Appendix A. List of Ambulyx Specimens Examined
Names of institutions in which specimens are deposited are listed after private collections and are abbreviated as follows: ZHJ—collection of Zhuo-Heng Jiang (Kunming, China); ZBX—collection of Zhen-Bang Xu (Beijing, China); CQL—collection of Chang-Qiu Liu (Guilin, China); DRC—collection of De-Rao Chou (Shanghai, China); KIZAS—collections of the Kunming Institute of Zoology, Chinese Academy of Science (Kunming, Yunnan, China); NHMUK—collection of the Natural History Museum (London, U.K.); NWAU—collections of the Northwest Agriculture and Forestry University (Shaanxi, China); SM—collections of the Sphingidae Museum (Příbram, Czech Republic).
♂, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2018 VII-23, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Rare Botanical Garden (1200 m), Ruili, Yunnan, China, 2007-VII-2, Xiao-Chen Zhang leg. [NWAU].
2♂♂, Donghe Township (560 m), Taitung County, Taiwan, China, 2018 VII-2, Wen-Yi Chou leg. [ZHJ]; 5♂♂, Nantou County, Taiwan, China, 2023 V-22, Local collector leg. [ZHJ]; 3♂♂, Puli, Nantou County, Taiwan, China, VI-8, Schnitzler leg. [SM]; ♀, Puli, Nantou County, Taiwan, China, VI-3, Schnitzler leg. [SM].
4♂♂, ♀, Yintiaoling Nature Reserve (1270 m), Wuxi County, Chongqing, China, 2022 VII-23, Zhuo-Heng Jiang leg. [ZHJ]; 4♂♂, 2♀♀, Ziqiu Township (1473 m), Changyang County, Hubei, China, 2022 V-30, Zi-Hao Shen leg. [ZHJ]; ♂, ♀, Mt. Wuyishan (1500 m), Jiangxi, China, 2005-VI-VII, Zhi-Yuan Qi leg. [SM]; ♂, Mt. Jiajinshan (1800 m), Xiaojin County, Sichuan, China, 2016 V-3, Zhi-Yuan Qi leg. [ZHJ]; ♀, Mentougou (1210 m), Beijing, China, 2018 VII-13, Kun Li leg. [ZHJ]; ♀, Baotianman (1170 m), NanYang, Henan, China, 2020 VI-19, Dan-Dan Xu leg. [ZHJ].
HOLOTYPE: ♂, Tacheng (2350 m), Weixi County, Yunnan, China, 2023 V-13, local collector leg. [KIZAS 0133744]. PARATYPES: 4 ♂♂, Tacheng (2510 m), Weixi County, Yunnan, China, 2023 V-17, local collector leg. [ZHJ]; 2♂♂, ♀, Tacheng (2510 m), Weixi County, Yunnan, China, 2024 V-12, local collector leg. [ZHJ].
♂, Mt. Baihuashan (1410 m), Beijing, China, 2016 VI-13, Kun Li leg. [ZHJ]; 2♂♂, ♀, Yintiaoling Nature Reserve (1270 m), Wuxi County, Chongqing, China, 2022 VII-22, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Donghe Township (560 m), Taitung County, Taiwan, China, 2018 VII-2, Wen-Yi Chou leg. [ZHJ]; 3♂♂, ♀, Mt. Tianmushan (920 m), Zhejiang, China, 2020 VII-13, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Mt. Linshan (134 m), Hangzhou, Zhejiang, China, 2021 VII-24, Zhuo-Heng Jiang leg. [ZHJ]; 3♂♂, 2♀♀, Maolan Nature Reserve (896 m), Libo, Guizhou, China, 2017 IV-27, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Hanjichong (810 m), Yuanjiang County, Yunnan, China, 2020 IX-27, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mengla County (847 m), Yunnan, China, 2013 VIII-30, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mt. Jianfengling (1012 m), Ledong County, Hainan, China, 2018 IV-17, Zhuo-Heng Jiang leg. [ZHJ].
3♂♂, 2♀♀, Hutiaoxia (2310 m), Lijiang, Yunnan, China, 2020 VII-26, Hao-Lin Gan leg. [ZHJ]; ♂, Renhe Villiage (2210 m), Lijiang, Yunnan, China, 2017 VI-20, Hui-Hong Zhang leg. [ZHJ]; ♂, Yanbian County (2130 m), Panzhihua, Sichuan, China, 2019 VIII-7, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Tacheng (2510 m), Weixi County, Yunnan, China, 2024 V-12, local collector leg. [ZHJ].
3♂♂, ♀, Youxi County (846 m), Sanming, Fujian, China, 2018 V-29, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, ♀, Mt. Tianmushan (920 m), Zhejiang, China, 2020 VII-16, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mengla County (847 m), Yunnan, China, 2013 VIII-30, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mt. Jianfengling (1012 m), Ledong County, Hainan, China, 2018 IV-17, Zhuo-Heng Jiang leg. [ZHJ].
♂, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2018 VII-23, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Nabanhe Nature Reserve (1093 m), Xishuangbanna, Yunnan, China, 2022 VII-7, Chang-Qiu Liu leg. [ZHJ]; ♂, Motuo (1210 m), Xizang, China, 2022 VII-21, Shi-Wei Guo leg. [ZHJ]; ♂, Pu’er (1608 m), Yunnan, China, 2021 VII-18, Dan-Dan Xu leg. [ZHJ]; ♀, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2022 VII, Wei-Zong Yang leg. [ZHJ].
3♂♂, Mt. Jianfengling (1012 m), Ledong County, Hainan, China, 2018 IV-17, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mengla County (847 m), Yunnan, China, 2013 VIII-30, Zhuo-Heng Jiang leg. [ZHJ]; ♀, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2018 VII-22, Zhuo-Heng Jiang leg. [ZHJ].
3♂♂, ♀, Yintiaoling Nature Reserve (1270 m), Wuxi County, Chongqing, China, 2022 VII-22, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Yanbian County (1130 m), Panzhihua, Sichuan, China, 2019 VIII-3, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Youxi County (846 m), Sanming, Fujian, China, 2018 V-29, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, ♀, Mt. Tianmushan (920 m), Zhejiang, China, 2020 VII-14, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Mt. Linshan (134 m), Hangzhou, Zhejiang, China, 2021 VII-24, Zhuo-Heng Jiang leg. [ZHJ]; 3♂♂, Maolan Nature Reserve (896 m), Libo, Guizhou, China, 2017 IV-28, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Mengla County (793 m), Yunnan, China, 2013 VIII-29, Zhuo-Heng Jiang leg. [ZHJ]; 2♂, Mt. Jianfengling (1012 m), Ledong County, Hainan, China, 2018 IV-17, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, ♀, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2018 VII-22, Zhuo-Heng Jiang leg. [ZHJ].
♂, Lixin Village (1370 m), Nyalam County, Xizang, China, 2020 VII-17, Si-Xun Ge leg. [ZHJ]; ♂, Motuo (1630 m), Xizang, China, 2023 VII-23, Yu-Fei Li leg. [ZHJ]; 3♂♂, Nyingchi City(1980 m), Xizang, China, 2024 VII-21, Xin Wang leg. [ZHJ].
2♂♂, Mt. Fushoushan (1097 m), Yueyang, Hunan, China, 2016 IX-23, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Jiading new town (4 m), Jiading District, Shanghai, China, 2021 X, De-Rao Chou leg. [DRC]; ♂, Menghai County, Xishuangbanna, Yunnan, China, 2020 V-23, Chang-Qiu Liu leg. [ZHJ]; 2♂♂, ♀, Yaoluoping (990 m), Yuexi County, Anhui, China, 2023 IX-7, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Mt. Laoshan (270 m), Nanjing, Jiangsu, China, 2023 VII-11, Zhuo-Heng Jiang leg. [ZHJ]; ♀, Zhenping County (1140 m), Shaanxi, China, 2023 VIII-13, Zhuo-Heng Jiang leg. [ZHJ].
2♂♂, Mt. Alishan (1450 m), Taiwan, China, 2017 V-6, Wen-Yi Chou leg. [ZHJ]; 6♂♂, 2♀♀, Nantou County, Taiwan, China, 2023 V-22, Local collector leg. [ZHJ]; 2♂♂, Nantou County, Taiwan, China, 2002 IV-18–19, D. Anstine leg. [SM]; ♀, Yilan County, Taiwan, China, 2011 X-6, Schnitzler leg. [SM]; ♀, Yilan County, Taiwan, China, 2011 X-6, Schnitzler leg. [SM].
♂, Shaoguan, Guangdong, China, 2017 V-2, Jin-Kun Zhang leg. [ZHJ]; 5♂♂, Mt. Leigongshan (1304 m), Suiyang County, Guizhou, China, 2021 V-3, Xin-Yi Zheng leg. [ZHJ]; ♀, Fengtongzhai (1802 m), Baoxing County, Sichuan, China, 2016 V-2, Zhi-Yuan Qi leg. [ZHJ].
♂, Pingbian County (1980 m), Yunnan, China, 2024 VI-25, Shi-Wei Guo leg. [ZHJ]; ♂, ♂, Pingbian County (2012 m), Yunnan, China, 2024 V-21, Peng Wang leg. [ZHJ]; ♂, ♀, Pingbian County (2040 m), Yunnan, China, 2024 V-4, Yong-Kang You leg. [ZHJ].
2♂♂, ♀, Yintiaoling Nature Reserve (1270 m), Wuxi County, Chongqing, China, 2022 VII-22, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Yanbian County (1130 m), Panzhihua, Sichuan, China, 2019 VIII-3, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Youxi County (846 m), Sanming, Fujian, China, 2018 V-29, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, ♀, Mt. Tianmushan (920 m), Zhejiang, China, 2020 VII-14, Zhuo-Heng Jiang leg. [ZHJ]; ♂, ♀, Mt. Alishan (1450 m), Taiwan, China, 2017 V-6, Wen-Yi Chou leg. [ZHJ]; 2♂♂, Mt. Fushoushan (1097 m), Yueyang, Hunan, China, 2016 IX-23, Zhuo-Heng Jiang leg. [ZHJ]; 3♂♂, ♀, Maolan Nature Reserve (896 m), Libo, Guizhou, China, 2017 IV-28, Zhuo-Heng Jiang leg. [ZHJ]; ♂, ♀, Lixin Village (1370 m), Nyalam County, Xizang, China, 2020 VII-17, Si-Xun Ge leg. [ZHJ].
3♂♂, 2♀♀, Sabah (1070 m), Borneo, Malaysia, 2023 IV-27, Zhuo-Heng Jiang leg. [ZHJ]; 2♂♂, Sabah (960 m), Borneo, Malaysia, 2018 V-16, Local collector leg. [ZHJ]; 4♂♂, ♀, Sumatra, Indonesia, 2023 VI, Local collector leg. [ZHJ].
3♂♂, Mt. Halimun (1020 m), Mt. Halimun, Java, Indonesia, 2022 V, Local collector leg.
3♂♂, Nabanhe Nature Reserve (1093 m), Xishuangbanna, Yunnan, China, 2022 VIII-29, Chang-Qiu Liu leg. [CQL, ZHJ]; ♂, Mt. Bulangshan (1450 m), Menghai County, Yunnan, China, 2021 IV-17, Chang-Qiu Liu leg. [CQL]; ♂, Xima Town (1500 m), Yingjiang County, Yunnan, China, 2022 VII, Wei-Zong Yang leg. [ZHJ].
