Remodeling of Cellular Respiration and Insulin Signaling Are Part of a Shared Stress Response in Divergent Bee Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Caging, Treatments, and Tissue Collection
2.2. Alfalfa Leafcutting Bee Adult Caging and Tissue Collection
2.3. RNA Isolation, Reverse-Transcription and Quantitative PCR for Gene Expression Analysis
2.4. RNA-Seq
2.5. Nuclear Magnetic Resonance (NMR) Metabolite Quantification
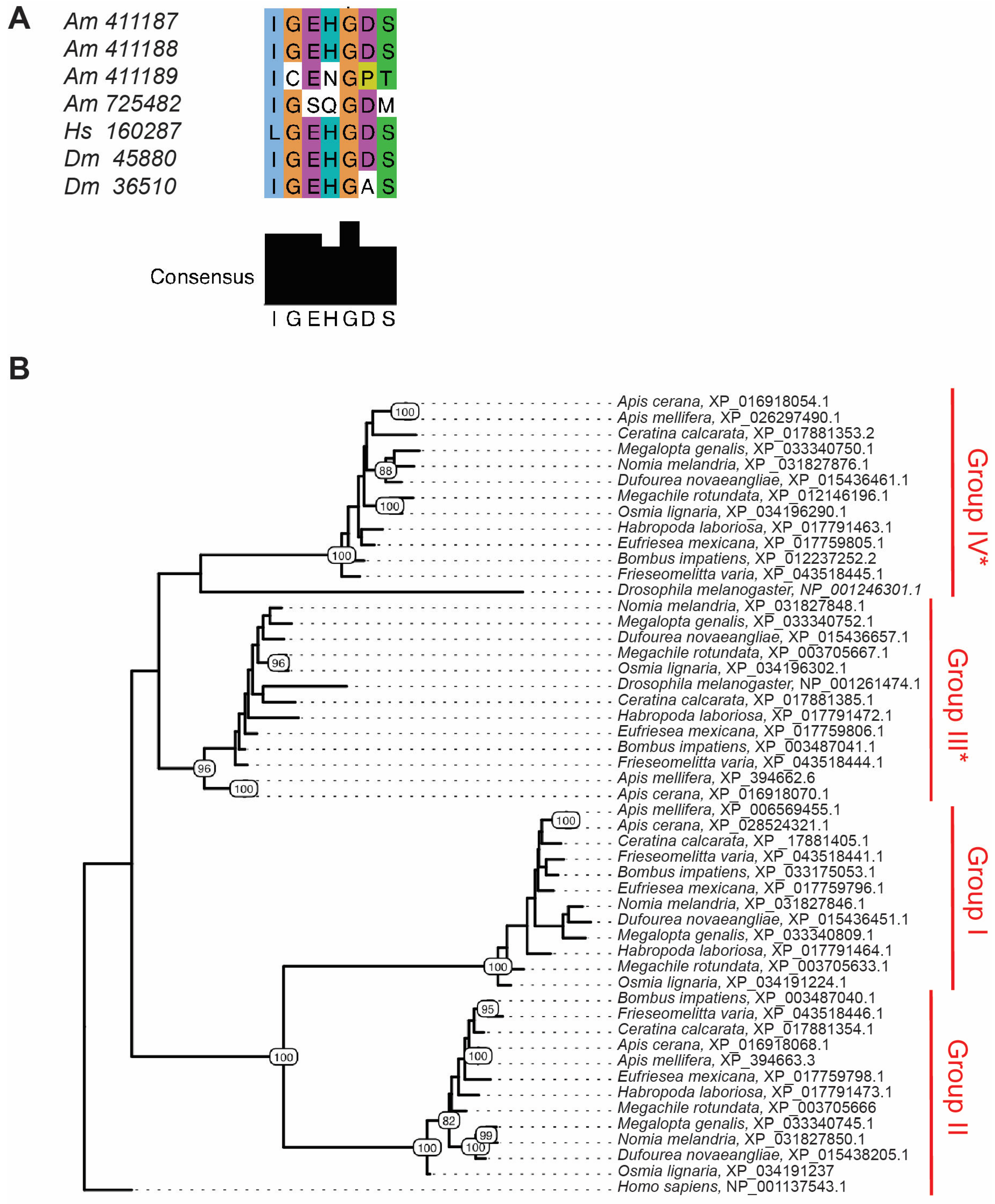
2.6. Identification and Analysis of Putative LDH Proteins in Bee (Hymenoptera: Apoidea: Anthophila) Genomes
2.7. Statistical Analysis
3. Results
3.1. Ldh Identified in Screen for Shared Stress Response Genes in Honey Bees
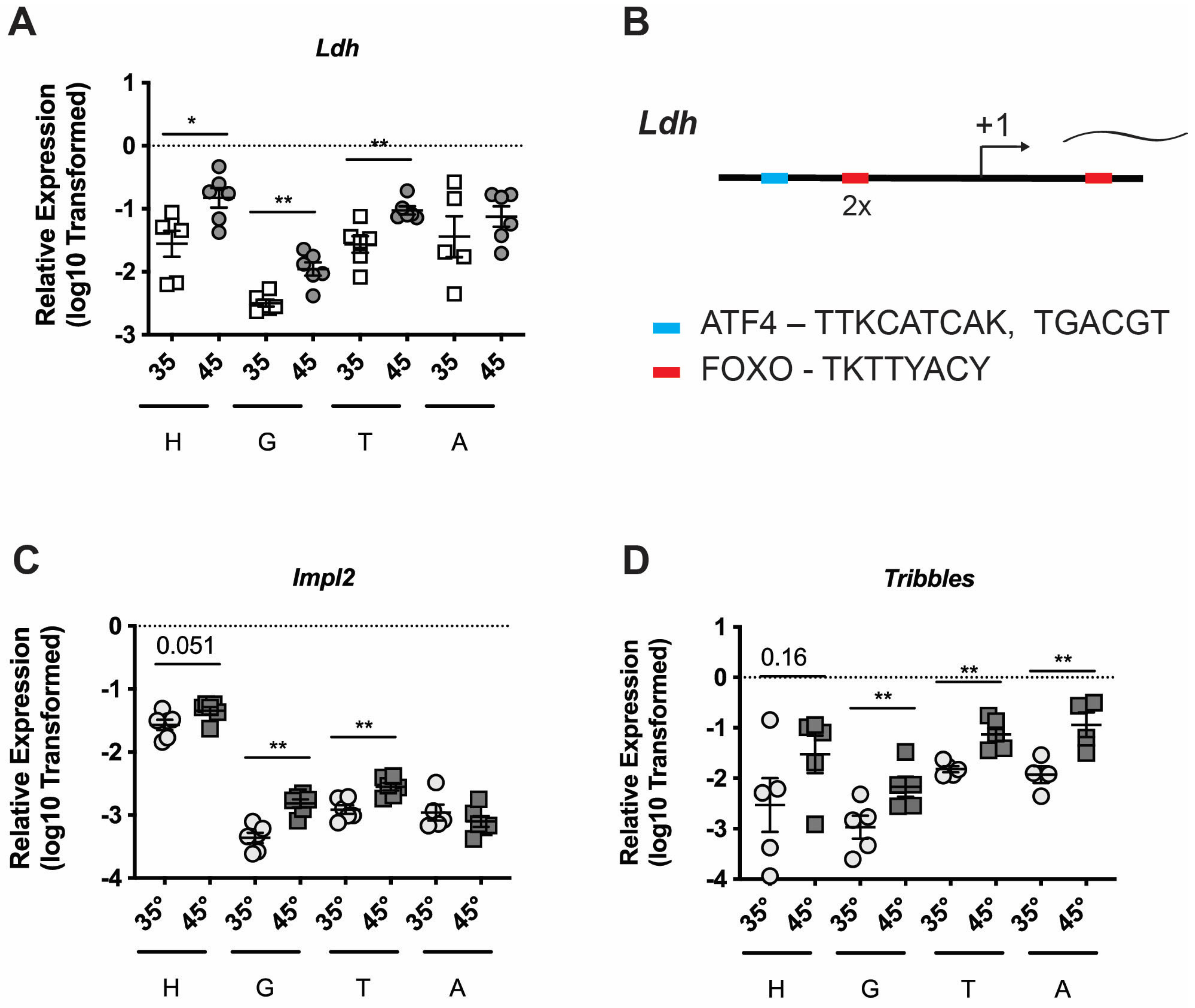
3.2. Additional Types of Cellular Stress Increase Ldh Expression in Honey Bees
3.3. Ldh Expression Is Induced in Multiple Tissues by Thermal Stress
3.4. Broader Transcriptional Remodeling of Cellular Respiration and Insulin Signaling Are Altered as Part of a Shared Stress Response in Honey Bees
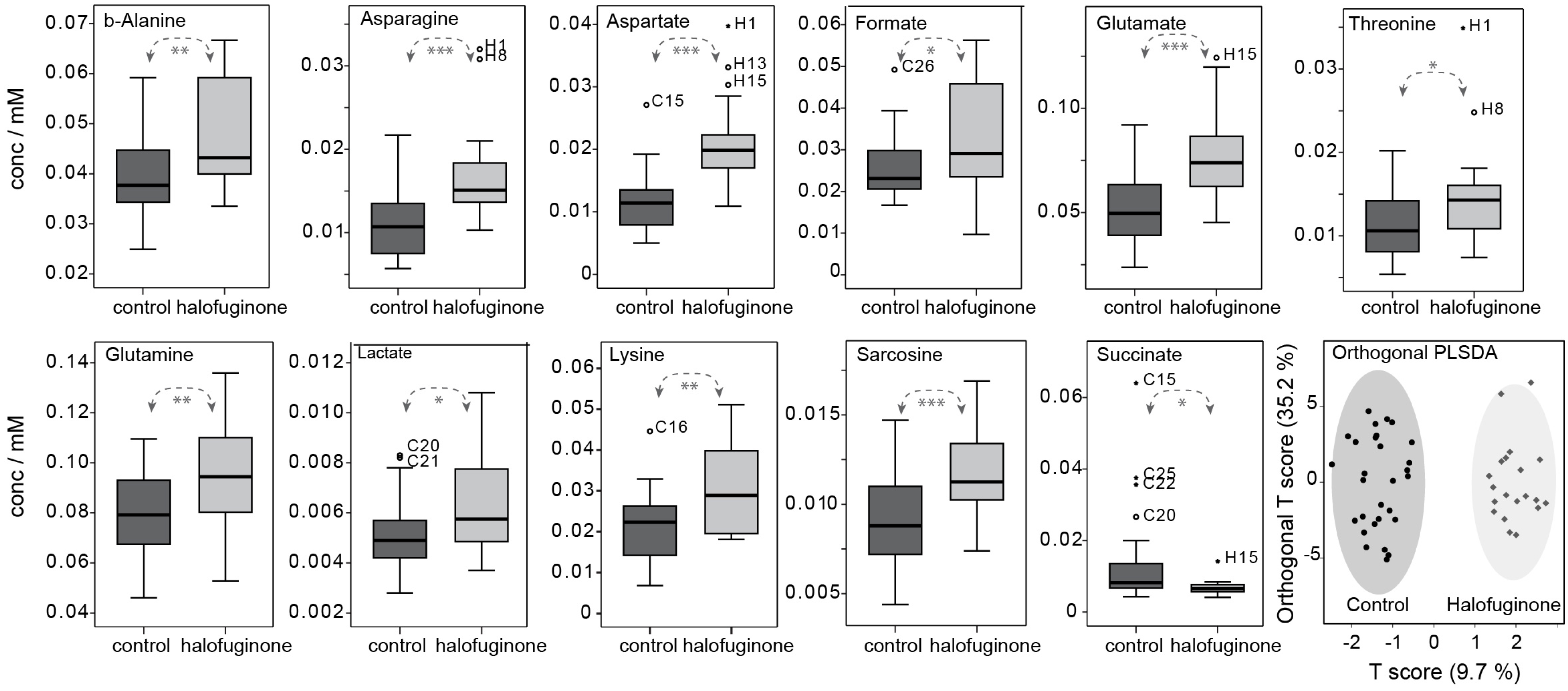
3.5. Changes in Metabolome Correlate with Transcriptional Changes in Cellular Respiration
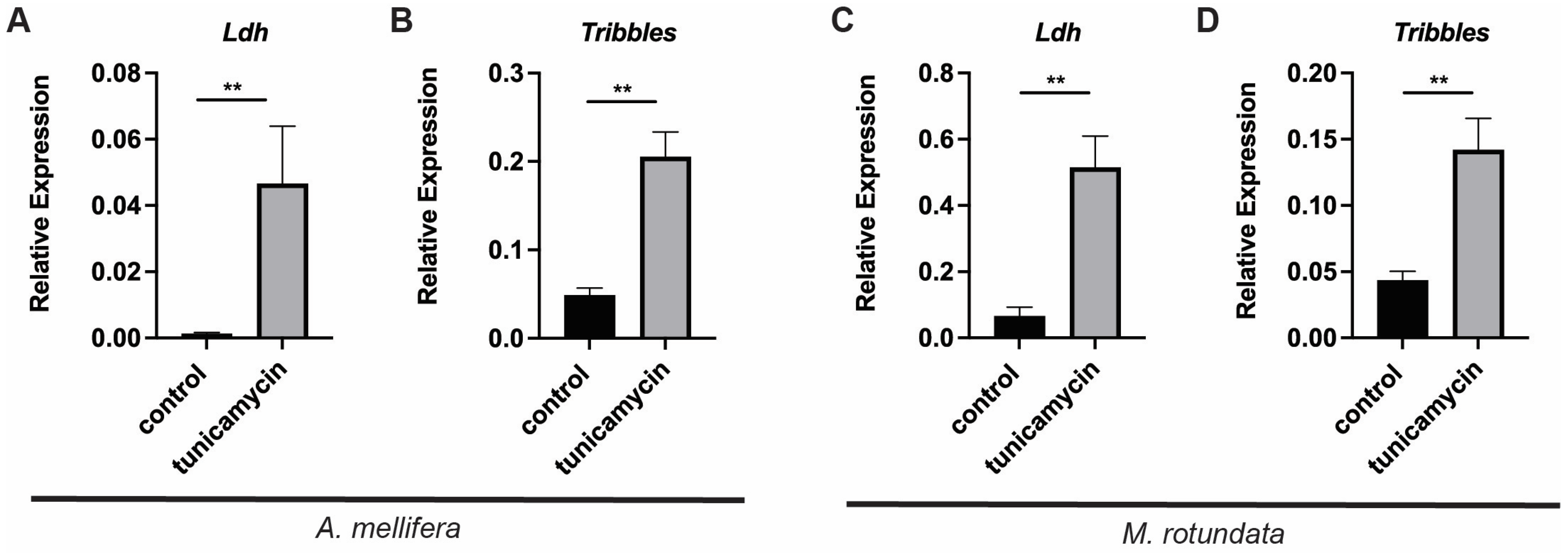
3.6. Stress Activates the Ldh Gene in the Alfalfa Leafcutting Bee
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; Vanengelsdorp, D. Drivers of Colony Losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Snow, J.W. What Does Cell Biology Have to Do with Saving Pollinators? In Transforming Sustainability Research and Teaching Discourses on Justice, Inclusion, and Authenticity; Maulucci, M.S.R., Callahan, H., Pfirman, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of Cellular Proteostasis in Aging and Disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.J.; Bott, L.C.; Morimoto, R.I. Shaping Proteostasis at the Cellular, Tissue, and Organismal Level. J. Cell Biol. 2017, 216, 1231–1241. [Google Scholar] [CrossRef]
- Adames, T.R.; Rondeau, N.C.; Kabir, M.T.; Johnston, B.A.; Truong, H.; Snow, J.W. The IRE1 Pathway Regulates Honey Bee Unfolded Protein Response Gene Expression. Insect Biochem. Mol. Biol. 2020, 121, 103368. [Google Scholar] [CrossRef]
- Flores, M.E.; McNamara-Bordewick, N.K.; Lovinger, N.L.; Snow, J.W. Halofuginone Triggers a Transcriptional Program Centered on Ribosome Biogenesis and Function in Honey Bees. Insect Biochem. Mol. Biol. 2021, 139, 03667. [Google Scholar] [CrossRef]
- Bach, D.M.; Holzman, M.A.; Wague, F.; Miranda, J.; Lopatkin, A.J.; Mansfield, J.H.; Snow, J.W. Thermal Stress Induces Tissue Damage and a Broad Shift in Regenerative Signaling Pathways in the Honey Bee Digestive Tract. J. Exp. Biol. 2021, 224, jeb242262. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of Labor in Honeybees: Form, Function, and Proximate Mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef]
- Ulgezen, Z.N.; van Dooremalen, C.; van Langevelde, F. Understanding Social Resilience in Honeybee Colonies. Curr. Res. Insect Sci. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Cremer, S. Social Immunity in Insects. Curr. Biol. 2019, 29, R458–R463. [Google Scholar] [CrossRef]
