Projecting Current and Future Habitat Suitability of the Pepper Weevil, Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae), in China: Implications for the Pepper Industry
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Screening of Species Geographic Distribution Points
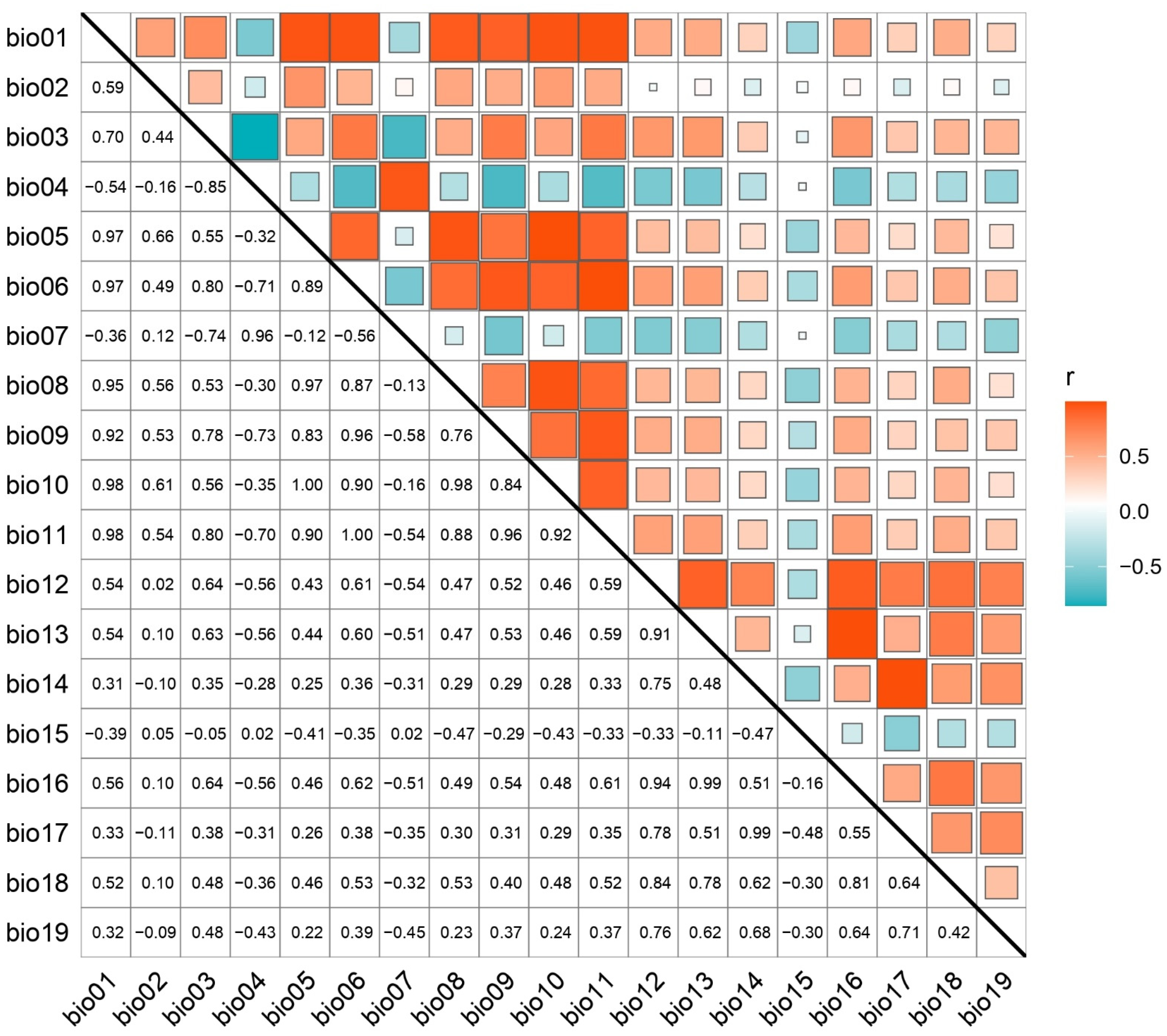
2.2. Environmental Variables and Selection Criteria
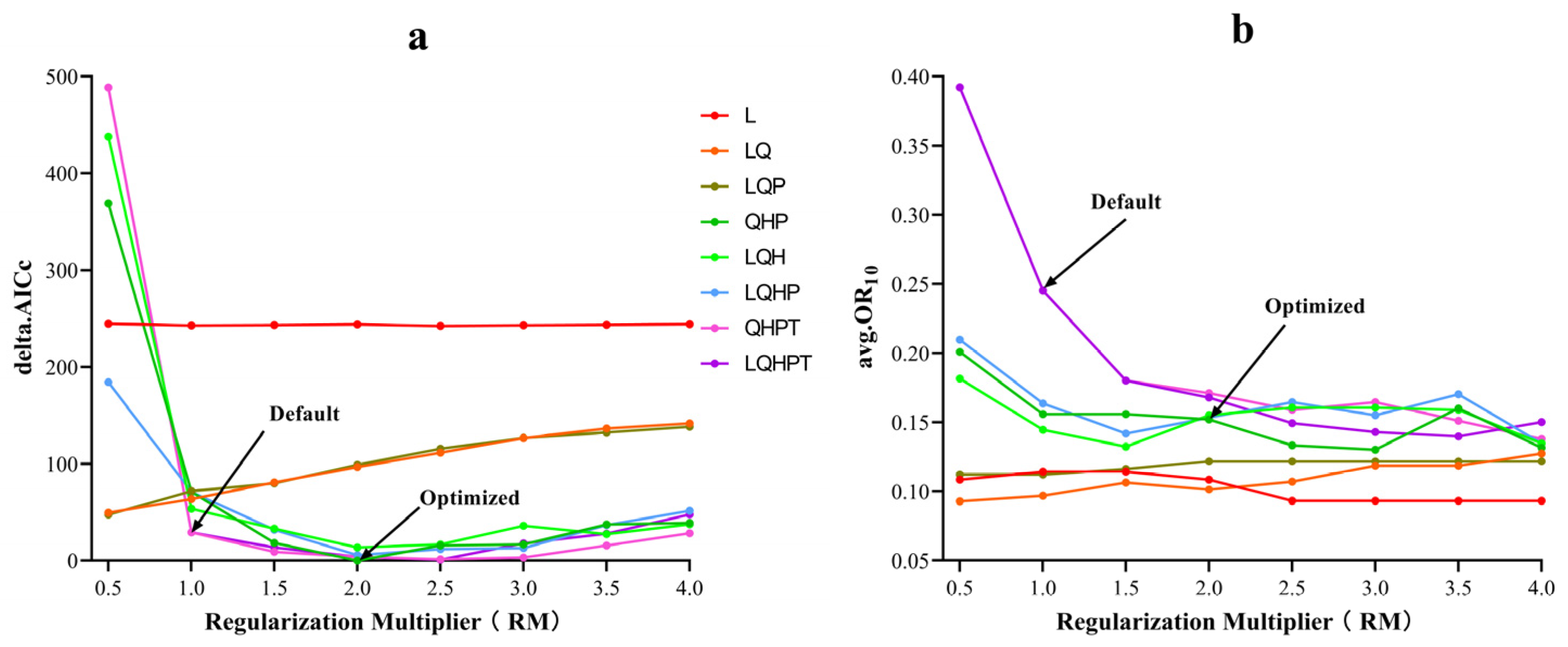
2.3. MaxEnt Model Optimization
2.4. MaxEnt Model Evaluation and Suitable Habitats Prediction
2.5. Centroid Shifts in Suitable Habitats
3. Results
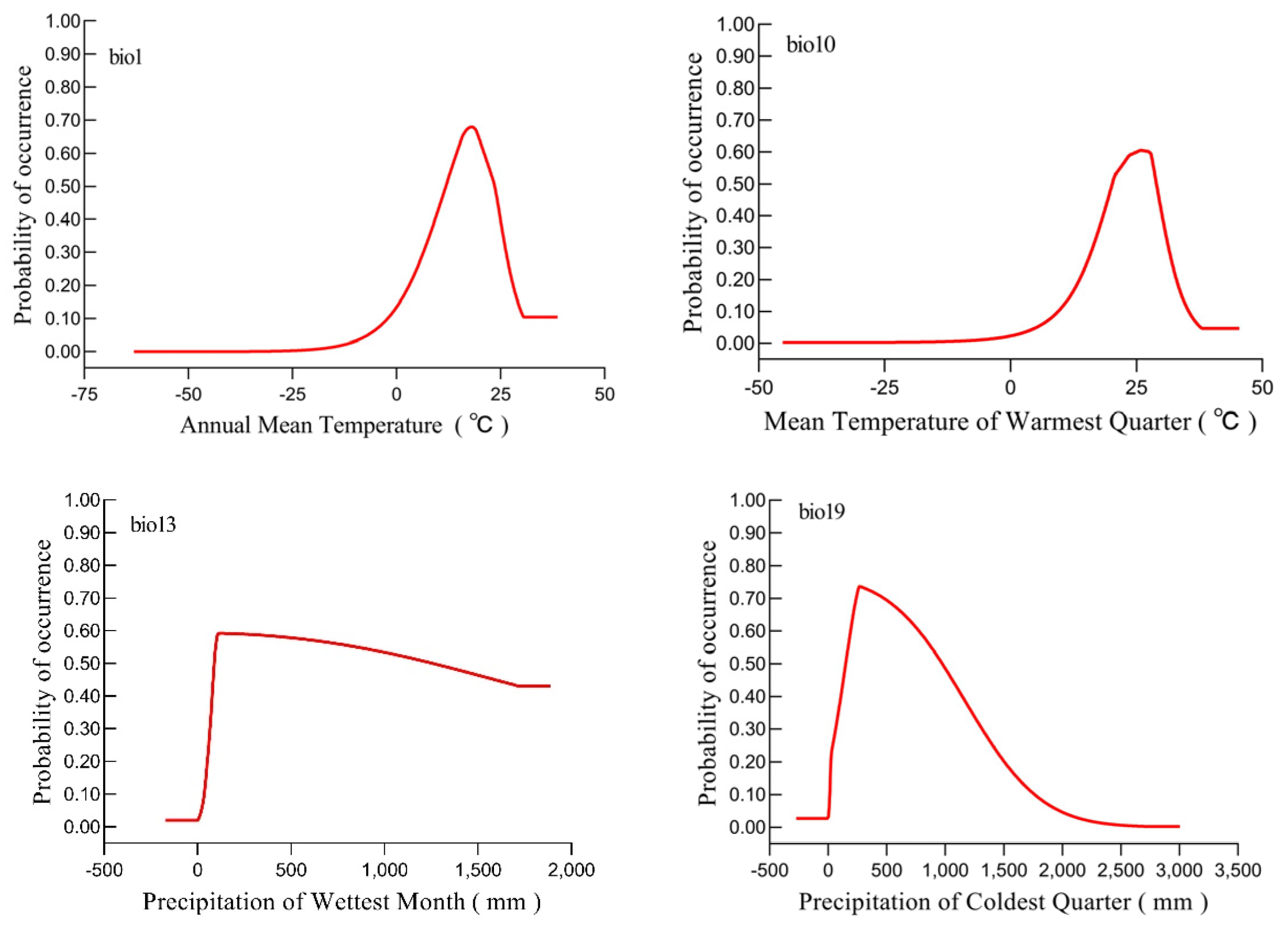
3.1. Key Bioclimate Variables and Response Curves
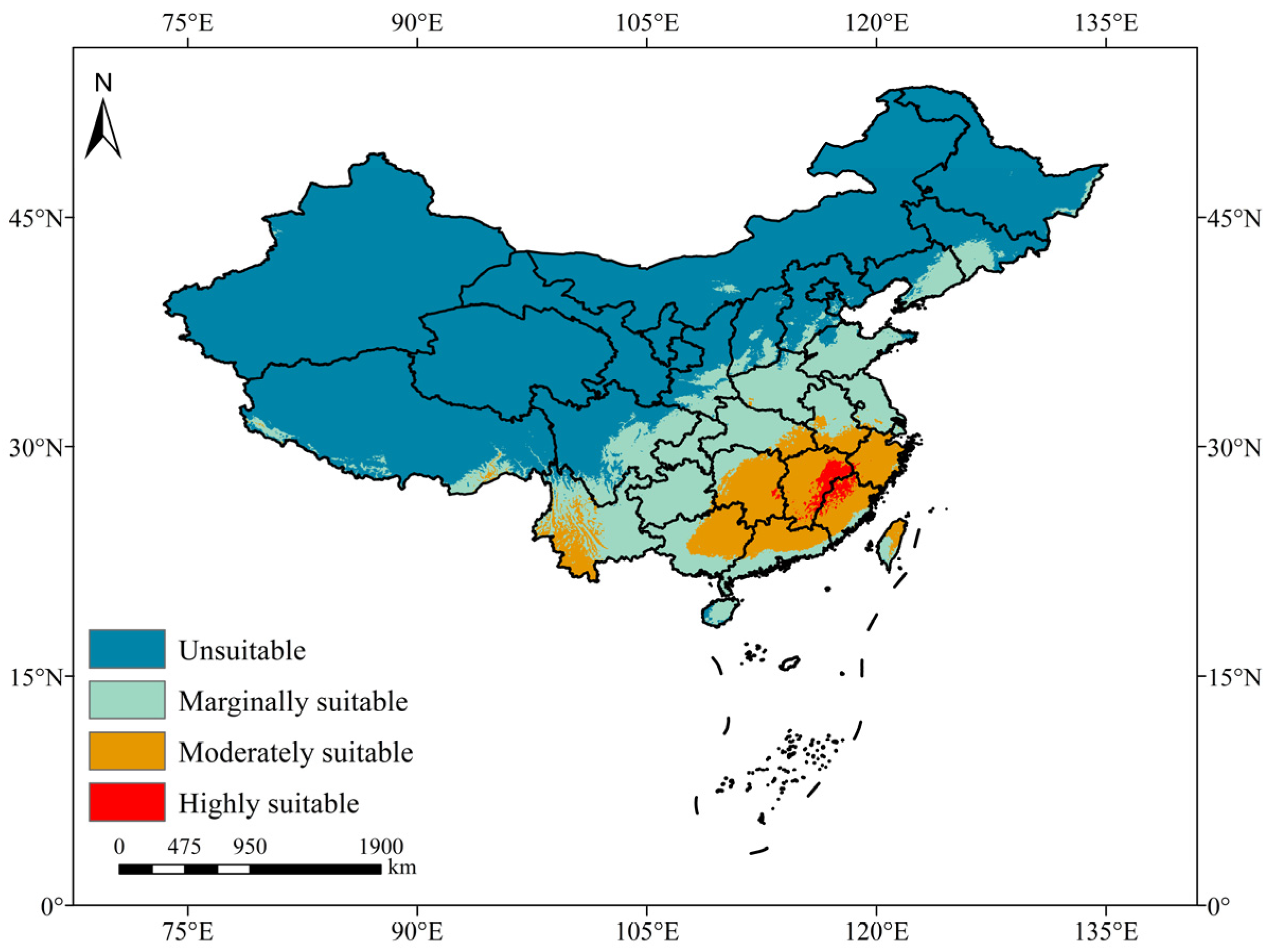
3.2. Current Distribution Prediction
3.3. Future Distribution Prediction
3.4. Centroid Change in the Suitable Habitats of A. eugenii
4. Discussion
4.1. Dominant Bioclimatic Variables Influencing the Suitable Areas of A. eugenii
4.2. Alterations in Suitable Habitats of A. eugenii
4.3. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ring, M.J.; Lindner, D.; Cross, E.F.; Schlesinger, M.E. Causes of the global warming observed since the 19th century. Atmos. Clim. Sci. 2012, 2, 401–415. [Google Scholar] [CrossRef]
- Stange, E.E.; Ayres, M.P. Climate change impacts: Insects. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–7. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I. Climate change 2021–the physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 2391. [Google Scholar]
- Nedvěd, O. Temperature, Effects on Development and Growth; Encyclopedia of Insects; Academic Press: New York, NY, USA, 2009; Chapter 252. [Google Scholar]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A.; Lovett, M.M.E. Some like it hot: The effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 2011, 214, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, A.M.; Harding, S.; Krokene, P.; Lange, H.; Lindelöw, Å.; Økland, B.; Ravn, H.P.; Schroeder, L.M. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Clim. Change 2011, 109, 695–718. [Google Scholar] [CrossRef]
- Huang, J.; Li, J. Effects of climate change on overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Int. J. Biometeorol. 2015, 59, 863–876. [Google Scholar] [CrossRef]
- Klockmann, M.; Fischer, K. Strong reduction in diapause survival under warm and humid overwintering conditions in a temperate-zone butterfly. Popul. Ecol. 2019, 61, 150–159. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 404. [Google Scholar] [CrossRef]
