An Evaluation of the Predatory Function of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae)
Simple Summary
Abstract
1. Introduction
2. Research Materials and Methodology
2.1. Materials and Methodology
2.2. The Predatory Function and Feeding Ability of O. strigicollis on Second-Instar Larvae and Adults of M. usitatus
2.3. Intraspecific Interference Experiment Determining O. strigicollis’s Predatory Activities on Adults of M. usitatus
2.4. Predation Preference of the O. strigicollis on Second-Instar Larvae and Adults of M. usitatus
2.5. Data Analysis
3. Results
3.1. Functional Responses and Search Efficiency During Predation by O. strigicollis on Second-Instar Larvae and Adults of M. usitatus
3.2. Intraspecific Interference Experiment with O. strigicollis and Adults of M. usitatus
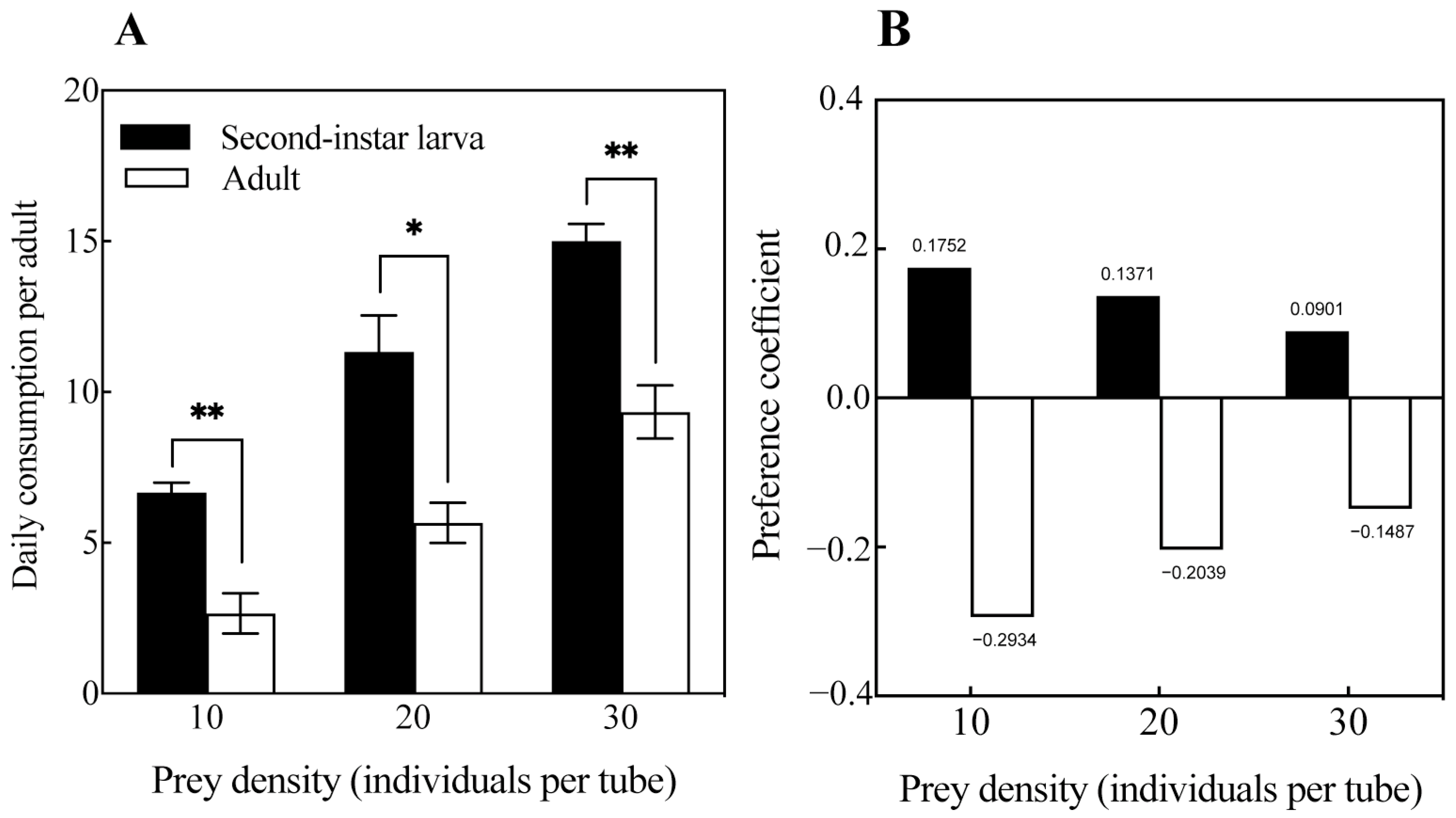
3.3. Predation Preference of O. strigicollis on Second-Instar Larvae and Adults of M. usitatus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michaud, R.; Lehman, W.F.; Rumbaugh, M. World distribution and historical development. Alfalfa Alfalfa Improv. 1988, 29, 25–91. [Google Scholar]
- Acharya, J.P.; Lopez, Y.; Gouveia, B.T.; de Bem Oliveira, I.; Resende, M.F.R., Jr.; Muñoz, P.R.; Rios, E.F. Breeding alfalfa (Medicago sativa L.) adapted to subtropical agroecosystems. Agronomy 2020, 10, 742. [Google Scholar] [CrossRef]
- Marković, J.; Štrbanović, R.; Cvetković, M.; Anđelković, B.; Živković, B. Effects of growth stage on the mineral concentrations in alfalfa (Medicago sativa L.) leaf, stem and the whole plant. Biotechnol. Anim. Husb. 2009, 25, 1225–1231. [Google Scholar]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, L.; Liu, Y.; Liu, Y.F.; López-Vicente, M.; Wei, X.H.; Wu, G.L. Alfalfa planting significantly improved alpine soil water infiltrability in the Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 285, 106606. [Google Scholar] [CrossRef]
- Fan, P.A.; Gao, L.J.; Zhu, K.H.; Du, G.L.; Zhu, M.M.; Li, Z.H.; Gao, Y.L.; Tu, X.B.; Zhang, Z.H. Regional selection of insecticides and fungal biopesticides to control aphids and thrips and improve the forage quality of alfalfa crops. J. Integr. Agric. 2023, 22, 185–194. [Google Scholar]