- Wueppenhorst, K.; Alkassab, A.T.; Beims, H.; Bischoff, G.; Ernst, U.; Friedrich, E.; Illies, I.; Janke, M.; Kehmstedt, J.; Kirchner, W.H.; et al. Nurse Honey Bees Filter Fungicide Residues to Maintain Larval Health. Curr. Biol. 2024, 34, 5570–5577.e11. [Google Scholar] [CrossRef] [PubMed]
- López-Uribe, M.M.; Ricigliano, V.A.; Simone-Finstrom, M. Defining Pollinator Health: Assessing Bee Ecological, Genetic, and Physiological Factors at the Individual, Colony, and Population Levels. Annu. Rev. Anim. Biosci. 2019, 8, 269–294. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.; Masson, P.; Mirzaei, Z.; Young, P. Identification and Characterization of a Drosophila Proteasome Regulatory Network. Mol. Cell. Biol. 2005, 25, 4662–4675. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 Signaling Regulates Oxidative Stress Tolerance and Lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Chow, C.Y.; Wolfner, M.F.; Clark, A.G. Using Natural Variation in Drosophila to Discover Previously Unknown Endoplasmic Reticulum Stress Genes. Proc. Natl. Acad. Sci. USA 2013, 110, 9013–9018. [Google Scholar] [CrossRef]
- Deliu, L.P.; Ghosh, A.; Grewal, S.S. Investigation of Protein Synthesis in Drosophila Larvae Using Puromycin Labelling. Biol. Open 2017, 6, 1229–1234. [Google Scholar] [CrossRef]
- Johnston, B.A.; Hooks, K.B.; McKinstry, M.; Snow, J.W. Divergent Forms of Endoplasmic Reticulum Stress Trigger a Robust Unfolded Protein Response in Honey Bees. J. Insect Physiol. 2016, 86, 1–10. [Google Scholar] [CrossRef]
- Shih, S.R.; Bach, D.M.; Rondeau, N.C.; Sam, J.; Lovinger, N.L.; Lopatkin, A.J.; Snow, J.W. Honey Bee SHSP Are Responsive to Diverse Proteostatic Stresses and Potentially Promising Biomarkers of Honey Bee Stress. Sci. Rep. 2021, 11, 22087. [Google Scholar] [CrossRef]
- Snow, J.W. Prolyl-TRNA Synthetase Inhibition Reduces Microsporidia Infection Intensity in Honey Bees. Apidologie 2020, 51, 557–569. [Google Scholar] [CrossRef]
- McKinstry, M.; Chung, C.; Truong, H.; Johnston, B.A.; Snow, J.W. The Heat Shock Response and Humoral Immune Response Are Mutually Antagonistic in Honey Bees. Sci. Rep. 2017, 7, 8850. [Google Scholar] [CrossRef]
- Kogan, H.V.; Macleod, S.G.; Rondeau, N.C.; Raup-Collado, J.; Cordero, V.A.; Rovnyak, D.; Marshalleck, C.A.; Mallapan, M.; Flores, M.E.; Snow, J.W. Transcriptional Control of a Metabolic Switch Regulating Cellular Methylation Reactions Is Part of a Common Response to Stress in Divergent Bee Species. J. Exp. Biol. 2024, 227, jeb246894. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- McDevitt, J.C.; Gupta, R.A.; Dickinson, S.G.; Martin, P.L.; Rieuthavorn, J.; Freund, A.; Pizzorno, M.C.; Capaldi, E.A.; Rovnyak, D. Methodology for Single Bee and Bee Brain 1H-NMR Metabolomics. Metabolites 2021, 11, 864. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Shih, S.R.; Huntsman, E.M.; Flores, M.E.; Snow, J.W. Reproductive Potential Does Not Cause Loss of Heat Shock Response Performance in Honey Bees. Sci. Rep. 2020, 10, 19610–19618. [Google Scholar] [CrossRef]
- Lee, J.E.; Oney, M.; Frizzell, K.; Phadnis, N.; Hollien, J. Drosophila melanogaster Activating Transcription Factor 4 Regulates Glycolysis during Endoplasmic Reticulum Stress. G3 Genes Genomes Genet. 2015, 5, 667–675. [Google Scholar] [CrossRef]
