, Y.L.A.; Chutimun, C.N. Amber from Khamti, Sagaing Region, Myanmar. J. Gemmol. 2020, 37, 314–322. [Google Scholar] [CrossRef]
- Nyunt, T.T.; Cho, C.; Kyaw, N.B.B.; Krishnaswamy, M.; Ying, L.H.; Sun, T.T.; Chanmuang, C. Geology, occurrence and gemmology of Khamti amber from Sagaing region, Myanmar. Thai Geosci. J. 2021, 2, 61–71. [Google Scholar]
- Peretti, A. Ethical guidelines for Burmese amber acquisitions. PMF J. 2020, 1, 4–78. [Google Scholar]
- Xing, L.D.; Qiu, L. Zircon U-Pb age constraints on the mid-Cretaceous Hkamti amber biota in northern Myanmar. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 558, 109960. [Google Scholar] [CrossRef]
- Sun, T.T.; Kleismantas, A.; Nyunt, T.T.; Zheng, M.; Krishnaswamy, M.; Ying, L.H. Burmese amber from Hti Lin. J. Gemmol. 2015, 34, 606–615. [Google Scholar] [CrossRef]
- Wu, W.J.; Wang, Y.M. Study on Raman spectrum characteristics of amber. J. Gems Gemmol. 2014, 161, 40–45. [Google Scholar]
- Bai, F.; Liang, H.F.; Qu, H.T. Structural evolution of Burmese amber during petrifaction based on a comparison of the spectral characteristics of amber, copal, and rosin. J. Spectrosc. 2019, 1, 6904541. [Google Scholar] [CrossRef]
- Musa, M.; Kaye, T.G.; Bieri, W.; Peretti, A. Burmese amber compared using micro-attenuated total reflection infrared spectroscopy and ultraviolet imaging. Appl. Spectrosc. 2021, 75, 839–845. [Google Scholar] [CrossRef]
- Peretti, A.; Bieri, W. PMF data depository of analysis FTIR-ATR, PL analysis, CT and UV imaging of amber containing holotype Yaksha perettii, Oculudentavis naga and comparative amber samples. PMF J. 2022, 2, 1–35. [Google Scholar]
- Li, X.P.; Wang, Y.M.; Shi, G.H.; Lu, R.; Li, Y. Evaluation of natural ageing responses on Burmese amber durability by FTIR spectroscopy with PLSR and ANN models. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121936. [Google Scholar] [CrossRef]
- Shi, Z.; Xin, C.; Wang, Y. Spectral characteristics of unique species of Burmese amber. Minerals 2023, 13, 151. [Google Scholar] [CrossRef]
- Shi, C.; Cai, H.H.; Jiang, R.X.; Wang, S.; Engel, M.S.; Yuan, J.; Bai, M.; Yang, D.; Long, C.L.; Zhao, Z.T.; et al. Balance scientific and ethical concerns to achieve a nuanced perspective on ‘blood amber’. Nat. Ecol. Evol. 2021, 5, 705–706. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar] [CrossRef]
- Szwedo, J.; Wang, B.; Soszyńska-Maj, A.; Azar, A.; Ross, A.J. International Palaeoentomological Society Statement. Palaeoentomology 2020, 3, 221–222. [Google Scholar] [CrossRef]
- Haug, J.T.; Azar, D.; Ross, A.J.; Szwedo, J.; Wang, B.; Arillo, A.; Baranov, V.; Bechteler, J.; Beutel, R.G.; Blagoderov, V.; et al. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated April 21, 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber. PalZ 2020, 94, 431–437. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Li, Y.D.; Su, Y.T.; Cai, C.Y.; Huang, D.Y. Application of confocal laser scanning microscopy to the study of amber bioinclusions. Palaeoentomology 2021, 4, 266–278. [Google Scholar] [CrossRef]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark. In Fauna Entomologica Scandinavica; Brill, E.J., Ed.; Scandinavian Science Press Ltd.: Leiden, The Netherlands; New York, NY, USA; Köln, Germany, 1992; Volume 26, p. 348. ISBN 978-90-04-27351-1. [Google Scholar] [CrossRef]
- Hollis, D. Australian Psylloidea. Jumping Plantlice and Lerp Insects; Australian Biological Resources Study: Canberra, Australia, 2004; p. xvi+216. ISBN 978-0-642-56836-6. [Google Scholar]
- Drohojowska, J. Thorax Morphology and Its Importance in Establishing Relationships Within Psylloidea (Hemiptera, Sternorrhyncha); Prace nauk. Uniw. Śląskiego 3414; University of Silesia Press: Katowice, Poland, 2015; pp. 1–171. ISBN 978-83-8012-825-5. [Google Scholar]
- Becker-Migdisova, E.E. Iskopaemye Nasekomye Psillomorfy; Akademiya Nauk SSSR, Trudy Paleontologicheskogo Instituta: Moscow, Russia, 1985; Volume 206, pp. 1–92. [Google Scholar]