- Sario, S.; Melo-Ferreira, J.; Santos, C. Winter is (Not) coming: Is climate change helping Drosophila suzukii overwintering? Biology 2023, 12, 907. [Google Scholar] [CrossRef]
- Raza, M.M.; Khan, M.A.; Arshad, M.; Sagheer, M.; Sattar, Z.; Shafi, J.; Haq, E.U.; Ali, A.; Aslam, U.; Mushtaq, A.; et al. Impact of global warming on insects. Arch. Phytopathol. Plant Prot. 2015, 48, 84–94. [Google Scholar] [CrossRef]
- Au, F.T.; Bonebrake, T.C. Increased suitability of poleward climate for a tropical butterfly (Euripus nyctelius) (Lepidoptera: Nymphalidae) accompanies its successful range expansion. J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Iannella, M.; D’Alessandro, P.; Biondi, M. Forecasting the spread associated with climate change in Eastern Europe of the invasive Asiatic flea beetle, Luperomorpha xanthodera (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2020, 117, 130–138. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Kean, J.M.; Phillips, C.B.; Senay, S.D.; Acosta, H.; Haye, T. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 2017, 90, 1033–1043. [Google Scholar] [CrossRef]
- Daly, E.Z.; Gerlich, H.S.; Frenot, Y.; Høye, T.T.; Holmstrup, M.; Renault, D. Climate change helps polar invasives establish and flourish: Evidence from long-term monitoring of the blowfly Calliphora vicina. Biology 2023, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.C.; Davis, A.C.; Campbell, R.E. The pepper weevil. In Technical Bulletin United States Department of Agriculture; United States Department of Agriculture: Washington, DC, USA, 1934. [Google Scholar]
- Patrock, R.J.; Schuster, D.J. Feeding, oviposition and development of the pepper weevil, (Anthonomus eugenii Cano), on selected species of Solanaceae. Trop. Pest Manag. 1992, 38, 65–69. [Google Scholar] [CrossRef]
- Aguilar, R.; Servín, R. Alternate wild host of the pepper weevil, Anthonomus eugenii Cano in Baja California Sur, Mexico. Southwest. Entomol. 2000, 25, 153–154. [Google Scholar]
- Addesso, K.M.; Mcauslane, H.J. Pepper weevil attraction to volatiles fromhost and nonhost plants. Environ. Entomol. 2009, 38, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Patrock, R.J.; Schuster, D.J.; Ozaki, H.; Everett, P.H. Field survey for the pepper weevil, Anthonomus eugenii, on nightshade. Proc. Florida State Hort. Soc. 1987, 100, 217–220. [Google Scholar]