- Wu, F.; Shi, S.; Li, Y.; Miao, J.; Kang, W.; Zhang, J.; Yun, A.; Liu, C. Physiological and biochemical response of different resistant alfalfa cultivars against thrips damage. Physiol. Mol. Biol. Plants 2021, 27, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xu, Y.; Zhang, H.; Wang, Y.; Zhang, R.; Wei, S.; Ban, L. Dynamic and correlation of major pests with natural enemies and response to climatic factors in alfalfa fields in Ningxia. J. Plant Prot. 2024, 51, 1169–1178. [Google Scholar]
- Mound, L.A.; Walker, A.K. Thysanoptera as tropical tramps: New records from New Zealand and the Pacific. N. Z. Entomol. 1987, 9, 70–85. [Google Scholar] [CrossRef]
- Palmer, J. Magalurothrips in the flowers of tropical legumes: A morphometric study. In Population Structure, Genetics and Taxonomy of Aphids and Thysanoptera; SPB Academic Publishing: Amsterdam, The Netherlands, 1987; pp. 480–495. [Google Scholar]
- Ke, T. Effect of Host Plants and Adults Sex Ratios on the Population of Megalurothrips usitatus. Ph.D. Thesis, Hainan University, Haikou, China, 2017. [Google Scholar]
- Tang, L.D.; Yan, K.L.; Fu, B.-L.; Wu, J.-H.; Liu, K.; Lu, Y.-Y. The life table parameters of Megalurothrips usitatus (Thysanoptera: Thripidae) on four leguminous crops. Fla. Entomol. 2015, 98, 620–625. [Google Scholar] [CrossRef]
- Duff, J.D.; Church, C.E.; Healey, M.A.; Senior, L. Thrips incidence in green beans and the degree of damage caused. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): 1105, Brisbane, QLD, Australia, 17–22 August 2014; pp. 19–26. [Google Scholar]
- Prasada Rao, R.D.; Reddy, A.S.; Reddy, S.V.; Thirumala-Devi, K.; Rao, S.C.; Manoj Kumar, V.; Subramaniam, K.; Yellamanda Reddy, T.; Nigam, S.N.; Reddy, D.V. The host range of Tobacco streak virus in India and transmission by thrips. Ann. Appl. Biol. 2003, 142, 365–368. [Google Scholar] [CrossRef]