- Birch-Machin, I.; Gao, S.; Huen, D.; McGirr, R.; White, R.A.H.; Russell, S. Genomic Analysis of Heat-Shock Factor Targets in Drosophila. Genome Biol. 2005, 6, R63. [Google Scholar] [CrossRef]
- Guertin, M.J.; Lis, J.T. Chromatin Landscape Dictates HSF Binding to Target DNA Elements. PLoS Genet. 2010, 6, e1001114. [Google Scholar] [CrossRef] [PubMed]
- Samali, A.; FitzGerald, U.; Deegan, S.; Gupta, S. Methods for Monitoring Endoplasmic Reticulum Stress and the Unfolded Protein Response. Int. J. Cell Biol. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barretto, E.C.; Polan, D.M.; Beevor-Potts, A.N.; Lee, B.; Grewal, S.S. Tolerance to Hypoxia Is Promoted by FOXO Regulation of the Innate Immunity Transcription Factor NF-ΚB/Relish in Drosophila. Genetics 2020, 215, 1013–1025. [Google Scholar] [CrossRef]
- Bai, H.; Kang, P.; Hernandez, A.M.; Tatar, M. Activin Signaling Targeted by Insulin/DFOXO Regulates Aging and Muscle Proteostasis in Drosophila. PLoS Genet. 2013, 9, e1003941-16. [Google Scholar] [CrossRef]
- Semaniuk, U.; Piskovatska, V.; Strilbytska, O.; Strutynska, T.; Burdyliuk, N.; Vaiserman, A.; Bubalo, V.; Storey, K.B.; Lushchak, O. Drosophila Insulin-like Peptides: From Expression to Functions—A Review. Entomol. Exp. Appl. 2021, 169, 195–208. [Google Scholar] [CrossRef]
- Semaniuk, U.; Strilbytska, O.; Malinovska, K.; Storey, K.B.; Vaiserman, A.; Lushchak, V.; Lushchak, O. Factors That Regulate Expression Patterns of Insulin-like Peptides and Their Association with Physiological and Metabolic Traits in Drosophila. Insect Biochem. Mol. Biol. 2021, 135, 103609. [Google Scholar] [CrossRef]
- Andersen, A.S.; Hansen, P.H.; Schäffer, L.; Kristensen, C. A New Secreted Insect Protein Belonging to the Immunoglobulin Superfamily Binds Insulin and Related Peptides and Inhibits Their Activities. J. Biol. Chem. 2000, 275, 16948–16953. [Google Scholar] [CrossRef]
- Kwon, Y.; Song, W.; Droujinine, I.A.; Hu, Y.; Asara, J.M.; Perrimon, N. Systemic Organ Wasting Induced by Localized Expression of the Secreted Insulin/IGF Antagonist ImpL2. Dev. Cell 2015, 33, 36–46. [Google Scholar] [CrossRef]
- Figueroa-Clarevega, A.; Bilder, D. Malignant Drosophila Tumors Interrupt Insulin Signaling to Induce Cachexia-like Wasting. Dev. Cell 2015, 33, 47–55. [Google Scholar] [CrossRef]
- Seher, T.C.; Leptin, M. Tribbles, a Cell-Cycle Brake That Coordinates Proliferation and Morphogenesis during Drosophila Gastrulation. Curr. Biol. 2000, 10, 623–629. [Google Scholar] [CrossRef]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. TRB3: A Tribbles Homolog That Inhibits Akt/PKB Activation by Insulin in Liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef]
- Das, R.; Sebo, Z.; Pence, L.; Dobens, L.L. Drosophila Tribbles Antagonizes Insulin Signaling-Mediated Growth and Metabolism via Interactions with Akt Kinase. PLoS ONE 2014, 9, e109530. [Google Scholar] [CrossRef]
- Pizzorno, M.C.; Field, K.; Kobokovich, A.L.; Martin, P.L.; Gupta, R.A.; Mammone, R.; Rovnyak, D.; Capaldi, E.A. Transcriptomic Responses of the Honey Bee Brain to Infection with Deformed Wing Virus. Viruses 2021, 13, 287. [Google Scholar] [CrossRef]