2♂♂, Nabanhe Nature Reserve (1093 m), Xishuangbanna, Yunnan, China, 2022 VIII-29, Chang-Qiu Liu leg. [CQL, ZHJ]; 2♂♂, Tongbiguan Township (1450 m), Yingjiang County, Yunnan, China, 2022 VIII-30, Chang-Qiu Liu leg. [CQL]; ♀, Xiajinchang Township, Malipo County, Yunnan, China, 2020 VII-26, Hao-Lin Gan leg. [ZHJ]; ♀, Bapo (1312 m), Dulongjiang, Yunnan, China, 2024 VI-6, Yi-Ting Lin leg. [ZBX]; ♂, Mt. Jianfengling (924 m), Ledong County, Hainan, China, 2018 IV-20, Zhuo-Heng Jiang leg. [ZHJ].
♂, Yiwu Town (1367 m), Mengla County, Xishuangbanna, Yunnan, China, 2013 VIII-31, Zhuo-Heng Jiang leg. [ZHJ]; ♂, ♀, Menghai County (1120 m), Xishuangbanna, Yunnan, China, 2022 IX-16, Dan-Dan Xu leg. [ZHJ].
2♂♂, ♀, Yintiaoling Nature Reserve (1270 m), Wuxi County, Chongqing, China, 2022 VII-22, Zhuo-Heng Jiang leg. [ZHJ]; ♂, Huairou County (1210 m), Beijing, China, 2023 VII-20, Li Tao leg. [ZHJ]; ♂, Mt. Lingyanshan (2130 m), Dujiangyan City, Sichuan, China, 2015 VII-20, Yi Zhang leg. [ZHJ]; 2♂♂, Mt. Fushoushan (1097 m), Yueyang, Hunan, China, 2016 IX-23, Zhuo-Heng Jiang leg. [ZHJ]; ♂, ♀, Mt. Leigongshan (1304 m), Suiyang County, Guizhou, China, 2021 V-13, Xin-Yi Zheng leg. [ZHJ]; ♂, Yanjin County (1107 m), Yunnan, China, 2016 VII-17, Zhuo-Heng Jiang leg. [ZHJ]; 3♂♂, Xiajinchang Township, Malipo County, Yunnan, China, 2020 VII-26, Hao-Lin Gan leg. [ZHJ].
♂, Yintiaoling Nature Reserve (1370 m), Wuxi County, Chongqing, China, 2022 IV-11, Tian-Yu Ren leg. [ZHJ]; ♀, Tapa Shan, China, 2001 V, Local collector leg. [SM]; ♀, Yu-Shan-Wu, Anji County, Zhejiang, China, 2001 V, Local collector leg. [SM].
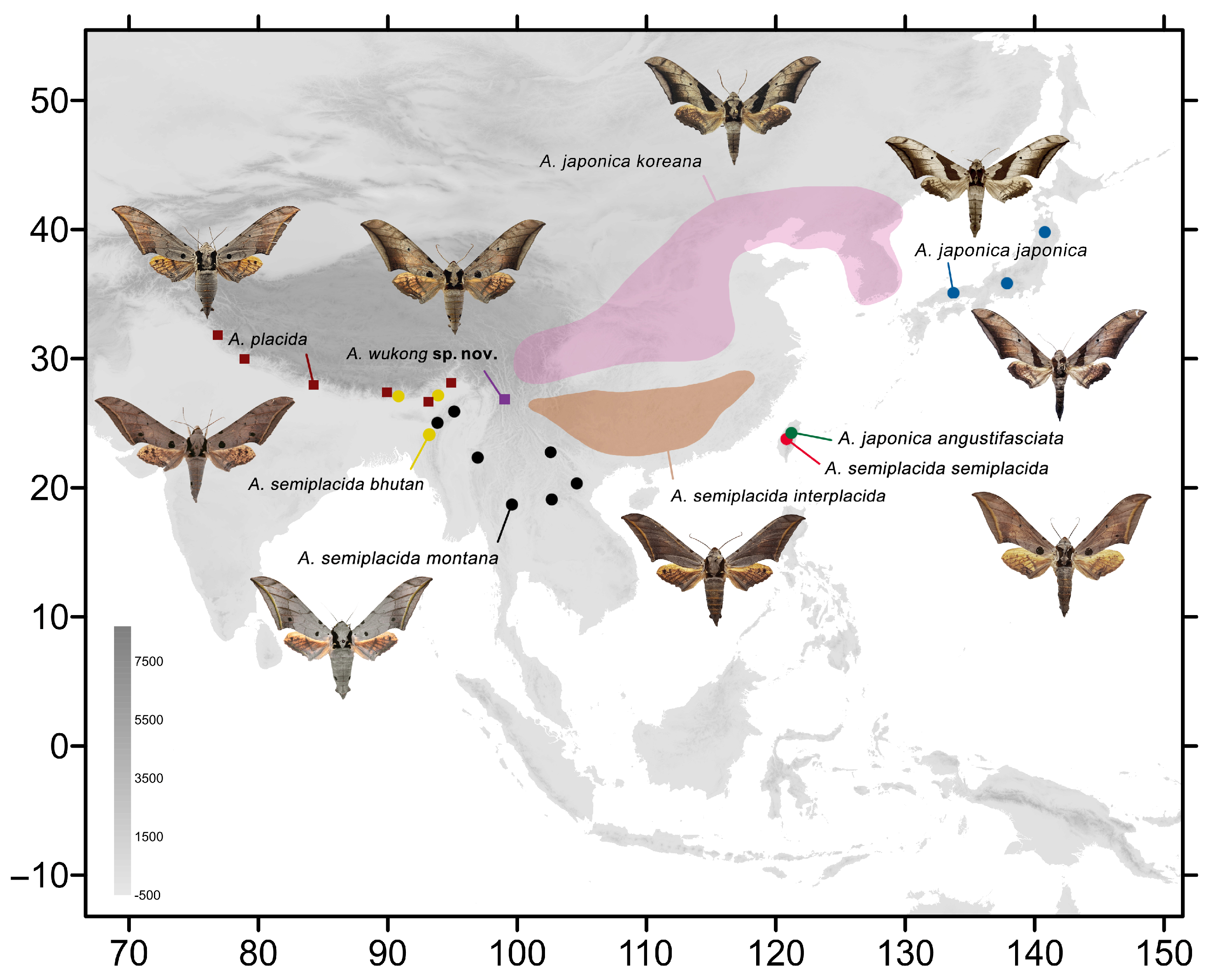
Figure 1.
Distribution of species of the Ambulyx placida-group: A. japonica japonica (blue dots); A. japonica koreana (pink patch); A. japonica angustifasciata (green dots); A. placida (ochre squares); A. semiplacida semiplacida (red dots); A. semiplacida montana (black dots); A. semiplacida interplacida (orange patch); A. semiplacida bhutana (yellow dots); and A. wukong sp. nov. (purple squares).
Figure 1.
Distribution of species of the Ambulyx placida-group: A. japonica japonica (blue dots); A. japonica koreana (pink patch); A. japonica angustifasciata (green dots); A. placida (ochre squares); A. semiplacida semiplacida (red dots); A. semiplacida montana (black dots); A. semiplacida interplacida (orange patch); A. semiplacida bhutana (yellow dots); and A. wukong sp. nov. (purple squares).
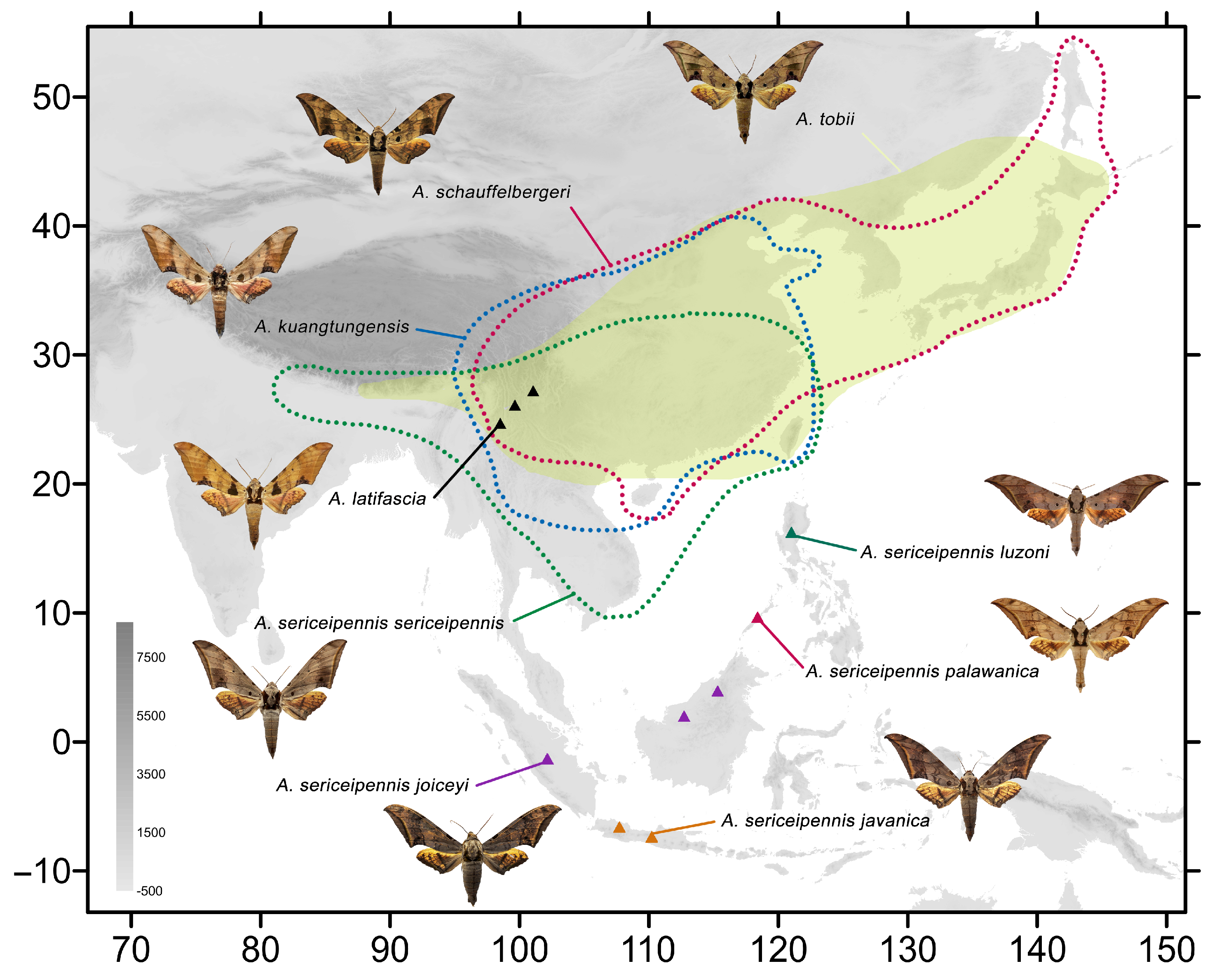
Figure 2.
Distribution of species of the Ambulyx schauffelbergeri- and kuangtungensis-groups included in this study: A. latifascia (black triangles); A. schauffelbergeri (red dotted line); A. sericeipennis sericeipennis (green dotted line); A. s. joiceyi (purple triangles); A. s. javanica (orange triangles); A. s. luzoni (green triangles); A. s. palawanica (red triangles); and A. tobii (yellow patch).
Figure 2.
Distribution of species of the Ambulyx schauffelbergeri- and kuangtungensis-groups included in this study: A. latifascia (black triangles); A. schauffelbergeri (red dotted line); A. sericeipennis sericeipennis (green dotted line); A. s. joiceyi (purple triangles); A. s. javanica (orange triangles); A. s. luzoni (green triangles); A. s. palawanica (red triangles); and A. tobii (yellow patch).