- van de Vossenberg, B.T.; Warbroek, T.; Ingerson-Mahar, J.; Waalwijk, C.; Van Der Gouw, L.P.; Eichinger, B.; Loomans, A.J. Tracking outbreak populations of the pepper weevil Anthonomus eugenii (Coleoptera; Curculionidae) using complete mitochondrial genomes. PLoS ONE 2019, 14, e0221182. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.R.; Clausen, C.P.; Debach, P.; Goeden, R.D.; Oatman, E.R. Introduced parasites and predators of arthropod pests and weeds: A world review. In Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1978; p. 480. [Google Scholar]
- Abreu, E.; Cruz, C. The occurrence of the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae) in Puerto Rico. J. Agric. Univ. Puerto Rico 1985, 69, 223–224. [Google Scholar] [CrossRef]
- Labbe, R.M.; Hilker, R.; Gagnier, D.; McCreary, C.; Gibson, G.A.P.; Fernandez-Triana, J.; Mason, P.G.; Gariepy, T.D. Natural enemies of Anthonomus eugenii (Coleoptera: Curculionidae) in Canada. Can. Entomol. 2018, 150, 404–411. [Google Scholar] [CrossRef]
- Speranza, S.; Colonnelli, E.; Garonna, A.P.; Laudonia, S. First record of Anthonomus eugenii (Coleoptera: Curculionidae) in Italy. Fla. Entomol. 2014, 97, 844–845. [Google Scholar] [CrossRef]
- Authority, E.F.S.; van der Gaag, D.J.; Schenk, M.; Loomans, A.; Delbianco, A.; Vos, S. Pest survey card on Anthonomus eugenii. EFSA Support. Publ. 2020, 17, 1887E. [Google Scholar]
- Chabaane, Y.; Haseeb, M.; Benrey, B. Domestication of chili pepper has altered fruit traits affecting the oviposition and feeding behavior of the pepper weevil. Insects 2021, 12, 630. [Google Scholar] [CrossRef]
- Fernández, D.C.; VanLaerhoven, S.L.; McCreary, C.; Labbe, R.M. An overview of the pepper weevil (Coleoptera: Curculionidae) as a Pest of greenhouse peppers. J. Integr. Pest Manag. 2020, 11, 26. [Google Scholar] [CrossRef]
- Chiu, G. Screening out more than pepper weevils. Greenhouse Can. 2020, 3, 40. [Google Scholar]
- Rodriguez-Leyva, E. Life history of Triaspis eugenii Wharton & Lopez-Martinez (Hymenoptera: Braconidae) and Evaluation of Its Potential for Biological Control of Pepper Weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2006. [Google Scholar]
- Labbé, R.M.; Gagnier, D.; Rizzato, R.; Tracey, A.; McCreary, C. Assessing new tools for management of the pepper weevil (Coleoptera: Curculionidae) in greenhouse and field pepper crops. J. Econ. Entomol. 2020, 113, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, V.O.; Seal, D.R.; Liburd, O.O.E.; Martini, X.; Meru, G. Integrated approach using insecticides in combination with reflective plastic mulch for the management of pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae). Environ. Entomol. 2023, 52, 391–398. [Google Scholar] [CrossRef]