- Shukla, S.; Kalyani, G.; Kulkarni, N.; Waliyar, F.; Nigam, S.N. Mechanism of transmission of Tobacco streak virus by Scirtothrips dorsalis, Frankliniella schultzei and Megalurothrips usitatus in groundnut, Arachis hypogaea L. J. Oilseeds Res. 2005, 22, 215–217. [Google Scholar]
- Oparaeke, A.M. The sensitivity of flower bud thrips, Megalurothrips sjostedti Trybom (Thysanoptera: Thripidae), on cowpea to three concentrations and spraying schedules of Piper guineense Schum. & Thonn. extracts. Plant Prot. Sci. 2006, 42, 106. [Google Scholar]
- Camara, I.; Cao, K.; Sangbaramou, R.; Wu, P.; Shi, W.; Tan, S. Screening of Beauveria bassiana (Bals.) (Hypocreales: Cordycipitaceae) strains against Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and conditions for large-scale production. Egypt. J. Biol. Pest Control 2022, 32, 85. [Google Scholar] [CrossRef]
- Wu, J.H.; Yang, B.; Zhang, X.C.; Cuthbertson, A.G.S.; Ali, S. Synergistic Interaction between the Entomopathogenic Fungus Akanthomyces attenuatus (Zare & Gams) and the Botanical Insecticide Matrine against Megalurothrips usitatus (Bagrall). J. Fungi 2021, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Li, Z.; Yue, Y.; Tian, Q.; Chen, W.; Ali, S.; Wu, J. Immune-Related Genes of Megalurothrips usitatus (Bagrall) Against Beauveria brongniartii and Akanthomyces attenuatus Identified Using RNA Sequencing. Front. Physiol. 2021, 12, 671599. [Google Scholar] [CrossRef] [PubMed]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Tang, L.D.; Guo, L.H.; Ali, A.; Desneux, N.; Zang, L.S. Synergism of adjuvants mixed with spinetoram for the management of bean flower thrips, Megalurothrips usitatus (Thysanoptera: Thripidae) in cowpeas. J. Econ. Entomol. 2022, 115, 2013–2019. [Google Scholar] [CrossRef]
- Yura, W.F.; Muhammad, F.R.; Mirza, F.F.; Maurend, Y.L.; Widyantoro, W.; Farida, S.S.; Aziz, Y.P.; Desti, A.; Edy, W.; Septy, M.; et al. Pesticide residues in food and potential risk of health problems: A systematic literature review. IOP Conf. Ser. Earth Environ. Sci. 2021, 894, 012025. [Google Scholar] [CrossRef]