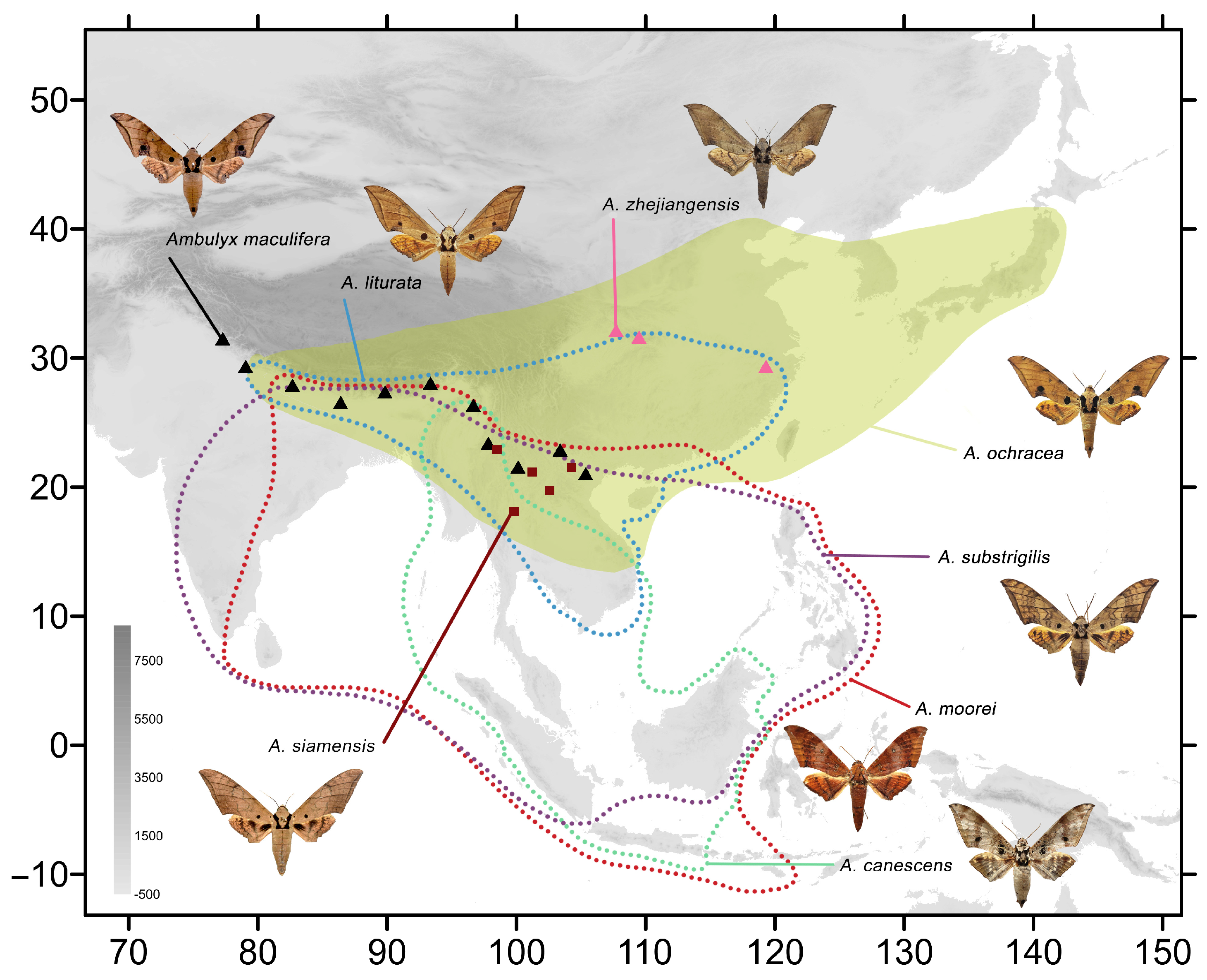
Figure 3.
Distribution of the remaining species of Ambulyx from China: A. substrigilis (purple dotted line); A. siamensis (ochre squares); A. liturata (blue dotted line); A. moorei (red dotted line); A. canescens (green dotted line); A. ochracea (yellow patch); A. maculifera (black triangles); and A. zhejiangensis (pink triangles).
Figure 3.
Distribution of the remaining species of Ambulyx from China: A. substrigilis (purple dotted line); A. siamensis (ochre squares); A. liturata (blue dotted line); A. moorei (red dotted line); A. canescens (green dotted line); A. ochracea (yellow patch); A. maculifera (black triangles); and A. zhejiangensis (pink triangles).
Figure 4.
Maximum likelihood phylogenetic tree of the genus Ambulyx based on cox1 DNA barcode sequences, reconstructed using IQ-TREE and rooted with Barbourion lemaii, Amplypterus panopus, and Anambulyx elwesi as outgroups. Nodes with SH-aLRT support values or ultrafast bootstrap (UFBoot2) values ≥90 are highlighted with black circles.
Figure 4.
Maximum likelihood phylogenetic tree of the genus Ambulyx based on cox1 DNA barcode sequences, reconstructed using IQ-TREE and rooted with Barbourion lemaii, Amplypterus panopus, and Anambulyx elwesi as outgroups. Nodes with SH-aLRT support values or ultrafast bootstrap (UFBoot2) values ≥90 are highlighted with black circles.
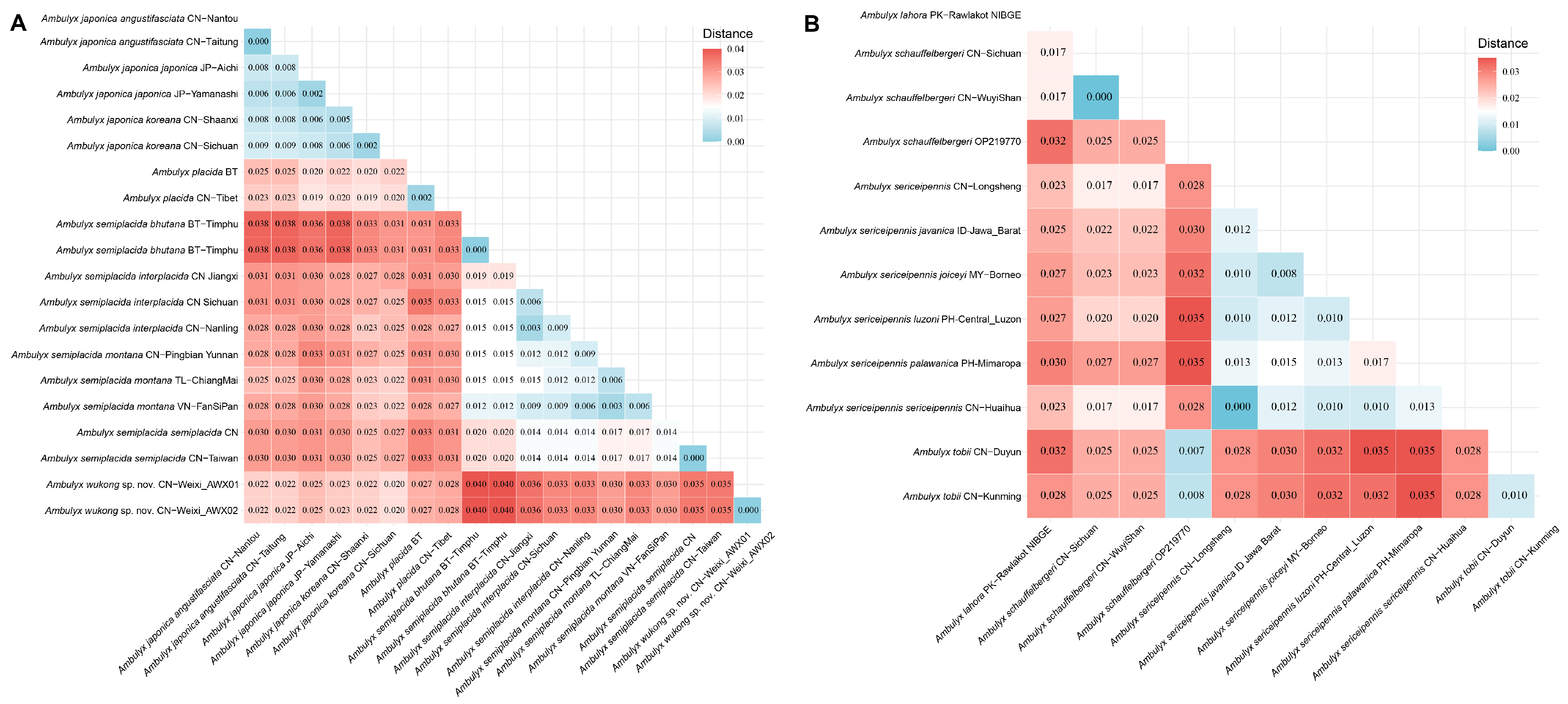
Figure 5.
Kimura two-parameter (K2P) distances (in percentages) between taxa of the
placida-group (
A) and
schauffelbergeri-group (
B) calculated from DNA barcode sequences (
cox1), with species identified as in the ggtree in
Figure 4.
Figure 5.
Kimura two-parameter (K2P) distances (in percentages) between taxa of the
placida-group (
A) and
schauffelbergeri-group (
B) calculated from DNA barcode sequences (
cox1), with species identified as in the ggtree in
Figure 4.