- ServÍN-Villegas, R.; GarcÍA-HernÁNdez, J.L.; Tejas-Romero, A.; MartÍNez-Carrillo, J.L.; Toapanta, M.A. Susceptibility of pepper weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) to seven insecticides in rural areas of Baja California Sur, Mexico. Acta Zool. Mex. 2008, 24, 45–54. [Google Scholar] [CrossRef][Green Version]
- Wei, X.J.; Xu, D.P.; Liu, Q.W.; Wu, Y.H.; Zhuo, Z.H. Predicting the potential distribution range of Batocera horsfieldi under CMIP6 climate change using the MaxEnt model. J. Econ. Entomol. 2024, 117, 187–198. [Google Scholar] [CrossRef]
- Deng, C.; Zhong, Q.; Shao, D.; Ren, Y.; Li, Q.; Wen, J.; Li, J. Potential suitable habitats of chili pepper in China under climate change. Plants 2024, 13, 1027. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Wang, X.; Wang, L.; Khoso, A.G.; Liu, D. Climate change and international trade can exacerbate the invasion risk of the red imported fire ant Solenopsis invicta around the globe. Entomol. Gen. 2023, 43, 315–323. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Yoon, S.; Lee, W.-H. Methodological analysis of bioclimatic variable selection in species distribution modeling with application to agricultural pests (Metcalfa pruinosa and Spodoptera litura). Comput. Electron. Agric. 2021, 190, 106430. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Li, X.; Wang, R. Distribution and invasion risk assessment of Oryctes rhinoceros (L.) in China under changing climate. J. Appl. Entomol. 2022, 146, 385–395. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, 46221. [Google Scholar] [CrossRef]
- Bowen, A.K.M.; Stevens, M.H.H. Temperature, topography, soil characteristics, and NDVI drive habitat preferences of a shade-tolerant invasive grass. Ecol. Evol. 2020, 10, 10785–10797. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Mao, J.; Meng, F.; Song, Y.; Li, D.; Ji, Q.; Hong, Y.; Lin, J.; Cai, P. Forecasting the expansion of Bactrocera tsuneonis (Miyake) (Diptera: Tephritidae) in China Using the MaxEnt Model. Insects 2024, 15, 417. [Google Scholar] [CrossRef]
- Song, Z.; Fan, G.; Deng, C.; Duan, G.; Li, J. Predicting the distribution of Neoceratitis asiatica (Diptera: Tephritidae), a primary pest of goji berry in China, under climate change. Insects 2024, 15, 558. [Google Scholar] [CrossRef]