- Burckhardt, D.; Poinar, G. The first jumping plant-louse from mid-Cretaceous Burmese amber and its impact on the classification of Mesozoic psylloids (Hemiptera: Sternorrhyncha: Psylloidea s. l.). Cretac. Res. 2019, 106, 104240. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordinus, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis; Editio decima, reformata, Salvii: Holmiae (Laurentii Salvii): Stockholm, Sweden, 1758; Volume 1, p. 824. [Google Scholar] [CrossRef]
- Amyot, C.J.-B.; Audinet-Serville, J.G. Deuxième Partie. Homoptères. Homoptera Latr. Histoire Naturelle des Insects. Hemiptères; Librairie encyclopédique de Roret: Paris, France, 1843; pp. 1–676. [Google Scholar] [CrossRef]
- Flor, G.A.A. Die Rhynchoten Livlands in systematischer Folge beschrieben. Zweiter Theil: Rhynchota gulaerostria Zett. (Homoptera Aut.). Cicadina und Psyllodea. Arch. Liv. Ehst. Kurl. ZweiteSer. Biol. Naturkunde 1861, 4, 1–567. [Google Scholar] [CrossRef]
- Verhoeff, C. Vergleichende Untersuchungen über die Abdominal-segmente der weiblichen Hemiptera-Heteroptera und Homoptera. Entomol. Nachrbl. 1893, 19, 369–378. [Google Scholar]
- Martynov, A.V. Jurassic fossil insects from Turkestan. 6. Homoptera and Psocoptera. Izv. Akad. Nauk SSSR/Bull. Acad. Sci. URSS (6 seriya) 1926, 20, 1349–1366. [Google Scholar]
- Liu, G.P.; Wang, X.L.; Zhuo, D.; Chen, J. A new non-jumping plant-louse (Hemiptera, Sternorrhyncha, Malmopsyllidae) in mid-Cretaceous Kachin amber, northern Myanmar. Cretac. Res. 2021, 124, 104816. [Google Scholar] [CrossRef]
- Ivanov, G.A.; Vorontsov, D.D.; Shcherbakov, D.E. A remarkable psyllomorph family from Cretaceous Burmese amber, Miralidae stat. nov. (= Dinglidae syn. nov.; Hemiptera: Sternorrhyncha). Cretac. Res. 2025, 168, 106069. [Google Scholar] [CrossRef]
- Shcherbakov, D.E. New Homoptera from the Early Cretaceous of Buryatia with notes on the insect fauna of Khasurty. Russ. Entomol. J. 2020, 29, 127–138. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): A global synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar] [CrossRef]
- Gushki, R.S.; Lashkari, M.; Mirzaei, S. Identification, sexual dimorphism, and allometric effects of three psyllid species of the genus Psyllopsis by geometric morphometric analysis (Hemiptera, Liviidae). ZooKeys 2018, 737, 57–73. [Google Scholar] [CrossRef]
- Štarhová Serbina, L.; Mennecart, B. Evolutionary pattern of the forewing shape in the Neotropical genus of jumping plant-lice (Hemiptera: Psylloidea: Russelliana). Org. Divers. Evol. 2018, 18, 313–325. [Google Scholar] [CrossRef]
- Lashkari, M.; Burckhardt, D.; Shamsi Gushki, R. Molecular and morphometric identification of pistachio psyllids with niche modeling of Agonoscena pistaciae (Hemiptera: Aphalaridae). Bull. Entomol. Res. 2020, 110, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, M.; Burckhardt, D.; Kashef, S. Molecular, morphometric and digital automated identification of three Diaphorina species (Hemiptera: Liviidae). Bull. Entomol. Res. 2021, 111, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, D.; Dalle Cort, G.; Queiroz, D.L. Jumping plant lice of the genus Aphalara (Hemiptera, Psylloidea, Aphalaridae) in the Neotropics. ZooKeys 2020, 980, 119–140. [Google Scholar] [CrossRef]
- Ge, Y.; Smith, O.M.; Chen, W.; Liu, P.; Yuan, Q.; Kang, C.; Wang, T.; Sun, J.; Yan, B.; Liu, X.; et al. Morphological characterization and sexual dimorphism of the antennal sensilla in Bactericera gobica Loginova (Hemiptera: Psyllidae)—A scanning and transmission electron microscopic study. PeerJ 2022, 10, e12888. [Google Scholar] [CrossRef]
- Ossiannilsson, F. Sound production in psyllids (Hem. Hom.). Opusc. Entomol. 1950, 15, 202. [Google Scholar]