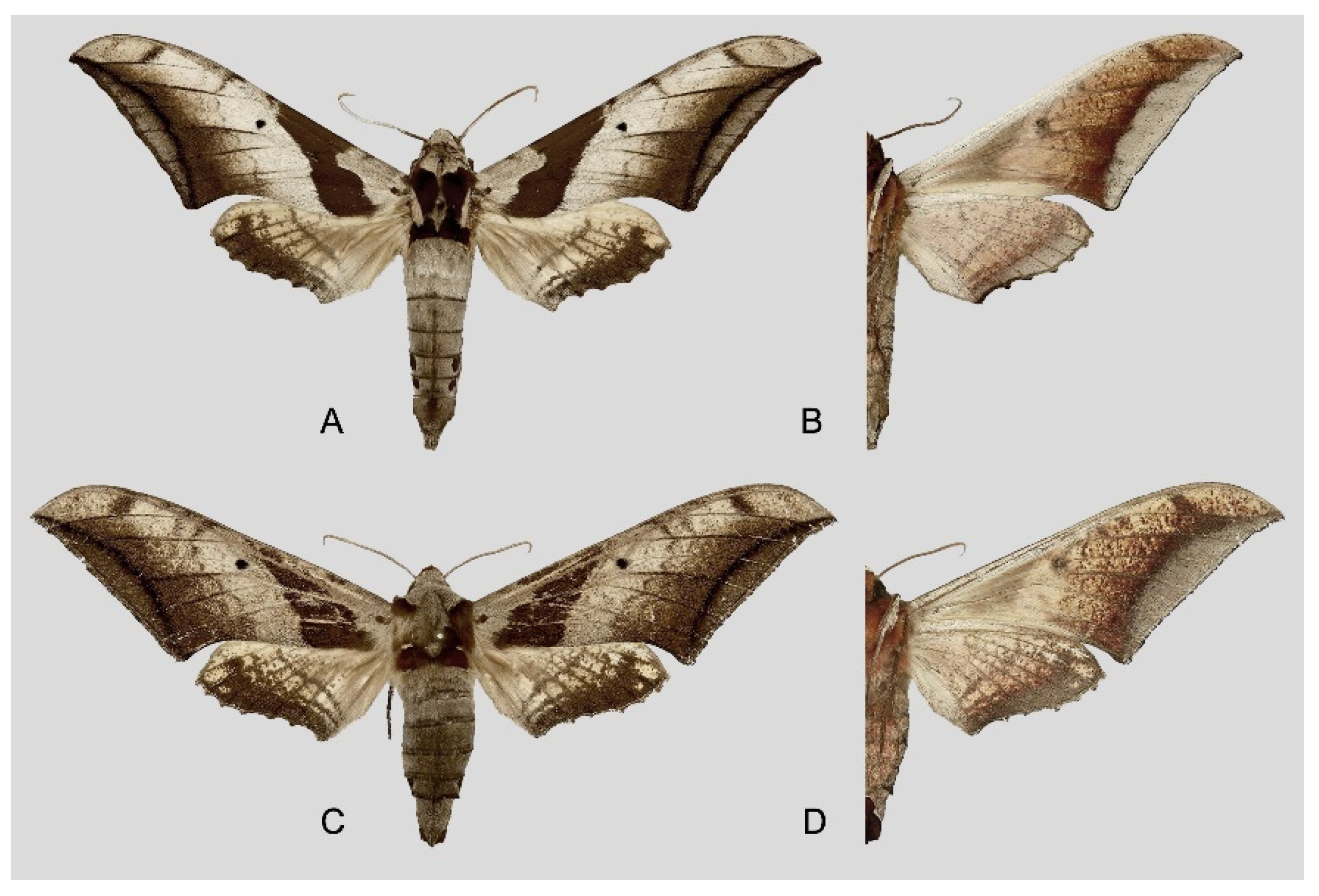
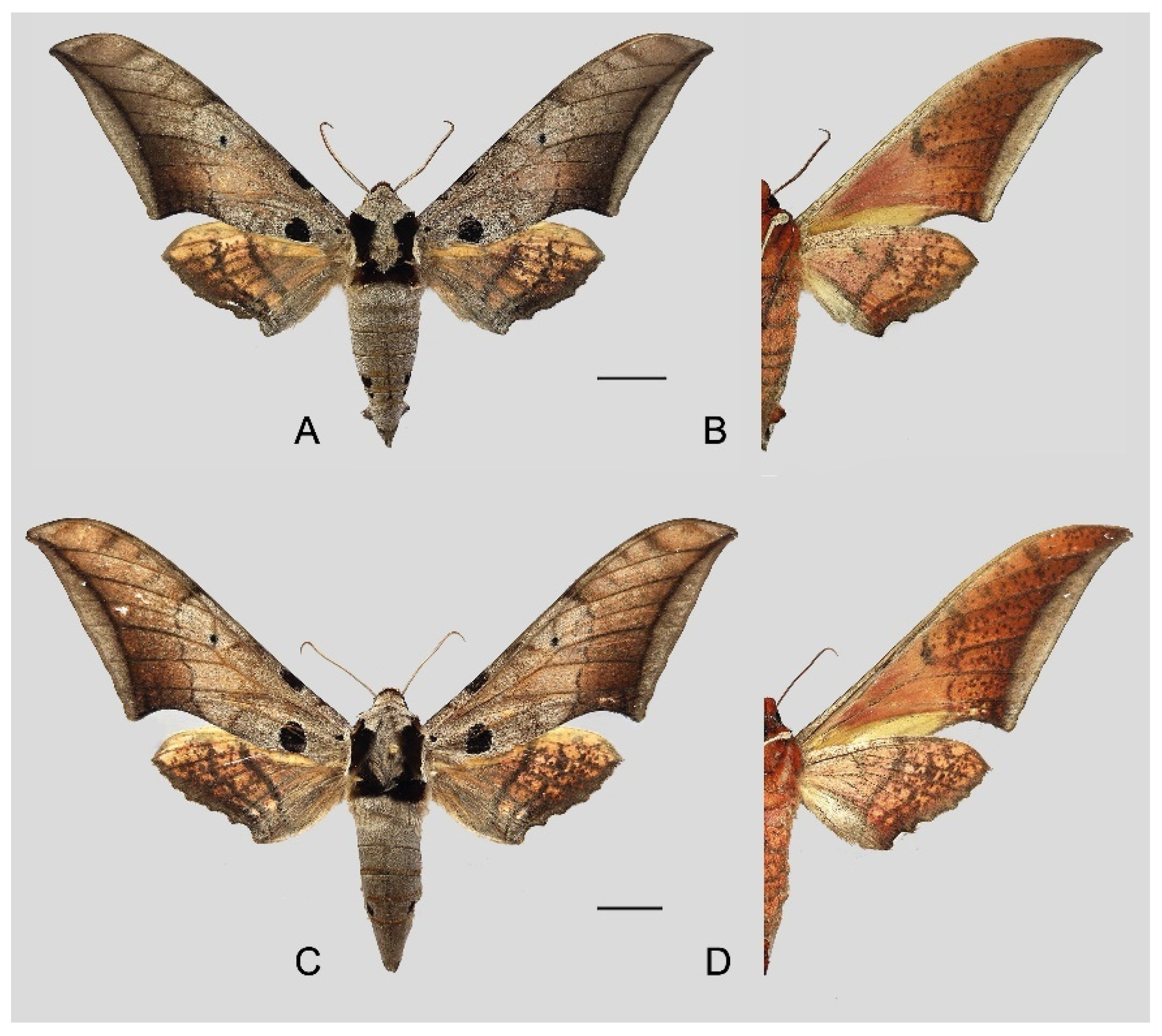
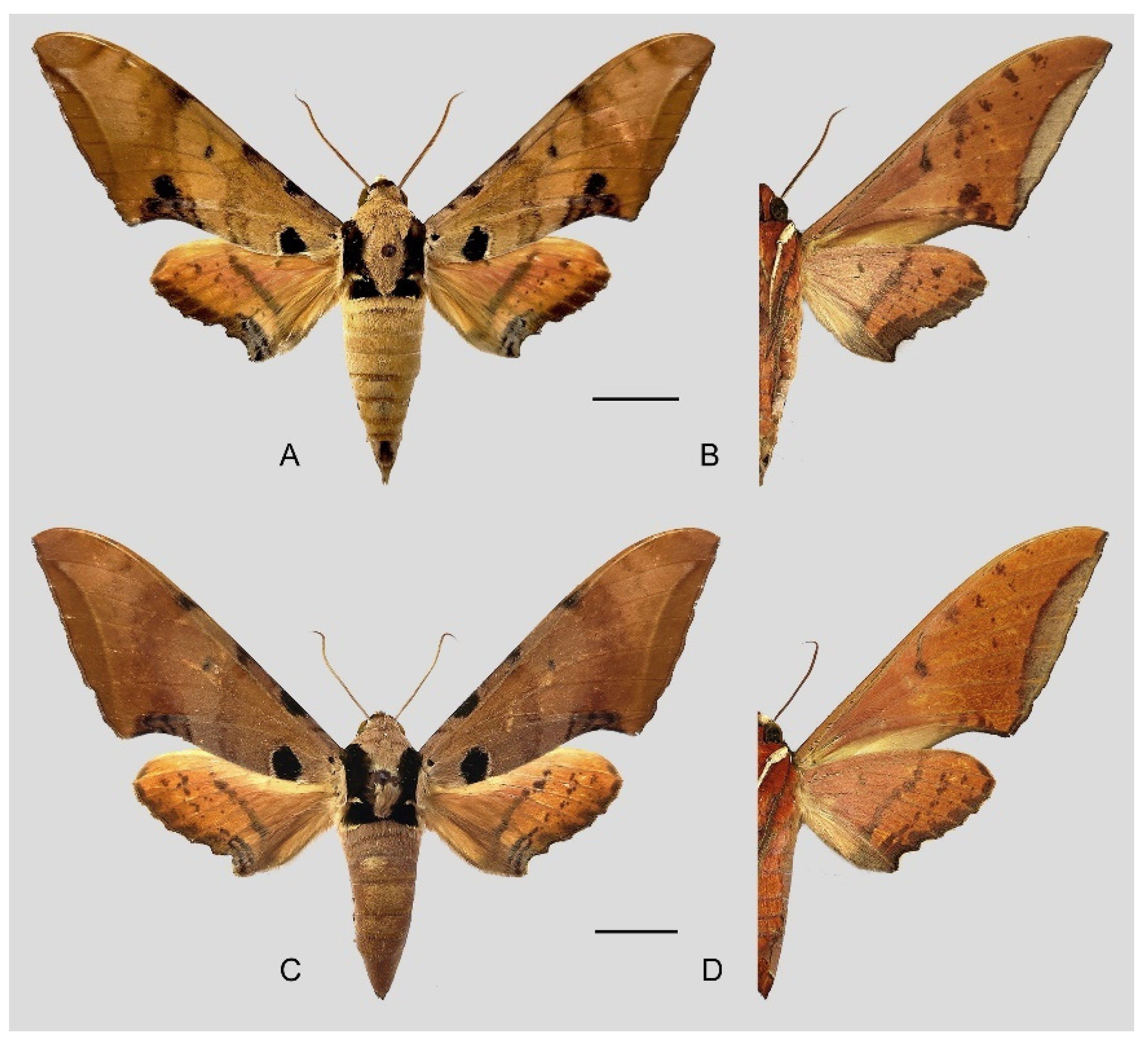
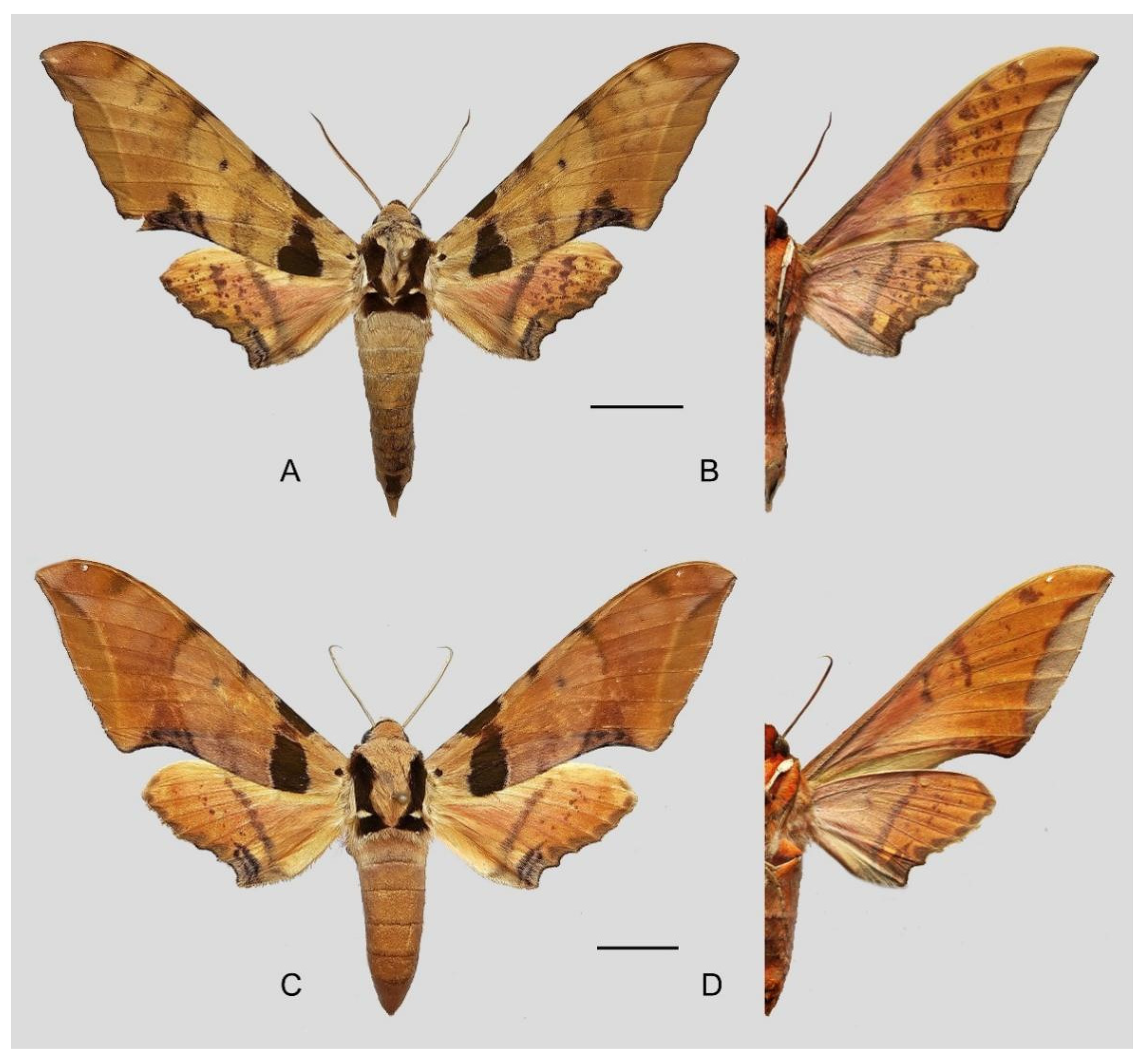
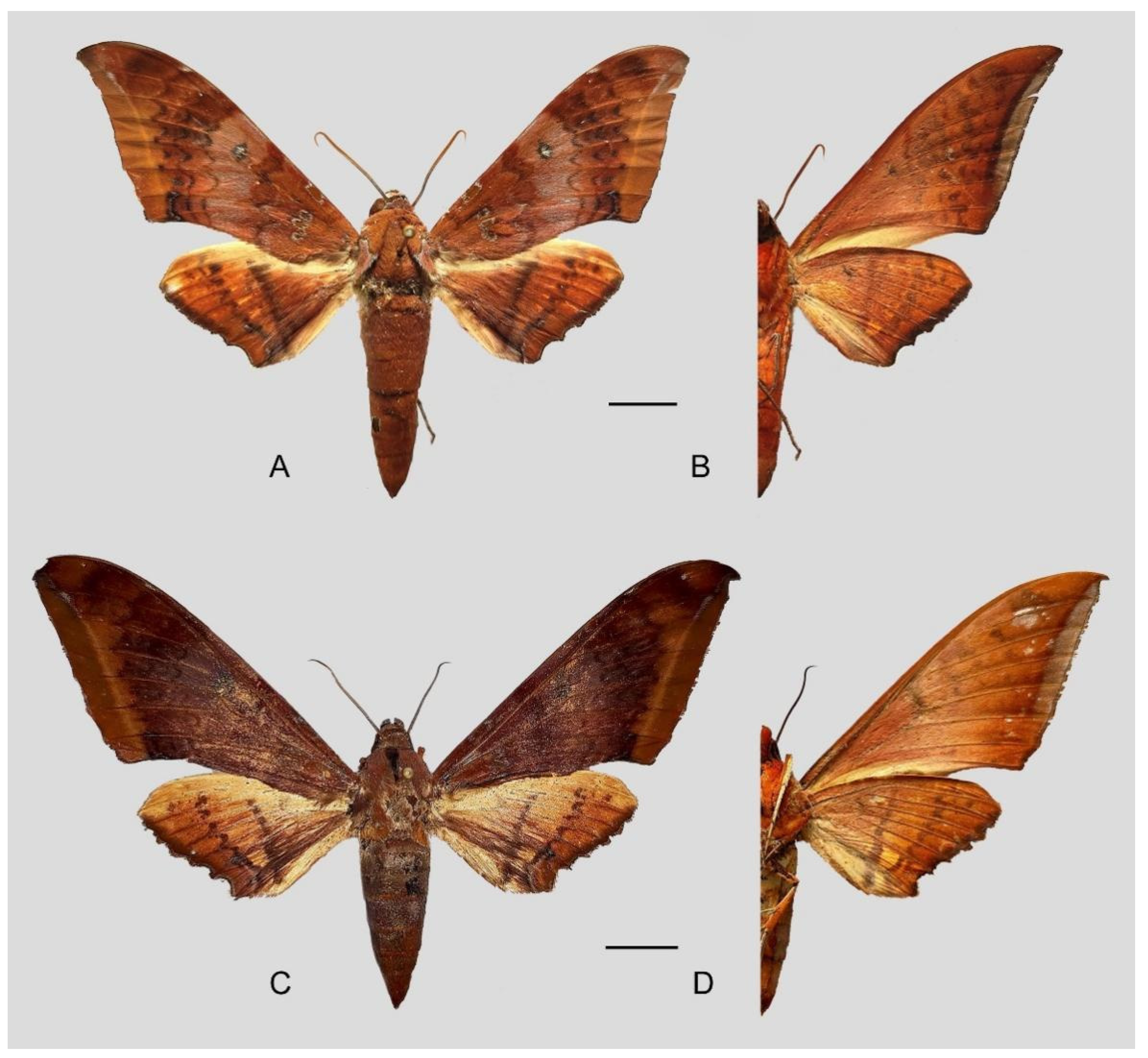