- Li, M.; Jin, Z.; Qi, Y.; Zhao, H.; Yang, N.; Guo, J.; Chen, B.; Xian, X.; Liu, W. Risk assessment of Spodoptera exempta against food security: Estimating the potential global overlapping areas of wheat, maize, and rice under climate change. Insects 2024, 15, 348. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, S.; Xu, C.; Wei, H.; Guo, K.; Xu, R.; Qiao, H.; Lu, P. Prediction of potential distribution of Carposina coreana in China under the current and future climate change. Insects 2024, 15, 411. [Google Scholar] [CrossRef]
- Eller, F.J.; Palmquist, D.E. Factors Affecting pheromone production by the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae) and collection efficiency. Insects 2014, 5, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Lechuga-Paredes, P.; Segura-Leon, O.L.; Cibrian-Tovar, J.; Torres-Huerta, B.; Velazquez-Gonzalez, J.C.; Cruz-Jaramillo, J.L. Odorant-binding and chemosensory proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and their tissue expression. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Kanchupati, N.M.; Seal, D.R.; Jangra, S.; Schaffer, B.; Liburd, O.E.; Beuzelin, J. Influence of parental age on reproductive potential and embryogenesis in the pepper weevil, Anthonomus eugenii (Cano) (Col.: Curculionidae). Insects 2024, 15, 562. [Google Scholar] [CrossRef]
- Wu, P.; Haseeb, M.; Diedrick, W.; Ouyang, H.; Zhang, R.; Kanga, L.H.B.; Legaspi, J.C. Influence of plant direction, layer, and spacing on the infestation levels of Anthonomus eugenii (Coleoptera: Curculionidae) in open jalapeno pepper fields in North Florida. Fla. Entomol. 2019, 102, 501–508. [Google Scholar] [CrossRef]
- Fernández, D.C.; VanLaerhoven, S.L.; Rodríguez-Leyva, E.; Zhang, Y.M.; Labbé, R. Population structure and genetic diversity of the pepper weevil (Coleoptera: Curculionidae) using the COI barcoding region. J. Insect Sci. 2022, 22, 25. [Google Scholar] [CrossRef]
- Adeleye, V.O.; Seal, D.R.; Martini, X.; Meru, G.; Liburd, O.E. Characterization of the spatial distribution of the pepper weevil, Anthonomus eugenii Cano (Col.: Curculionidae), in pepper fields in South Florida. Insects 2024, 15, 579. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.V.; Labbe, R.; Scott-Dupree, C. Assessing the sterility and quality of gamma-irradiated pepper weevils, Anthonomus eugenii (Coleoptera: Curculionidae), toward the development of the sterile insect technique. Pest Manag. Sci. 2024, 80, 1692–1701. [Google Scholar] [CrossRef]
- Leo, S.; Labbe, R.; Scott-Dupree, C. How plant and insect host characteristics affect pepper weevil Anthonomus eugenii parasitism efficacy by the pteromalid Jaliscoa hunteri. BioControl 2024, 69, 589–601. [Google Scholar] [CrossRef]