- Liu, P.; Qin, Z.; Feng, M.; Zhang, L.; Huang, X.; Shi, W. The male-produced aggregation pheromone of the bean flower thrips Megalurothrips usitatus in China: Identification and attraction of conspecifics in the laboratory and field. Pest Manag. Sci. 2020, 76, 2986–2993. [Google Scholar] [CrossRef]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Khan, R.; Seal, D.; Adhikari, R. Bean Flower Thrips Megalurothrips usitatus (Bagnall) (Insecta: Thysanoptera: Thripidae); EDIS: Gainesville, FL, USA, 2022. [Google Scholar]
- Kim, D.I.; Park, J.D.; Kim, S.G.; Kim, S.S.; Paik, C.H. Biological control of Thrips palmi (Thysanoptera: Thripidae) with Orius strigicollis (Hemiptera: Anthocoridae) on cucumber in plastic houses in the southern region of Korea. J. Asia Pac. Entomol. 2004, 7, 311–315. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lin, Y.H.; Peng, S.C.; Lai, W.H. Predatory efficacy of Orius strigicollis (Hemiptera: Anthocoridae) against Tetranychus urticae (Acarina: Tetranychidae) on strawberry. J. Asia-Pac. Entomol. 2016, 19, 109–114. [Google Scholar] [CrossRef]
- Wang, C.; Lee, P.; Wu, Y. Field augmentation of Orius strigicollis (Heteroptera: Anthocoridae) for the control of thrips in Taiwan. In International Seminar on Biological Control of Insect Pests in Economic Crops; FFTC: Taipei City, Taiwan, 2001; Volume 500, pp. 141–152. [Google Scholar]
- Dai, X.; Wang, R.; Liu, Y.; Su, L.; Yin, Z.; Wu, M.; Chen, H.; Zheng, L.; Zhai, Y. Control effect and field application of four predatory Orius species on Megalurothrips usitatus (Thysanoptera: Thripidae). J. Econ. Entomol. 2024, 117, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zeng, Z.; Chen, Y.; You, Y.; Hu, J.; Yang, F.; Wei, H. Compatibility of six reduced-risk insecticides with Orius strigicollis (Heteroptera: Anthocoridae) predators for controlling Thrips hawaiiensis (Thysanoptera: Thripidae) pests. Ecotoxicol. Environ. Saf. 2021, 226, 112812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.R.; Liu, M.; Ban, F.X.; Shang, X.L.; Liu, S.L.; Mao, T.T.; Zhang, X.Y.; Zhi, J.R. Establishment