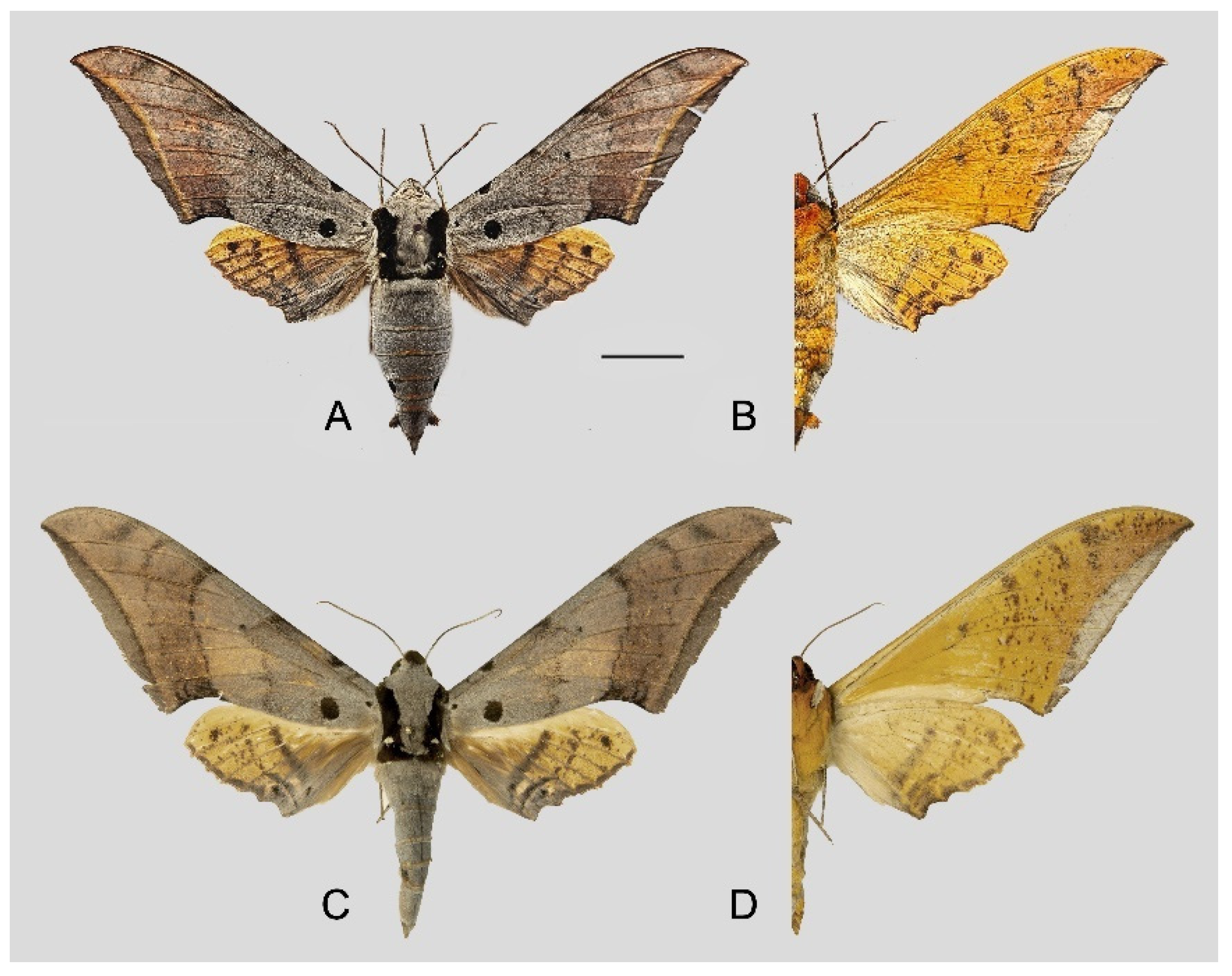
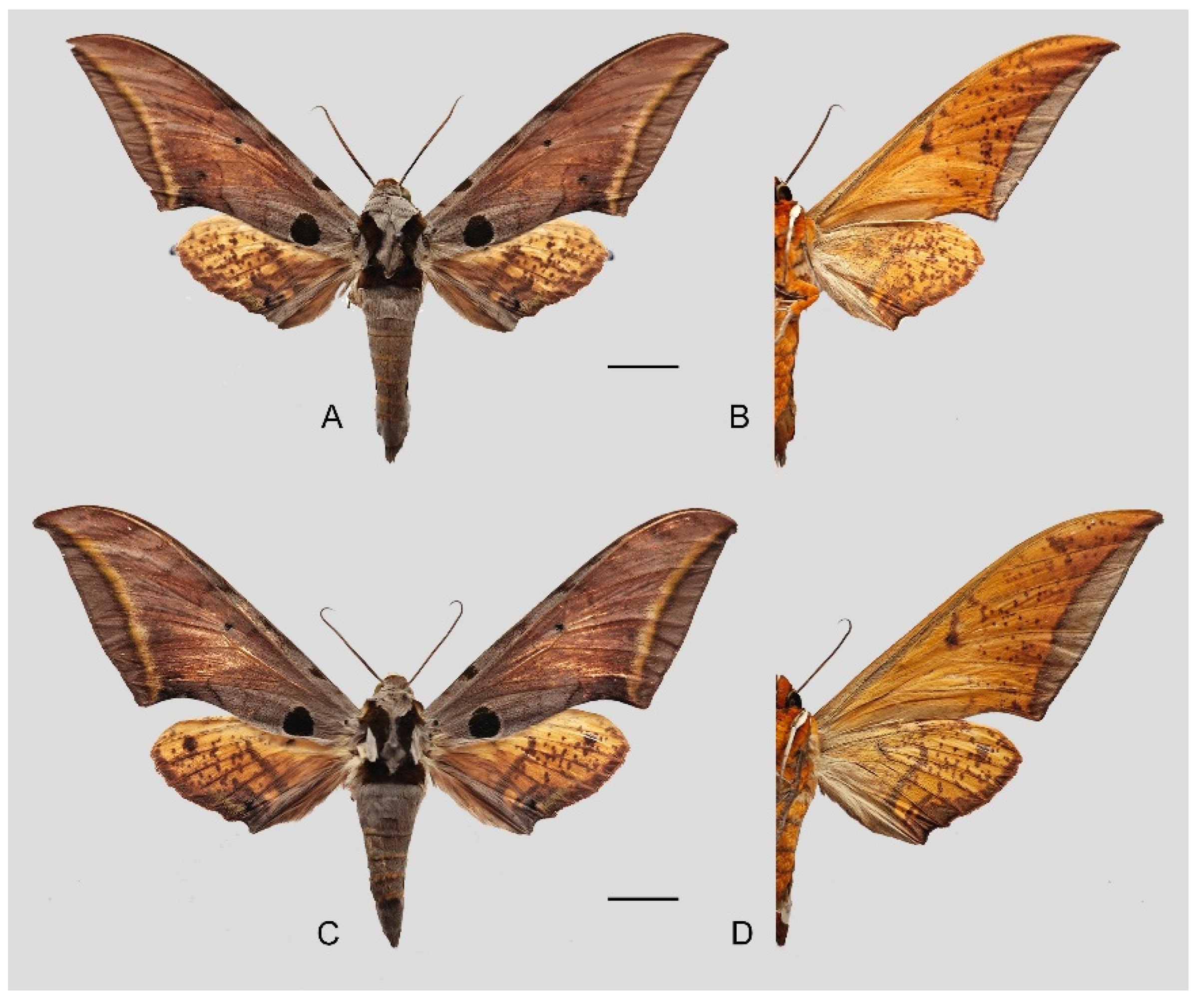
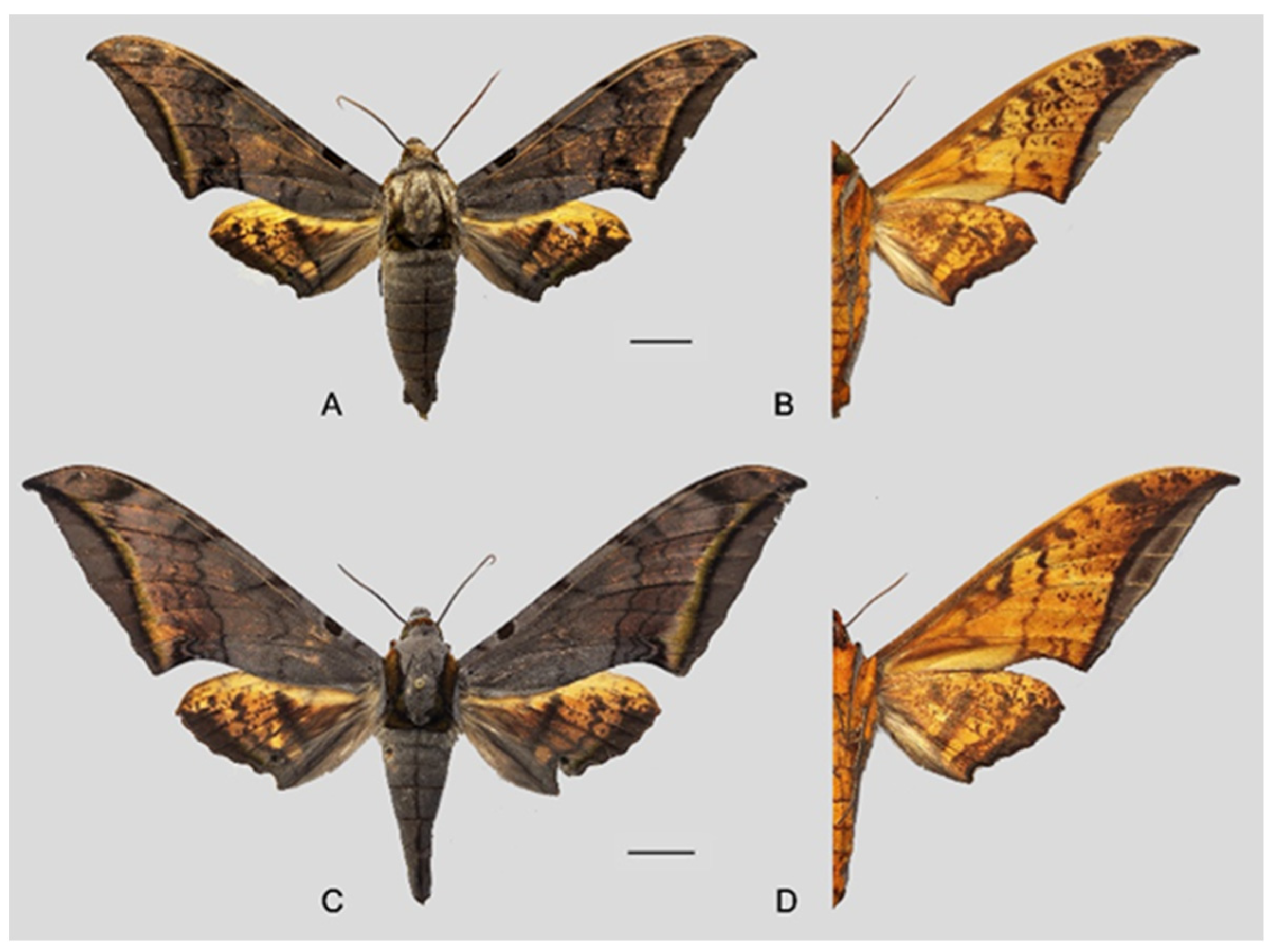
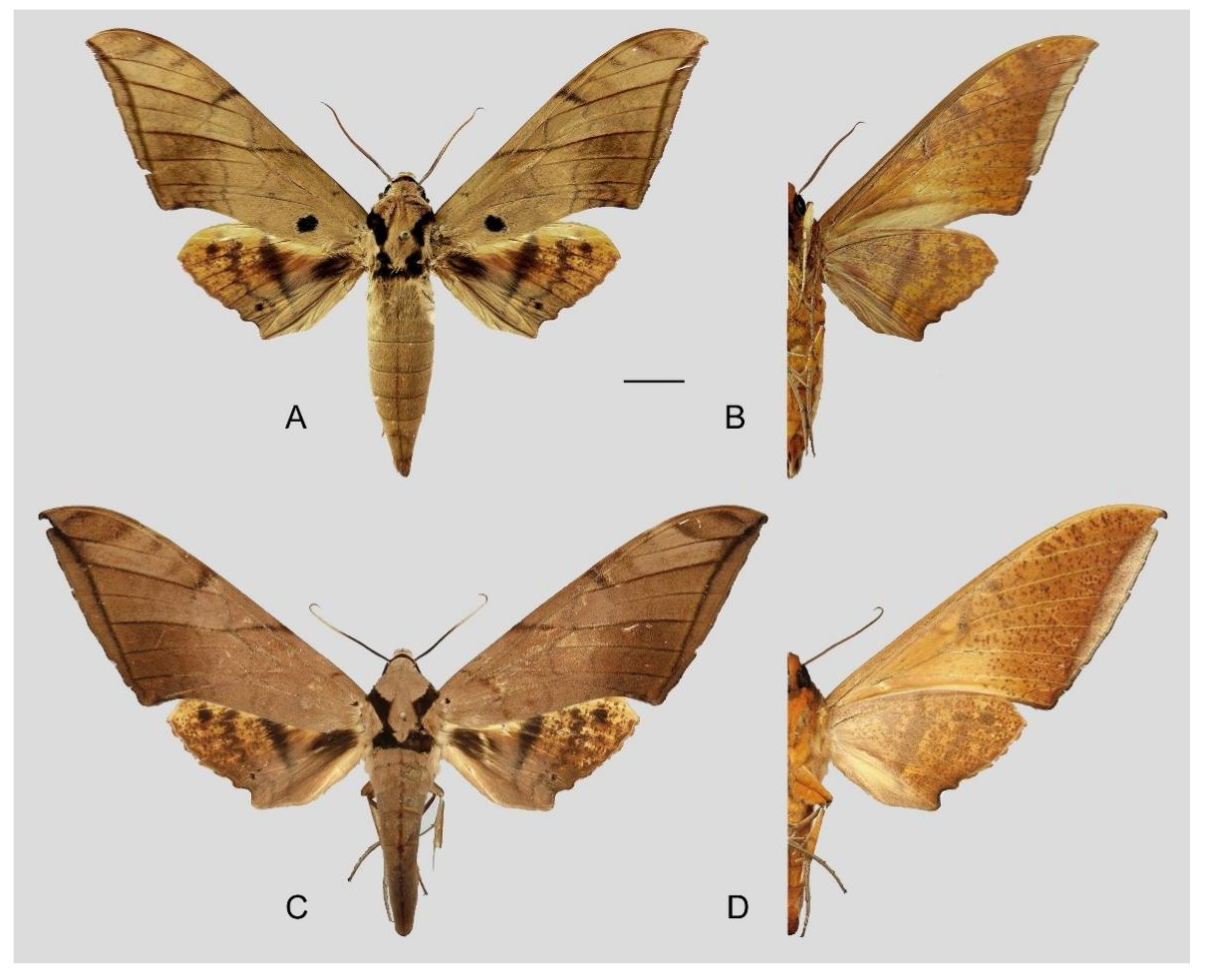