- Rubio-Aragón, W.A.; López-Urquídez, G.A.; PayánArzapalo, M.A.; Cruz-Mendivil, A.; Hernández-Verdugo, S.; RetesManjarrez, J.E. A rapid screening method for resistance to Anthonomus eugenii (Coleoptera: Curculionidae) in Capsicum (Solanaceae) spp. plants. Fla. Entomol. 2022, 105, 101–107. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Ji, W.; Gao, G.; Wei, J. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, Y.; Chang, N.; Gao, T.; Ma, D.; Li, C.; Liu, Q. Projecting the potential distribution of Glossina morsitans (Diptera: Glossinidae) under climate change using the MaxEnt model. Biology 2021, 10, 1150. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model. Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Qiao, H.; Soberón, J.; Peterson, A.T. No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference—Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Moreno, R.; Zamora, R.; Ramon Molina, J.; Vasquez, A.; Angel Herrera, M. Predictive modeling of microhabitats for endemic birds in South Chilean temperate forests using Maximum entropy (Maxent). Ecol. Inform. 2011, 6, 364–370. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Castex, V.; Beniston, M.; Calanca, P.; Fleury, D.; Moreau, J. Pest management under climate change: The importance of understanding tritrophic relations. Sci. Total Environ. 2018, 616, 397–407. [Google Scholar] [CrossRef]
- Toapanta, M.A.; Schuster, D.J.; Stansly, P.A. Development and life history of Anthonomus eugenii (Coleoptera: Curculionidae) at constant temperatures. Environ. Entomol. 2005, 34, 999–1008. [Google Scholar] [CrossRef]
- Fernández, D.C. An Integrated Approach to Understanding Anthonomus eugenii Cano (Coleoptera: Curculionidae): An Exotic Pest of Greenhouse and Field Pepper Crops. Ph.D. Thesis, University of Windsor, Windsor, ON, Canada, 2021. [Google Scholar]
- Staley, J.T.; Hodgson, C.J.; Mortimer, S.R.; Morecroft, M.D.; Masters, G.J.; Brown, V.K.; Taylor, M.E. Effects of summer rainfall manipulations on the abundance and vertical distribution of herbivorous soil macro-invertebrates. Eur. J. Soil Biol. 2007, 43, 189–198. [Google Scholar] [CrossRef]
- Qian, W.; Ding, T.; Hu, H.; Lin, X.; Qin, A. An overview of dry-wet climate variability among monsoon-westerly regions and the monsoon northernmost marginal active zone in China. Adv. Atmos. Sci. 2009, 26, 630–641. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e01107. [Google Scholar] [CrossRef]