- Williamson, J.M.; Brown, G.M. Purification and Properties of L-Aspartate-Alpha-Decarboxylase, an Enzyme That Catalyzes the Formation of Beta-Alanine in Escherichia Coli. J. Biol. Chem. 1979, 254, 8074–8082. [Google Scholar] [CrossRef]
- Cronan, J.E. Beta-Alanine Synthesis in Escherichia Coli. J. Bacteriol. 1980, 141, 1291–1297. [Google Scholar] [CrossRef]
- Perchat, N.; Dubois, C.; Mor-Gautier, R.; Duquesne, S.; Lechaplais, C.; Roche, D.; Fouteau, S.; Darii, E.; Perret, A. Characterization of a Novel β-Alanine Biosynthetic Pathway Consisting of Promiscuous Metabolic Enzymes. J. Biol. Chem. 2022, 298, 102067. [Google Scholar] [CrossRef]
- Landis, G.; Shen, J.; Tower, J. Gene Expression Changes in Response to Aging Compared to Heat Stress, Oxidative Stress and Ionizing Radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef]
- McAfee, A.; Milone, J.; Chapman, A.; Foster, L.J.; Pettis, J.S.; Tarpy, D.R. Candidate Stress Biomarkers for Queen Failure Diagnostics. BMC Genom. 2020, 21, 571. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Higo, H.; Underwood, R.; Milone, J.; Foster, L.J.; Guarna, M.M.; Tarpy, D.R.; Pettis, J.S. Vulnerability of Honey Bee Queens to Heat-Induced Loss of Fertility. Nat. Sustain. 2020, 3, 367–376. [Google Scholar] [CrossRef]
- Goblirsch, M.; Adamczyk, J.J. Using the Honey Bee (Apis mellifera L.) Cell Line, AmE-711, to Evaluate Insecticide Toxicity. Environ. Toxicol. Chem. 2022, 42, 88–99. [Google Scholar] [CrossRef]
- Cho, R.M.; Kogan, H.V.; Elikan, A.B.; Snow, J.W. Paromomycin Reduces Vairimorpha (Nosema) ceranae Infection in Honey Bees but Perturbs Microbiome Levels and Midgut Cell Function. Microorganisms 2022, 10, 1107. [Google Scholar] [CrossRef]
- Parrella, P.; Elikan, A.B.; Kogan, H.V.; Wague, F.; Marshalleck, C.A.; Snow, J.W. Bleomycin Reduces Vairimorpha (Nosema) ceranae Infection in Honey Bees with Some Evident Host Toxicity. Microbiol. Spectr. 2024, 12, e0334923. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and DsRNA-Triggered Transcriptional Responses Reveal Key Components of Honey Bee Antiviral Defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef]
- McMenamin, A.; Daughenbaugh, K.; Parekh, F.; Pizzorno, M.; Flenniken, M. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395. [Google Scholar] [CrossRef]
- Quirós, P.M.; Mottis, A.; Auwerx, J. Mitonuclear Communication in Homeostasis and Stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Rittschof, C.C.; Djukovic, D.; Gu, H.; Raftery, D.; Price, N.D.; Robinson, G.E. Aggression Is Associated with Aerobic Glycolysis in the Honey Bee Brain 1. Genes Brain Behav. 2015, 14, 158–166. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Bukhari, S.A.; Sloofman, L.G.; Troy, J.M.; Caetano-Anollés, D.; Cash-Ahmed, A.; Kent, M.; Lu, X.; Sanogo, Y.O.; Weisner, P.A.; et al. Neuromolecular Responses to Social Challenge: Common Mechanisms across Mouse, Stickleback Fish, and Honey Bee. Proc. Natl. Acad. Sci. USA 2014, 111, 17929–17934. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Vekaria, H.J.; Palmer, J.H.; Sullivan, P.G. Brain Mitochondrial Bioenergetics Change with Rapid and Prolonged Shifts in Aggression in the Honey Bee, Apis mellifera. J. Exp. Biol. 2018, 221, jeb176917. [Google Scholar] [CrossRef]