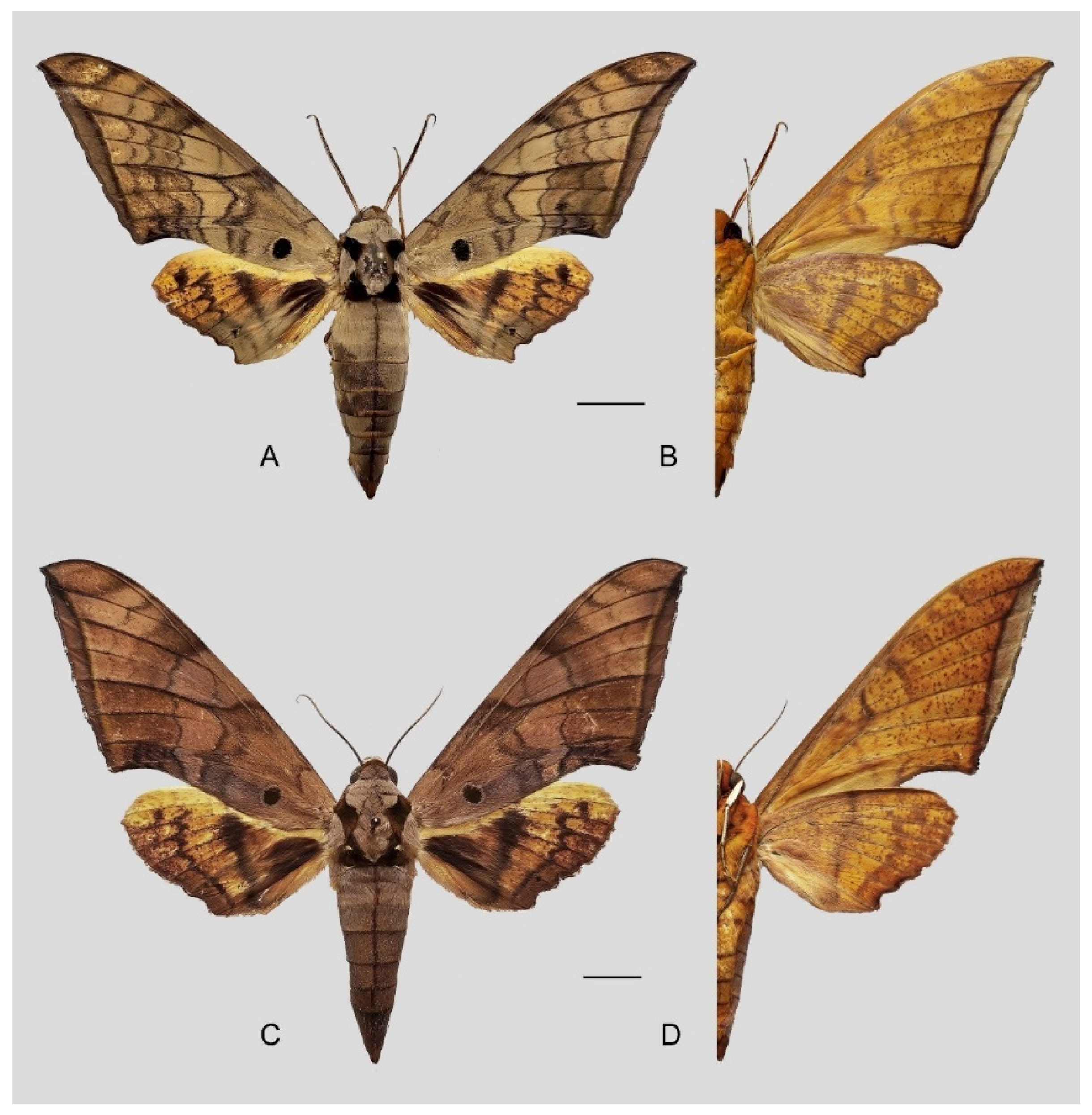
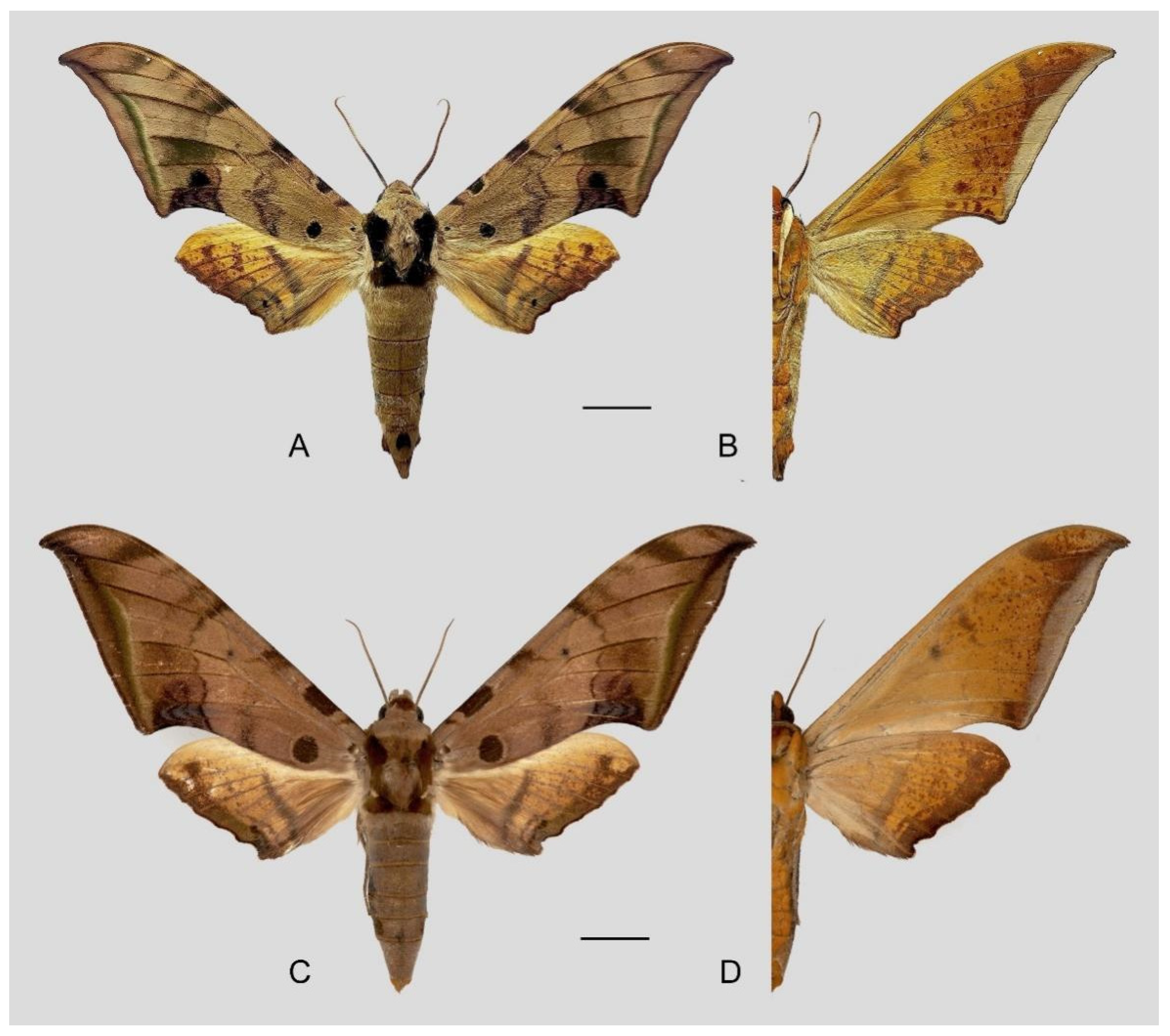
Figure 6.
Ambulyx canescens. (A,B) Male, Yingjiang, Yunnan, China; (C,D) female, Chiang Mai, Thailand. Scale bar = 10 mm.