- Hu, J.; Pan, C.; Gu, S. Effects of climate conditions on hot pepper yield in northern Guizhou and prediction model of meteorological yield. Southwest China J. Agric. Sci. 2024, 37, 1322–1328. [Google Scholar]
- Yang, T.; Lu, X.; Xiao, D.; Han, X. Comprehensive sesponse study of climate and hydrological system in southwest Karst region under Global Climate Change—A case study of Guizhou Province. J. Anhui Agric. Sci. 2024, 52, 195–199. [Google Scholar]
- Lin, Q.; Xin, Z.; Kong, L.; Wang, X.; Yang, X.; He, W. Current situation of pepper industry development and breeding countermeasures in China. J. China Agric. Univ. 2023, 28, 82–95. [Google Scholar]
- Poland, T.M.; Rassati, D. Improved biosecurity surveillance of non-native forest insects: A review of current methods. J. Pest Sci. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Alborn, H.T. Attraction of pepper weevil to volatiles from damaged pepper plants. Entomol. Exp. Appl. 2011, 138, 1–11. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]


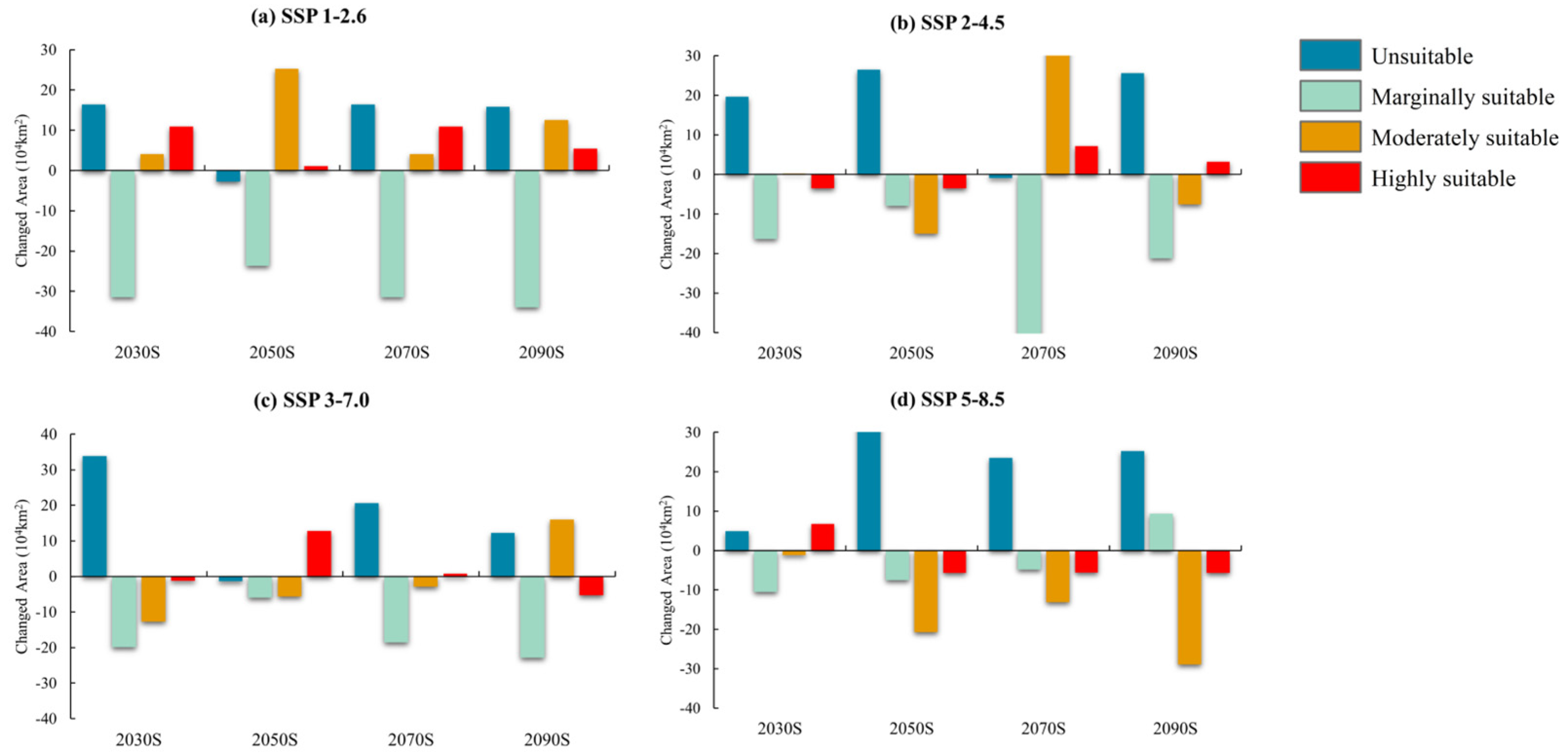

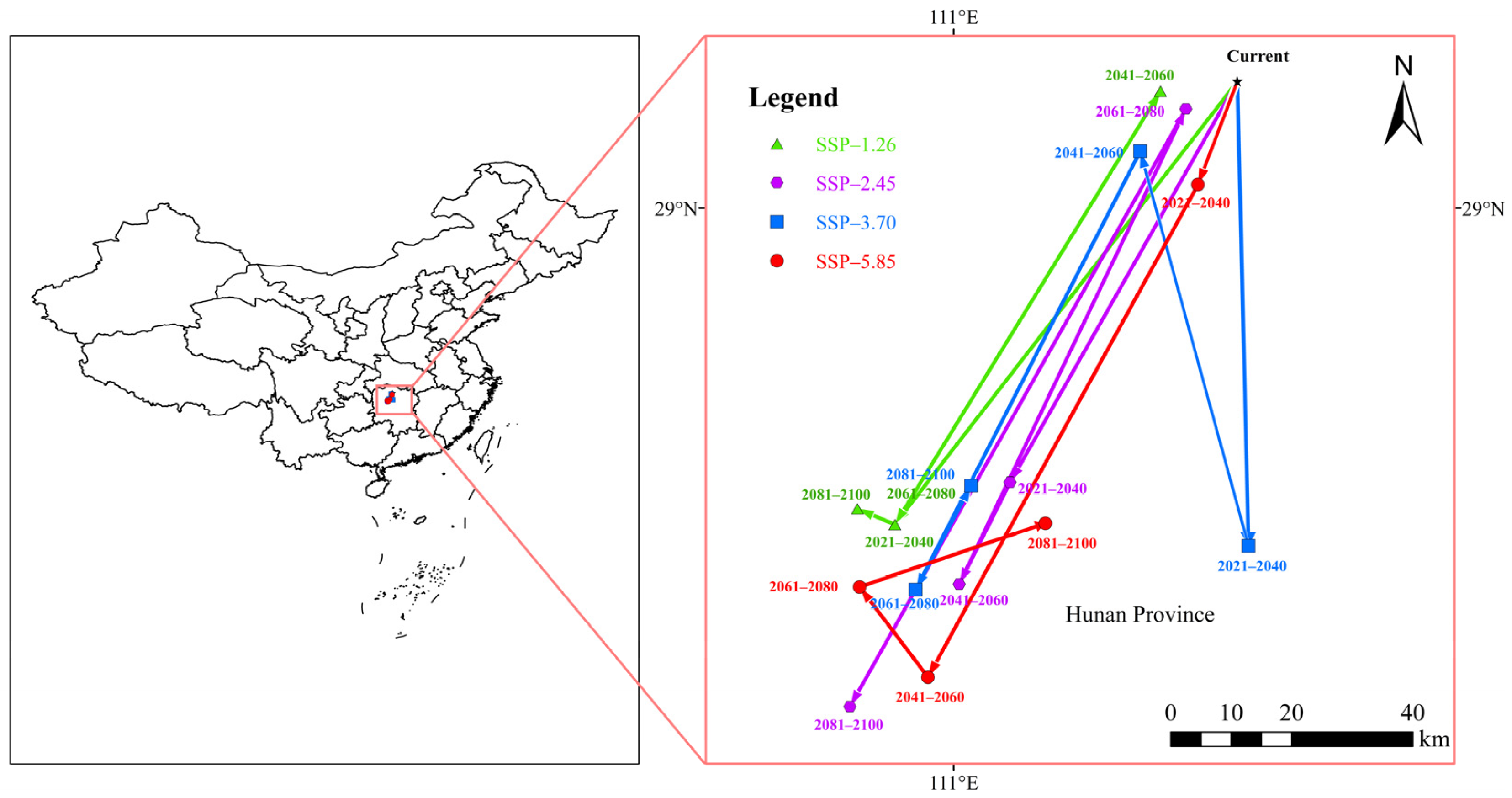
| Variables | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|
| Bio1 | 52.2 | 43.1 |
| Bio19 | 27.6 | 27.3 |
| Bio2 | 11.3 | 11.8 |
| Bio13 | 8 | 7.3 |
| Bio10 | 0.8 | 10.6 |
| Scenario | Decade | Predicted Area (104 km2) | Comparison with Current Distribution (%) | ||||
|---|---|---|---|---|---|---|---|
| High Suitable | Medium Suitable | Low Suitable | High Suitable | Medium Suitable | Low Suitable | ||
| - | Current | 5.68 | 83.43 | 184.63 | —— | —— | —— |
| SSP1-2.6 | 2030s | 16.60 | 87.56 | 153.18 | 192.30% | 4.94% | −17.04% |
| 2050s | 6.83 | 108.69 | 160.95 | 20.29% | 30.28% | −12.83% | |
| 2070s | 16.60 | 87.56 | 153.18 | 192.30% | 4.94% | −17.04% | |
| 2090s | 11.15 | 96.03 | 150.69 | 96.33% | 15.10% | −18.38% | |
| SSP2-4.5 | 2030s | 2.17 | 83.65 | 168.29 | −61.83% | 0.27% | −8.85% |
| 2050s | 2.16 | 68.49 | 176.65 | −62.01% | −17.90% | −4.32% | |
| 2070s | 12.88 | 117.75 | 144.09 | 126.68% | 41.13% | −21.96% | |
| 2090s | 8.90 | 75.81 | 163.44 | 56.60% | −9.13% | −11.48% | |
| SSP3-7.0 | 2030s | 4.47 | 70.72 | 164.73 | −21.24% | −15.23% | −10.78% |
| 2050s | 18.53 | 77.84 | 178.72 | 226.22% | −6.70% | −3.20% | |
| 2070s | 6.50 | 80.58 | 166.07 | 14.49% | −3.41% | −10.05% | |
| 2090s | 0.35 | 99.38 | 161.75 | −93.92% | 19.12% | −12.40% | |
| SSP5-8.5 | 2030s | 12.41 | 82.25 | 174.18 | 118.43% | −1.41% | −5.66% |