- Li-Byarlay, H.; Rittschof, C.C.; Massey, J.H.; Pittendrigh, B.R.; Robinson, G.E. Socially Responsive Effects of Brain Oxidative Metabolism on Aggression. Proc. Natl. Acad. Sci. USA 2014, 111, 12533–12537. [Google Scholar] [CrossRef]
- Hosios, A.M.; Heiden, M.G.V. The Redox Requirements of Proliferating Mammalian Cells. J. Biol. Chem. 2018, 293, 7490–7498. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Li, Z.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased Demand for NAD+ Relative to ATP Drives Aerobic Glycolysis. Mol. Cell 2021, 81, 691–707.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rai, M.; Buddika, K.; Sterrett, M.C.; Luhur, A.; Mahmoudzadeh, N.H.; Julick, C.R.; Pletcher, R.C.; Chawla, G.; Gosney, C.J.; et al. Lactate Dehydrogenase and Glycerol-3-Phosphate Dehydrogenase Cooperatively Regulate Growth and Carbohydrate Metabolism during Drosophila melanogaster larval Development. Development 2019, 146, dev175315. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Wang, B.; Liu, H.; Shah, H.; Carmona-Fontaine, C.; Rustenburg, A.S.; Salah, S.; Gunner, M.R.; Chodera, J.D.; Cross, J.R.; et al. L-2-Hydroxyglutarate Production Arises from Noncanonical Enzyme Function at Acidic PH. Nat. Chem. Biol. 2017, 13, 494–500. [Google Scholar] [CrossRef]
- Li, H.; Chawla, G.; Hurlburt, A.J.; Sterrett, M.C.; Zaslaver, O.; Cox, J.; Karty, J.A.; Rosebrock, A.P.; Caudy, A.A.; Tennessen, J.M. Drosophila Larvae Synthesize the Putative Oncometabolite L-2-Hydroxyglutarate during Normal Developmental Growth. Proc. Natl. Acad. Sci. USA 2017, 114, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, N.H.; Fitt, A.J.; Schwab, D.B.; Martenis, W.E.; Nease, L.M.; Owings, C.G.; Brinkley, G.J.; Li, H.; Karty, J.A.; Sudarshan, S.; et al. The Oncometabolite L-2-Hydroxyglutarate Is a Common Product of Dipteran Larval Development. Insect Biochem. Mol. Biol. 2020, 127, 103493. [Google Scholar] [CrossRef]
- Volkenhoff, A.; Weiler, A.; Letzel, M.; Stehling, M.; Klämbt, C.; Schirmeier, S. Glial Glycolysis Is Essential for Neuronal Survival in Drosophila. Cell Metab. 2015, 22, 437–447. [Google Scholar] [CrossRef]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; Snippert, H.J.; et al. Interplay between Metabolic Identities in the Intestinal Crypt Supports Stem Cell Function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Strachecka, A.; Grzybek, M.; Ptaszyńska, A.A.; Los, A.; Chobotow, J.; Rowinski, R. Comparison of Lactate Dehydrogenase Activity in Hive and Forager Honeybees May Indicate Delayed Onset Muscle Soreness—Preliminary Studies. Biochemistry 2019, 84, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Droujinine, I.A.; Perrimon, N. Interorgan Communication Pathways in Physiology: Focus on Drosophila. Annu. Rev. Genet. 2016, 50, 539–570. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, L.H. Organ-to-Organ Communication: A Drosophila Gastrointestinal Tract Perspective. Front. Cell Dev. Biol. 2017, 5, 29. [Google Scholar] [CrossRef]
- Wong, A.C.N.; Vanhove, A.S.; Watnick, P.I. The Interplay between Intestinal Bacteria and Host Metabolism in Health and Disease: Lessons from Drosophila melanogaster. Dis. Model Mech. 2016, 9, 271–281. [Google Scholar] [CrossRef]