of a faba bean banker plant system with predator Orius strigicollis for the control of thrips Dendrothrips minowai on tea plants under laboratory conditions. Insects 2021, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Ohta, I. Effect of temperature on development of Orius strigicollis (Heteroptera: Anthocoridae) fed on Frankliniella occidentalis (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2001, 36, 483–488. [Google Scholar] [CrossRef][Green Version]
- Liu, P.; Jia, W.; Zheng, X.; Zhang, L.; Sangbaramou, R.; Tan, S.; Liu, Y.; Shi, W. Predation functional response and life table parameters of Orius sauteri (Hemiptera: Anthocoridae) feeding on Megalurothrips usitatus (Thysanoptera: Thripidae). Fla. Entomol. 2018, 101, 254–259. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Ding, Y.Q. Insect Mathematical Ecology; Science Press: Beijing, China, 1994; pp. 257–258, 303–304. [Google Scholar]
- Hassell, M.P.; Varley, G.C. New inductive population model for insect parasites and its bearing on biological control. Nature 1969, 223, 1133–1137. [Google Scholar] [CrossRef]
- Zou, Y.; Geng, J.; Chen, G.; Meng, Q.; Wang, G. Predation of Harmonia axyridis nymph on Schizaphis graminum. Chin. J. Appl. Ecol. 1996, 7, 197–200. [Google Scholar]
- Zhou, J.Z.; Chen, C.M. Quantitative measurement of selectivityof predator for prey. Acta Ecol. Sin. 1987, 7, 50–56. [Google Scholar]
- Jeschke, J.M.; Kopp, M.; Tollrian, R. Predator functional responses: Discriminating between handling and digesting prey. Ecol. Monogr. 2002, 72, 95–112. [Google Scholar] [CrossRef]
- Bell, W.J. Searching behavior patterns in insects. Annu. Rev. Entomol. 1990, 35, 447–467. [Google Scholar] [CrossRef]
- Zhang, F.G.; Cai, X.M.; Xiu, C.L.; Wang, G.C.; Chen, Z.M. Predatory function of mulberry flower bug Orius sauteri on tea stick thrips Dendrothrips minowai adults. J. Plant Prot. 2023, 50, 668–675. [Google Scholar]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. The components of arthropod predation: I. The prey death rate. J. Anim. Ecol. 1976, 45, 135–164. [Google Scholar] [CrossRef]