Figure 6.
Ambulyx canescens. (A,B) Male, Yingjiang, Yunnan, China; (C,D) female, Chiang Mai, Thailand. Scale bar = 10 mm.
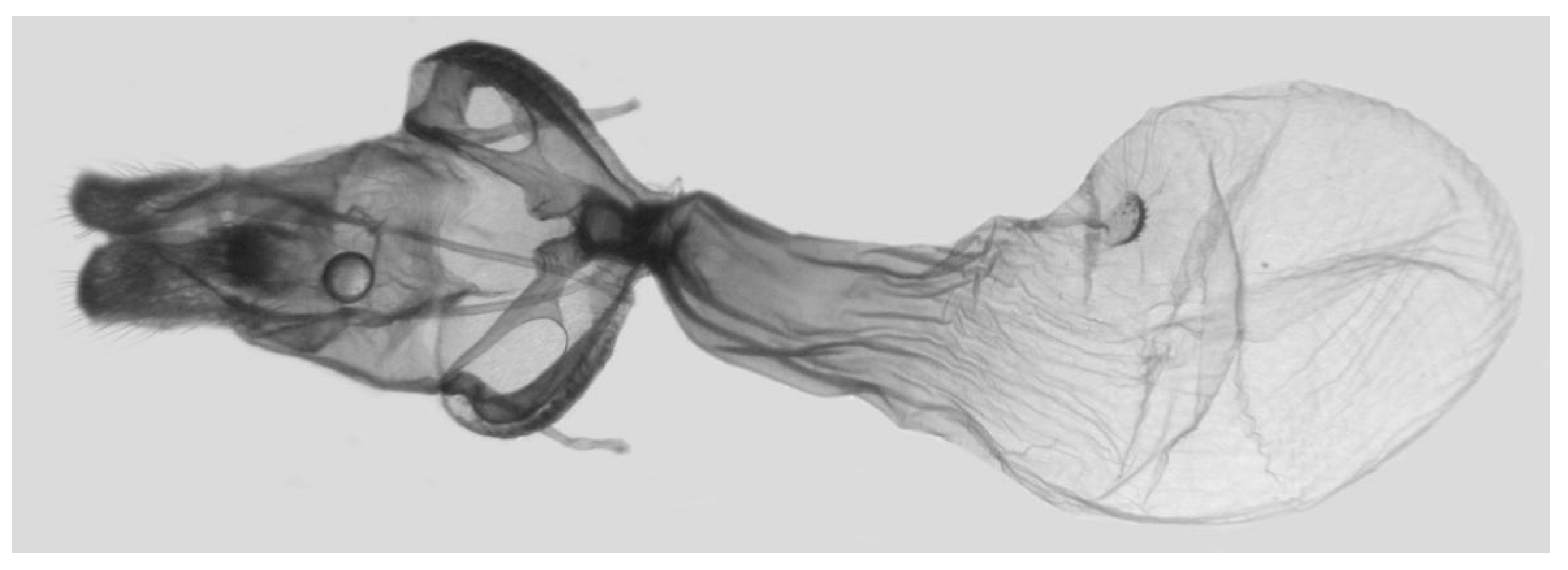