- Raymann, K.; Moran, N.A. The Role of the Gut Microbiome in Health and Disease of Adult Honey Bee Workers. Curr. Opin. Insect Sci. 2018, 26, 97–104. [Google Scholar] [CrossRef]
- Kwong, W.K.; Engel, P.; Koch, H.; Moran, N.A. Genomics and Host Specialization of Honey Bee and Bumble Bee Gut Symbionts. Proc. Natl. Acad. Sci. USA 2014, 111, 11509–11514. [Google Scholar] [CrossRef]
- Lee, F.J.; Miller, K.I.; McKinlay, J.B.; Newton, I.L.G. Differential Carbohydrate Utilization and Organic Acid Production by Honey Bee Symbionts. FEMS Microbiol. Ecol. 2018, 94, fiy113. [Google Scholar] [CrossRef]
- Iatsenko, I.; Boquete, J.-P.; Lemaitre, B. Microbiota-Derived Lactate Activates Production of Reactive Oxygen Species by the Intestinal NADPH Oxidase Nox and Shortens Drosophila Lifespan. Immunity 2018, 49, 929–942.e5. [Google Scholar] [CrossRef]
- de Groot, A.P. Amino Acid Requirements for Growth of the Honeybee (Apis mellifica L.). Experientia 1952, 8, 192–194. [Google Scholar] [CrossRef]
- Hunt, R.J.; Granat, L.; McElroy, G.S.; Ranganathan, R.; Chandel, N.S.; Bateman, J.M. Mitochondrial Stress Causes Neuronal Dysfunction via an ATF4-Dependent Increase in L-2-Hydroxyglutarate. J. Cell Biol. 2019, 218, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Sorge, S.; Theelke, J.; Yildirim, K.; Hertenstein, H.; McMullen, E.; Müller, S.; Altbürger, C.; Schirmeier, S.; Lohmann, I. ATF4-Induced Warburg Metabolism Drives Over-Proliferation in Drosophila. Cell Rep. 2020, 31, 107659. [Google Scholar] [CrossRef]
- Nargund, A.M.; Fiorese, C.J.; Pellegrino, M.W.; Deng, P.; Haynes, C.M. Mitochondrial and Nuclear Accumulation of the Transcription Factor ATFS-1 Promotes OXPHOS Recovery during the UPRmt. Mol. Cell 2015, 58, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pirooznia, M.; Xu, H. Mitochondria Regulate Intestinal Stem Cell Proliferation and Epithelial Homeostasis through FOXO. Mol. Biol. Cell 2020, 31, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Tain, L.S.; Jain, C.; Nespital, T.; Froehlich, J.; Hinze, Y.; Grönke, S.; Partridge, L. Longevity in Response to Lowered Insulin Signaling Requires Glycine N-Methyltransferase-Dependent Spermidine Production. Aging Cell 2020, 19, e13043. [Google Scholar] [CrossRef]
- Obata, F.; Kuranaga, E.; Tomioka, K.; Ming, M.; Takeishi, A.; Chen, C.H.; Soga, T.; Miura, M. Necrosis-Driven Systemic Immune Response Alters SAM Metabolism through the FOXO-GNMT Axis. Cell Rep. 2014, 7, 821–833. [Google Scholar] [CrossRef]
- Obata, F.; Miura, M. Enhancing S-Adenosyl-Methionine Catabolism Extends Drosophila Lifespan. Nat. Commun. 2015, 6, 8332. [Google Scholar] [CrossRef]
- Jünger, M.A.; Rintelen, F.; Stocker, H.; Wasserman, J.D.; Végh, M.; Radimerski, T.; Greenberg, M.E.; Hafen, E. The Drosophila Forkhead Transcription Factor FOXO Mediates the Reduction in Cell Number Associated with Reduced Insulin Signaling. J. Biol. 2003, 2, 20. [Google Scholar] [CrossRef]
- Puig, O.; Marr, M.T.; Ruhf, M.L.; Tjian, R. Control of Cell Number by Drosophila FOXO: Downstream and Feedback Regulation of the Insulin Receptor Pathway. Genes Dev. 2003, 17, 2006–2020. [Google Scholar] [CrossRef]