- Qiu, H.; Fu, B.; He, S.; Liu, K. Functional response and predation preference of Orius tantillus to Megalurothrips usitatus. Chin. J. Biol. Control 2022, 38, 1443. [Google Scholar]
- Luo, Z.W.; Gong, X.N.; Yu, X.S.; Wang, X.S.; Shen, S.Q.; Long, Y.Q. Evaluation of predation effectiveness of Arma chinensis to Agriophara rhombata larvae. For. Pest Dis. 2023, 42, 19–25. [Google Scholar]
- Wang, L.P.; Wang, Y.M.; Du, J.P.; Zhang, G.A. Predation of amblyseius swirskii on tetranychus cinnabarinus nymphs. Chin. J. Biol. Control 2011, 27, 171–175. [Google Scholar]
- Osawa, N. Sex-dependent effects of sibling cannibalism on life history traits of the ladybird beetle Harmonia axyridis. Biol. J. Linn. Soc. 2002, 76, 349–360. [Google Scholar] [CrossRef]
- Getto, P.; Diekmann, O.; de Roos, A.M. On the (dis) advantages of cannibalism. J. Math. Biol. 2005, 51, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.X.; Lei, Z.Y.; Zhang, Q.; Fan, W.; Yin, H.H.; Xu, T.Y.; Chen, G.H.; Zhang, X.M. Predatory function of minute pirate bug Orius strigicollis against western flower thrips Frankliniella occidentalis adults and its intraspecific cannibalism behavior. J. Plant Prot. 2022, 49, 758–766. [Google Scholar]
- Schmitz, O.J. Effects of predator hunting mode on grassland ecosystem function. Science 2008, 319, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Wiegert, R.G.; Petersen, C.E. Energy transfer in insects. Annu. Entomol. 1983, 28, 455–486. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Deng, B.D.; Zhang, W.J.; Ma, G.L.; Gao, G.Z. Predation of Harmonia axyridis (Coleoptera: Coccinellidae) on two walnut aphids, Chromaphis juglandicola and Panaphis juglandis. J. Appl. Entomol. 2024, 61, 827–834. [Google Scholar]
- Chailleux, A.; Mohl, E.K.; Teixeira Alves, M.; Messelink, G.J.; Desneux, N. Natural enemy-mediated indirect interactions among prey species: Potential for enhancing biocontrol services in agroecosystems. Pest Manag. Sci. 2014, 70, 1769–1779. [Google Scholar] [CrossRef]