| 2050s | 0.01 | 62.73 | 177.10 | −99.76% | −24.81% | −4.08% | |
| 2070s | 0.05 | 70.33 | 179.84 | −99.05% | −15.71% | −2.60% | |
| 2090s | 0.00 | 54.56 | 193.95 | −100.00% | −34.60% | 5.05% | |
| Current Centroid Location | Climate Scenario | Future Centroid Location | |||
|---|---|---|---|---|---|
| 2030s | 2050s | 2070s | 2090s | ||
| Changde City, Hunan Province (111.421° E, 29.188° N) | SSP1-2.6 | Huaihua City (110.912° E, 28.528° N) | Changde City (111.308° E, 29.172° N) | Huaihua City (110.912° E, 28.528° N) | Huaihua City (110.857° E, 28.552° N) |
| SSP2-4.5 | Changde City (111.085° E, 28.593° N) | Yiyang City (111.008° E, 28.44° N) | Changde City (111.345° E, 29.147° N) | Yiyang City (110.846° E, 28. 26° N) | |
| SSP3-7.0 | Yiyang City (111.438° E, 28.498° N) | Changde City (111.277°E, 29.084°N) | Changde City (110.943° E, 28.433° N) | Huaihua City (111.026° E, 28.588° N) | |
| SSP5-8.5 | Changde City (111.363° E, 29.036° N) | Yiyang City (110.962° E, 28.302° N) | Huaihua City (110.86° E, 28.437° N) | Changde City (111.137° E, 28.532° N) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Mao, J.; Wang, W.; Liu, R.; Xie, Q.; Su, S.; Wang, Z.; Song, Y.; Hong, Y.; Cai, P. Projecting Current and Future Habitat Suitability of the Pepper Weevil, Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae), in China: Implications for the Pepper Industry. Insects 2025, 16, 227. https://doi.org/10.3390/insects16020227
Li Q, Mao J, Wang W, Liu R, Xie Q, Su S, Wang Z, Song Y, Hong Y, Cai P. Projecting Current and Future Habitat Suitability of the Pepper Weevil, Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae), in China: Implications for the Pepper Industry. Insects. 2025; 16(2):227. https://doi.org/10.3390/insects16020227
Chicago/Turabian StyleLi, Qisong, Jianxiang Mao, Weifeng Wang, Ruijun Liu, Qiufan Xie, Shiyao Su, Zhong Wang, Yunzhe Song, Yongcong Hong, and Pumo Cai. 2025. "Projecting Current and Future Habitat Suitability of the Pepper Weevil, Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae), in China: Implications for the Pepper Industry" Insects 16, no. 2: 227. https://doi.org/10.3390/insects16020227
APA StyleLi, Q., Mao, J., Wang, W., Liu, R., Xie, Q., Su, S., Wang, Z., Song, Y., Hong, Y., & Cai, P. (2025). Projecting Current and Future Habitat Suitability of the Pepper Weevil, Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae), in China: Implications for the Pepper Industry. Insects, 16(2), 227. https://doi.org/10.3390/insects16020227