- Heslop-Harrison, G. Sound production in the Homoptera with special reference to sound producing mechanisms in the Psyllidae. Ann. Mag. Nat. Hist. (Ser. 13) 1961, 3, 633–640. [Google Scholar] [CrossRef]
- Taylor, K.L. A possible stridulatory organ in some Psylloidea (Homoptera). Aust. J. Entomol. 1985, 24, 77–80. [Google Scholar] [CrossRef]
- Liao, Y.C.; Wu, Z.Z.; Yang, M.M. Vibrational behavior of psyllids (Hemiptera: Psylloidea): Functional morphology and mechanisms. PLoS ONE 2019, 14, e0215196. [Google Scholar] [CrossRef]
- Liao, Y.C.; Percy, D.M.; Yang, M.M. Biotremology: Vibrational communication of Psylloidea. Arthropod Struct. Dev. 2022, 66, 101138. [Google Scholar] [CrossRef]
- Avosani, S.; Mankin, R.W.; Sullivan, T.E.S.; Polajnar, J.; Suckling, D.M.; Mazzoni, V. Vibrational communication in psyllids. In Biotremology: Physiology, Ecology, and Evolution; Hill, P.S.M., Mazzoni, V., Stritih-Peljhan, N., Virant-Doberlet, M., Wessel, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 529–546. ISBN 978-3-030-97418-3. [Google Scholar] [CrossRef]
- Polajnar, J.; Kvinikadze, E.; Harley, A.W.; Malenovský, I. Wing buzzing as a mechanism for generating vibrational signals in psyllids (Hemiptera: Psylloidea). Insect Sci. 2024, 31, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Tishechkin, D.Y. Vibratory communication in Psylloidea (Homoptera). In Insects Sounds and Communication. Physiology, Behaviour, Ecology and Evolution; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2005; pp. 357–363. ISBN 9780429122002. [Google Scholar] [CrossRef]
- Tishechkin, D.Y. On the structure of stridulatory organs in jumping plant lice (Homoptera: Psyllinea). Russ. Entomol. J. 2006, 15, 335–340. [Google Scholar]
- Klimaszewski, S.M. The structure of hind wings in Psyllodea (Homoptera) and its possible significance in recognizing the relationships within this suborder. Acta Biol. Siles. 1993, 22, 57–68, Prace naukowe Uniwersytetu Śląskiego 1336. [Google Scholar]
- Kwon, J.H.; Kwon, Y.J. Insect Fauna of Korea: Psylloidea (Arthropoda: Insecta: Hemiptera: Sternorrhyncha); National Institute of Biological Resources: Incheon, Republic of Korea, 2020; Volume 9, p. 405. ISBN 9788968114533. [Google Scholar]
- Luo, X.; Cai, W.; Qiao, G. Half-jumping plant lice—A taxonomic revision of the distinctive psyllid genus Togepsylla Kuwayama with a reassessment of morphology (Hemiptera, Psylloidea). ZooKeys 2017, 716, 63–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouvrard, D.; Burckhardt, D.; Azar, D.; Grimaldi, D. Non-jumping plant-lice in Cretaceous amber (Hemiptera: Sternorrhyncha: Psylloidea). Syst. Entomol. 2010, 35, 172–180. [Google Scholar] [CrossRef]
- Burrows, M. Jumping mechanisms in jumping plant lice (Hemiptera, Sternorrhyncha, Psyllidae). J. Exp. Biol. 2012, 215, 3612–3621. [Google Scholar] [CrossRef]
- Kuwayama, S. A revision of the Psyllidae of Taiwan. Insecta Matsumurana 1931, 5, 117–133. Available online: http://hdl.handle.net/2115/9213 (accessed on 6 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drohojowska, J.; Hakim, M.; Huang, D.; Szwedo, J. A New Species Amecephala micra sp. nov. (Hemiptera: Liadopsyllidae) from Mid-Cretaceous Myanmar Amber. Insects 2025, 16, 302. https://doi.org/10.3390/insects16030302
Drohojowska J, Hakim M, Huang D, Szwedo J. A New Species Amecephala micra sp. nov. (Hemiptera: Liadopsyllidae) from Mid-Cretaceous Myanmar Amber. Insects. 2025; 16(3):302. https://doi.org/10.3390/insects16030302
Chicago/Turabian StyleDrohojowska, Jowita, Marina Hakim, Diying Huang, and Jacek Szwedo. 2025. "A New Species Amecephala micra sp. nov. (Hemiptera: Liadopsyllidae) from Mid-Cretaceous Myanmar Amber" Insects 16, no. 3: 302. https://doi.org/10.3390/insects16030302
APA StyleDrohojowska, J., Hakim, M., Huang, D., & Szwedo, J. (2025). A New Species Amecephala micra sp. nov. (Hemiptera: Liadopsyllidae) from Mid-Cretaceous Myanmar Amber. Insects, 16(3), 302. https://doi.org/10.3390/insects16030302