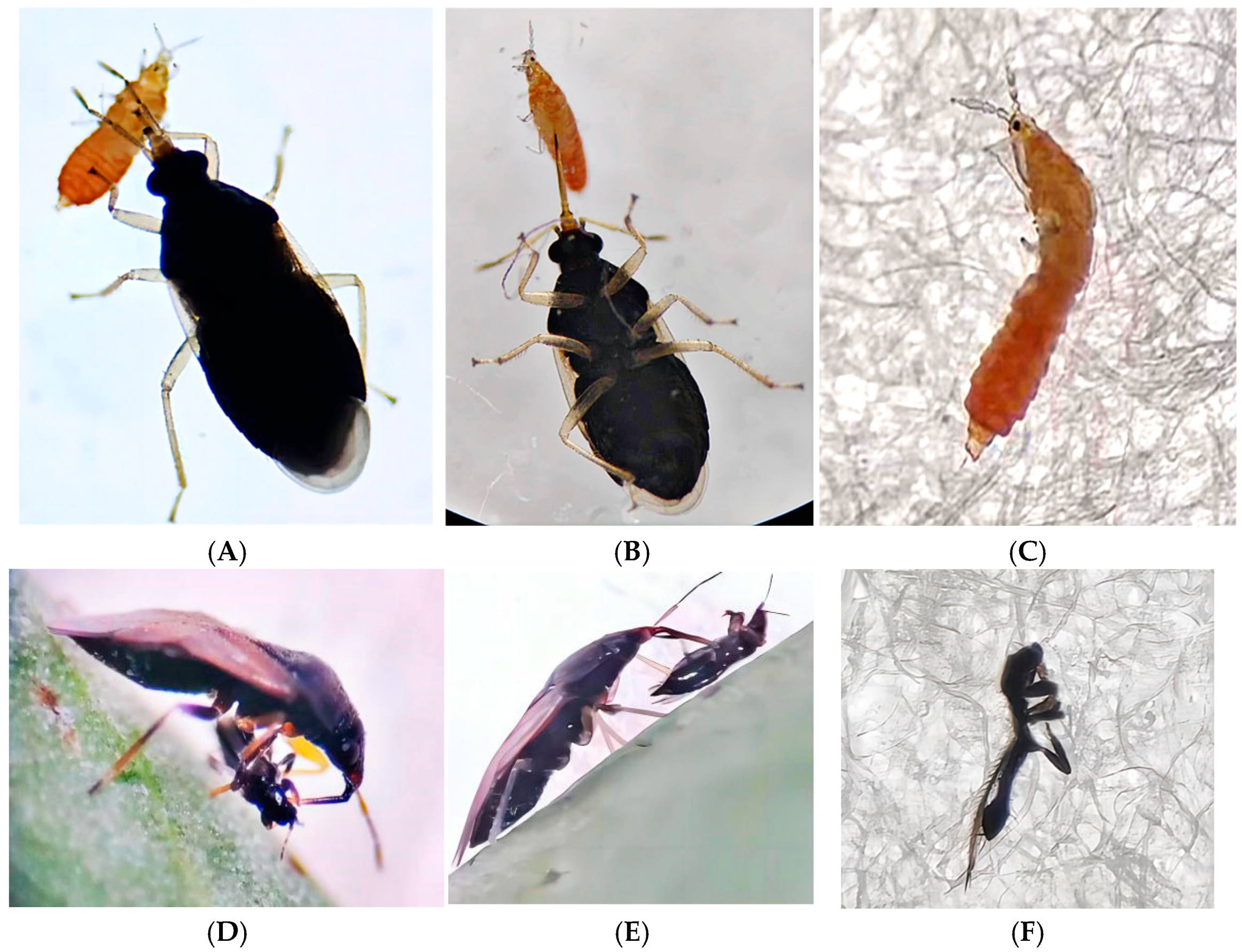
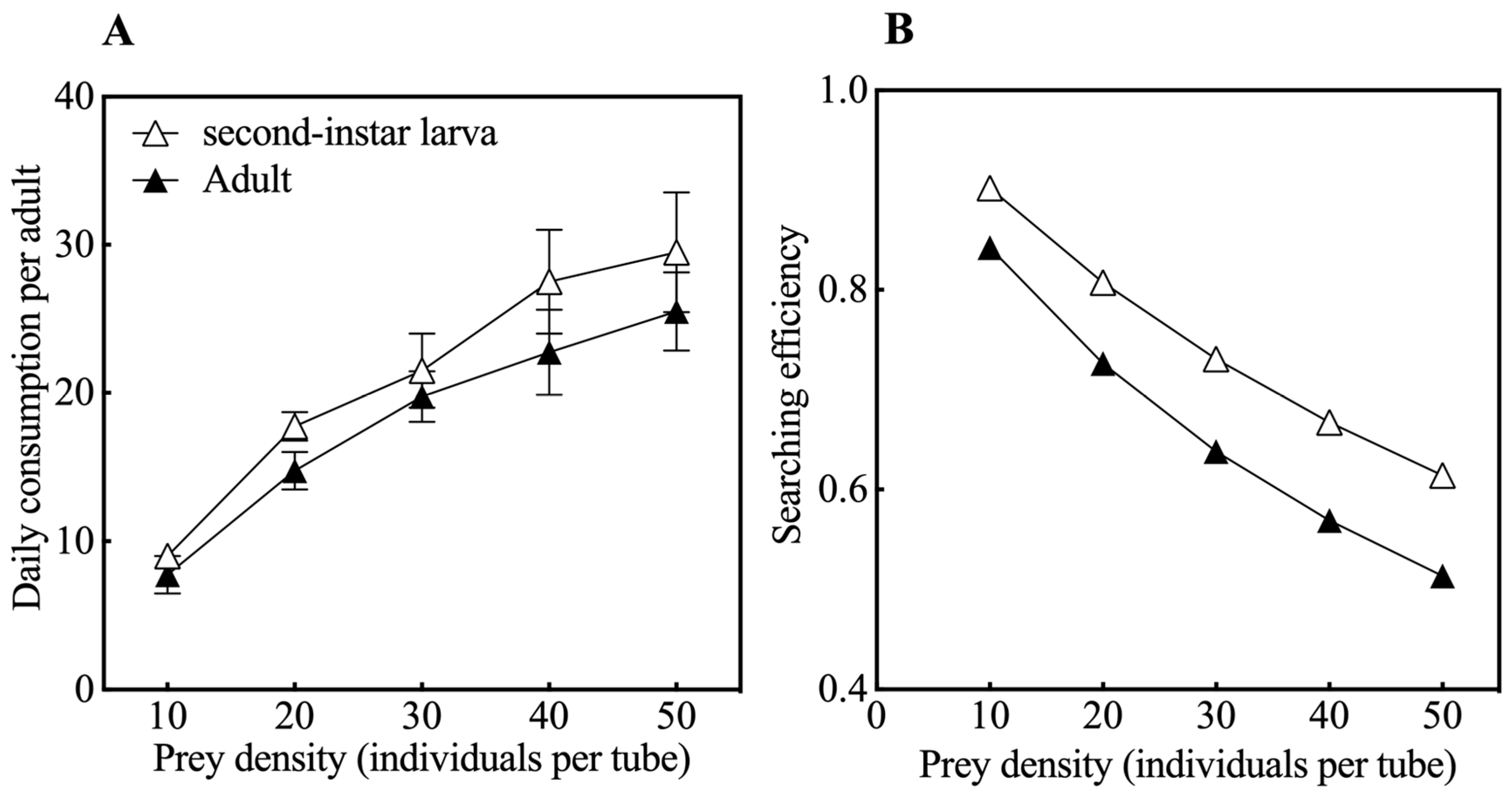
| M. usitatus’ Age | Predation Functional Response Equation | R2 | Instantaneous Attack Rate (a) | Th Handling Time (d) | Predation Capacity (a/Th) | Daily Maximum Prey Consumed (1/Th) |
|---|---|---|---|---|---|---|
| Second-instar larva | Na = 1.022N/(1 + 0.013N) | 0.929 | 1.022 | 0.013 | 78.62 | 76.92 |
| Adult | Na = 1.003N/(1 + 0.019N) | 0.921 | 1.003 | 0.019 | 52.79 | 52.62 |
| Density of Predators (Individuals per Tube) (p) | Predation Capacity (Number of Preys/Predators) (Na) | Predation Rate (E) | Intensity of Apportioned Competition (I) | F | df | p |
|---|---|---|---|---|---|---|
| 1 | 39.25 ± 2.17 b | 0.393 | 0.000 | 12.896 | 19 | 0.017 |
| 2 | 23.75 ± 2.90 c | 0.238 | 0.395 | |||
| 3 | 18.42 ± 1.25 a | 0.184 | 0.531 | |||
| 4 | 14.13 ± 1.84 a | 0.141 | 0.640 | |||
| 5 | 11.60 ± 2.42 a | 0.116 | 0.704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Cheng, Y.; Cui, Y.; Xiong, C.; Cao, Z.; Wang, Y.; Zhang, R.; Liu, C.; Sun, W.; Ban, L.; et al. An Evaluation of the Predatory Function of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Insects 2025, 16, 236. https://doi.org/10.3390/insects16030236
Fu Z, Cheng Y, Cui Y, Xiong C, Cao Z, Wang Y, Zhang R, Liu C, Sun W, Ban L, et al. An Evaluation of the Predatory Function of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Insects. 2025; 16(3):236. https://doi.org/10.3390/insects16030236
Chicago/Turabian StyleFu, Zuying, Yuanrun Cheng, Yifan Cui, Changyu Xiong, Ziyu Cao, Ying Wang, Rong Zhang, Chang Liu, Wei Sun, Liping Ban, and et al. 2025. "An Evaluation of the Predatory Function of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae)" Insects 16, no. 3: 236. https://doi.org/10.3390/insects16030236
APA StyleFu, Z., Cheng, Y., Cui, Y., Xiong, C., Cao, Z., Wang, Y., Zhang, R., Liu, C., Sun, W., Ban, L., Tan, Y., & Wei, S. (2025). An Evaluation of the Predatory Function of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Insects, 16(3), 236. https://doi.org/10.3390/insects16030236