- Mattila, J.; Kokki, K.; Hietakangas, V.; Boutros, M. Stem Cell Intrinsic Hexosamine Metabolism Regulates Intestinal Adaptation to Nutrient Content. Dev. Cell 2018, 47, 112–121.e3. [Google Scholar] [CrossRef]
- Altintas, O.; Park, S.; Lee, S.J.V. The Role of Insulin/IGF-1 Signaling in the Longevity of Model Invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Song, W.; Perrimon, N. Muscle Mitohormesis Promotes Longevity via Systemic Repression of Insulin Signaling. Cell 2013, 155, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Long, D.M.; Frame, A.K.; Reardon, P.N.; Cumming, R.C.; Hendrix, D.A.; Kretzschmar, D.; Giebultowicz, J.M. Lactate Dehydrogenase Expression Modulates Longevity and Neurodegeneration in Drosophila melanogaster. Aging 2020, 12, 10041–10058. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.C.; Demontis, F. Age-Related Increase in Lactate Dehydrogenase Activity in Skeletal Muscle Reduces Life Span in Drosophila. J. Gerontol. Ser. A 2022, 2, 259–267. [Google Scholar] [CrossRef]
- Statzer, C.; Meng, J.; Venz, R.; Bland, M.; Robida-Stubbs, S.; Patel, K.; Petrovic, D.; Emsley, R.; Liu, P.; Morantte, I.; et al. ATF-4 and Hydrogen Sulfide Signalling Mediate Longevity in Response to Inhibition of Translation or MTORC1. Nat. Commun. 2022, 13, 967. [Google Scholar] [CrossRef]
- Wang, L.; Ryoo, H.D.; Qi, Y.; Jasper, H. PERK Limits Drosophila Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress. PLoS Genet. 2015, 11, e1005220. [Google Scholar] [CrossRef]
- Alic, N.; Hoddinott, M.P.; Vinti, G.; Partridge, L. Lifespan Extension by Increased Expression of the Drosophila Homologue of the IGFBP7 Tumour Suppressor. Aging Cell 2011, 10, 137–147. [Google Scholar] [CrossRef]
- Paoli, P.P.; Wakeling, L.A.; Wright, G.A.; Ford, D. The Dietary Proportion of Essential Amino Acids and Sir2 Influence Lifespan in the Honeybee. Age 2014, 36, 9649. [Google Scholar] [CrossRef]
- Rascón, B.; Hubbard, B.P.; Sinclair, D.A.; Amdam, G.V. The Lifespan Extension Effects of Resveratrol Are Conserved in the Honey Bee and May Be Driven by a Mechanism Related to Caloric Restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef]
- Flatt, T.; Amdam, G.V.; Kirkwood, T.B.L.; Omholt, S.W. Life-History Evolution and the Polyphenic Regulation of Somatic Maintenance and Survival. Q. Rev. Biol. 2013, 88, 185–218. [Google Scholar] [CrossRef]
- Ament, S.; Corona, M.; Pollock, H.; Robinson, G. Insulin Signaling Is Involved in the Regulation of Worker Division of Labor in Honey Bee Colonies. Proc. Natl. Acad. Sci. USA 2008, 105, 4226. [Google Scholar] [CrossRef] [PubMed]
- Ament, S.; Wang, Y.; Robinson, G. Nutritional Regulation of Division of Labor in Honey Bees: Toward a Systems Biology Perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mutti, N.S.; Ihle, K.E.; Siegel, A.; Dolezal, A.G.; Kaftanoglu, O.; Amdam, G.V. Down-Regulation of Honey Bee IRS Gene Biases Behavior toward Food Rich in Protein. PLoS Genet. 2010, 6, e1000896. [Google Scholar] [CrossRef] [PubMed]
- Ihle, K.E.; Mutti, N.S.; Kaftanoglu, O.; Amdam, G.V. Insulin Receptor Substrate Gene Knockdown Accelerates Behavioural Maturation and Shortens Lifespan in Honeybee Workers. Insects 2019, 10, 390. [Google Scholar] [CrossRef]
- Mengoni Goñalons, C.; Guiraud, M.; de Brito Sanchez, M.G.; Farina, W.M. Insulin Effects on Honeybee Appetitive Behaviour. J. Exp. Biol. 2016, 219, 3003–3008. [Google Scholar] [CrossRef]
- Nilsen, K.A.; Ihle, K.E.; Frederick, K.; Fondrk, M.K.; Smedal, B.; Hartfelder, K.; Amdam, G.V. Insulin-like Peptide Genes in Honey Bee Fat Body Respond Differently to Manipulation of Social Behavioral Physiology. J. Exp. Biol. 2011, 214, 1488–1497. [Google Scholar] [CrossRef]
- Ihle, K.E.; Baker, N.A.; Amdam, G.V. Insulin-like Peptide Response to Nutritional Input in Honey Bee Workers. J. Insect Physiol. 2014, 69, 49–55. [Google Scholar] [CrossRef]
- Perry, C.J.; Søvik, E.; Myerscough, M.R.; Barron, A.B. Rapid Behavioral Maturation Accelerates Failure of Stressed Honey Bee Colonies. Proc. Natl. Acad. Sci. USA 2015, 112, 3427–3432. [Google Scholar] [CrossRef]
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in Defence: Honeybee Workers Exhibit Conserved Molecular Responses to Diverse Pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef]
- Erban, T.; Sopko, B.; Kadlikova, K.; Talacko, P.; Harant, K. Varroa Destructor Parasitism Has a Greater Effect on Proteome Changes than the Deformed Wing Virus and Activates TGF-β Signaling Pathways. Sci. Rep. 2019, 9, 9400. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Rubin, B.E.R.; Palmer, J.H. The Transcriptomic Signature of Low Aggression in Honey Bees Resembles a Response to Infection. BMC Genom. 2019, 20, 1029. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L.; Fawcett, F. The Solitary Bees; Princeton University Press: Princeton, NJ, USA, 2019; ISBN 0691189323. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondeau, N.C.; Raup-Collado, J.; Kogan, H.V.; Cho, R.; Lovinger, N.; Wague, F.; Lopatkin, A.J.; Texeira, N.G.; Flores, M.E.; Rovnyak, D.; et al. Remodeling of Cellular Respiration and Insulin Signaling Are Part of a Shared Stress Response in Divergent Bee Species. Insects 2025, 16, 300. https://doi.org/10.3390/insects16030300
Rondeau NC, Raup-Collado J, Kogan HV, Cho R, Lovinger N, Wague F, Lopatkin AJ, Texeira NG, Flores ME, Rovnyak D, et al. Remodeling of Cellular Respiration and Insulin Signaling Are Part of a Shared Stress Response in Divergent Bee Species. Insects. 2025; 16(3):300. https://doi.org/10.3390/insects16030300
Chicago/Turabian StyleRondeau, Nicole C., Joanna Raup-Collado, Helen V. Kogan, Rachel Cho, Natalie Lovinger, Fatoumata Wague, Allison J. Lopatkin, Noelle G. Texeira, Melissa E. Flores, David Rovnyak, and et al. 2025. "Remodeling of Cellular Respiration and Insulin Signaling Are Part of a Shared Stress Response in Divergent Bee Species" Insects 16, no. 3: 300. https://doi.org/10.3390/insects16030300
APA StyleRondeau, N. C., Raup-Collado, J., Kogan, H. V., Cho, R., Lovinger, N., Wague, F., Lopatkin, A. J., Texeira, N. G., Flores, M. E., Rovnyak, D., & Snow, J. W. (2025). Remodeling of Cellular Respiration and Insulin Signaling Are Part of a Shared Stress Response in Divergent Bee Species. Insects, 16(3), 300. https://doi.org/10.3390/insects16030300