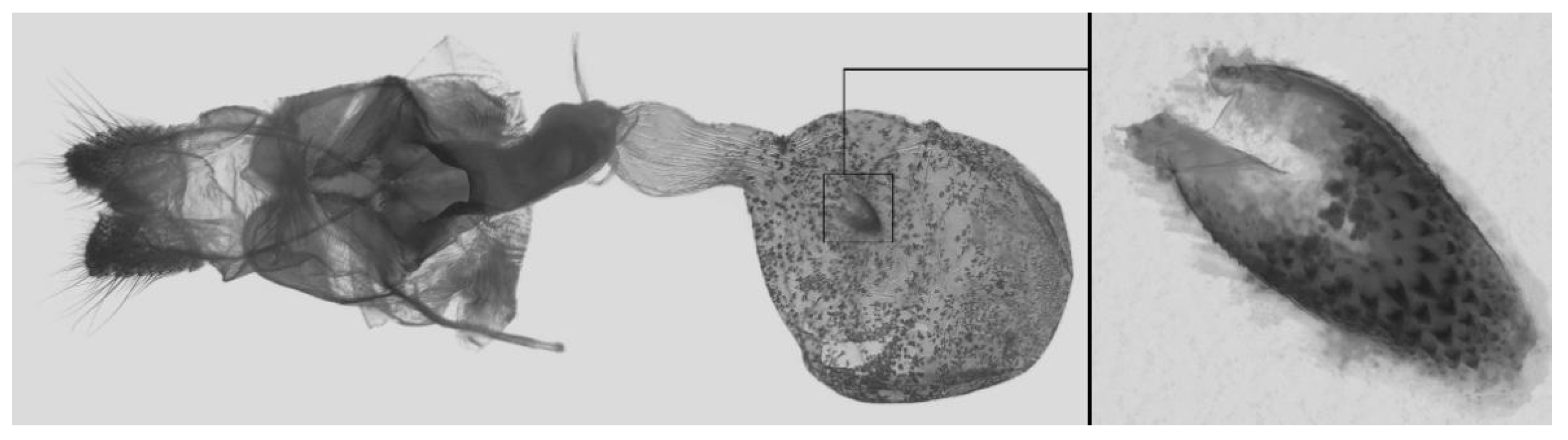
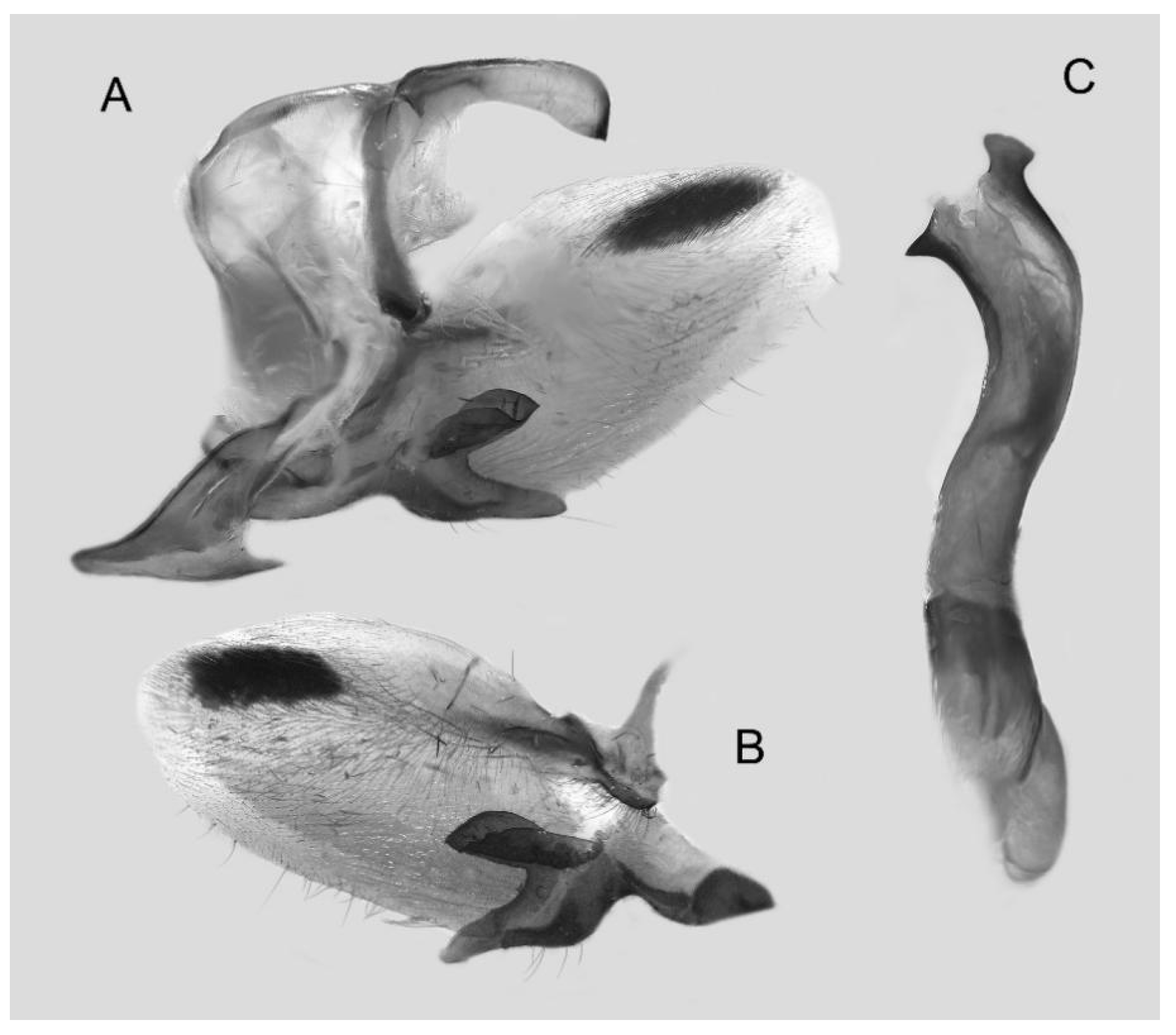
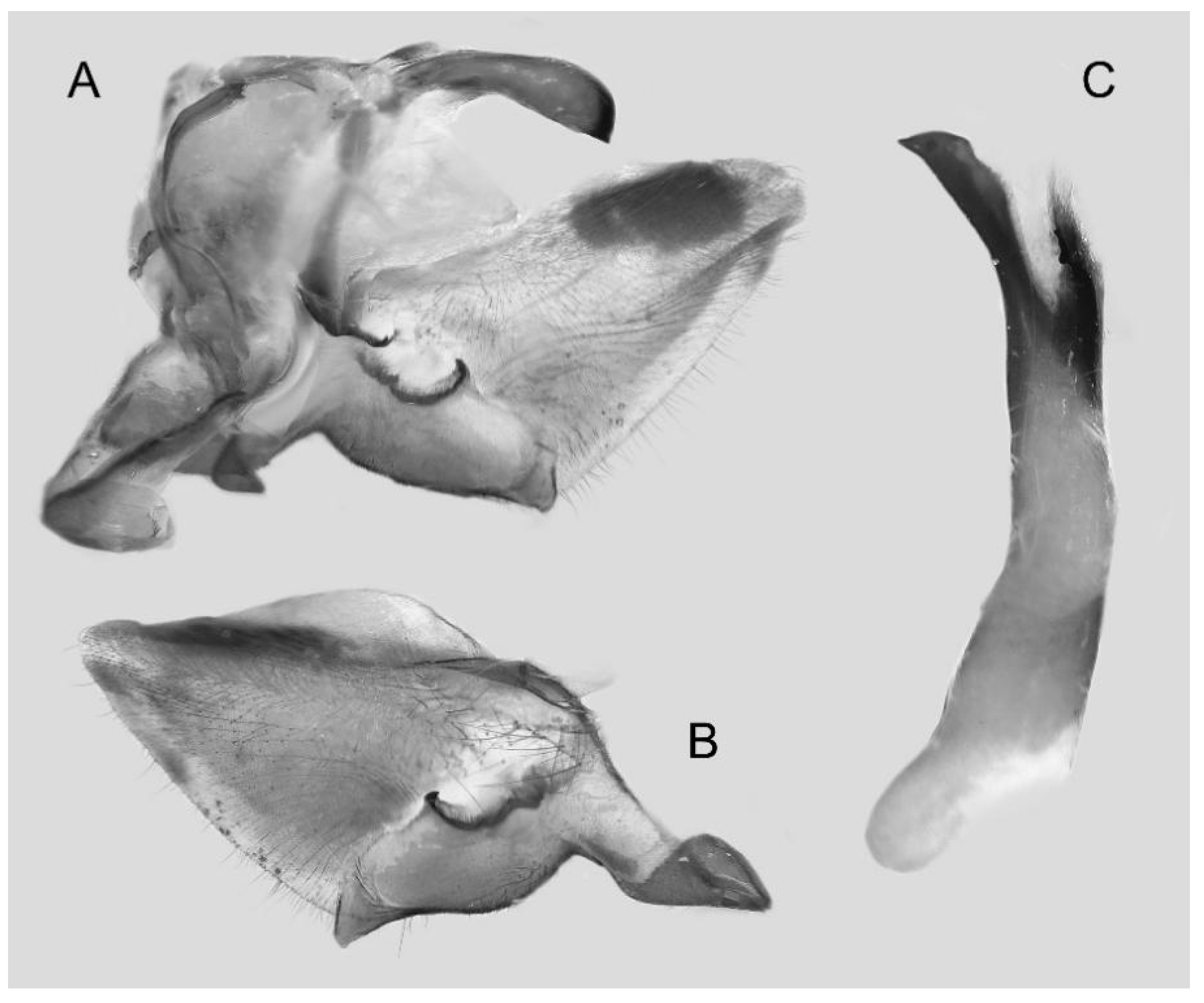
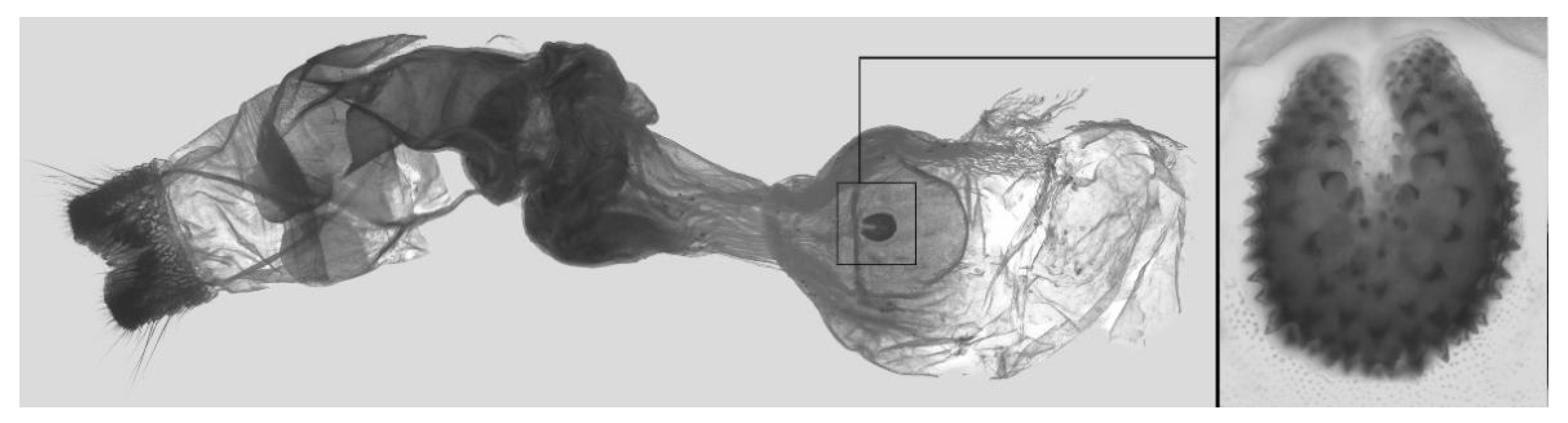
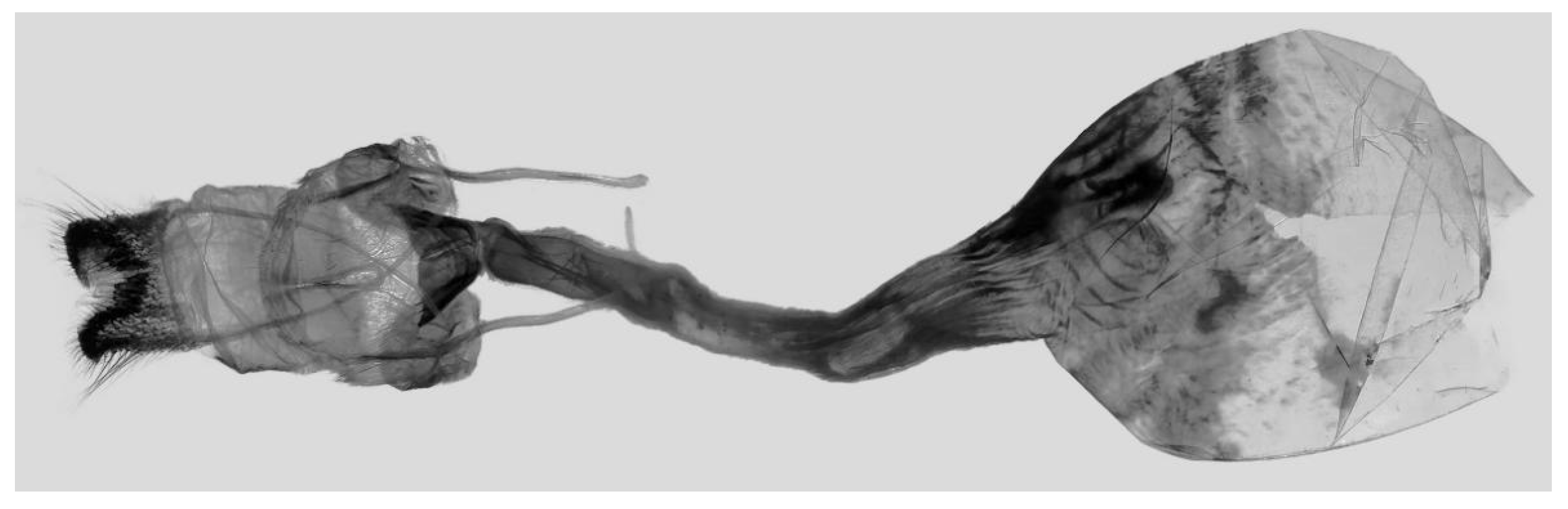
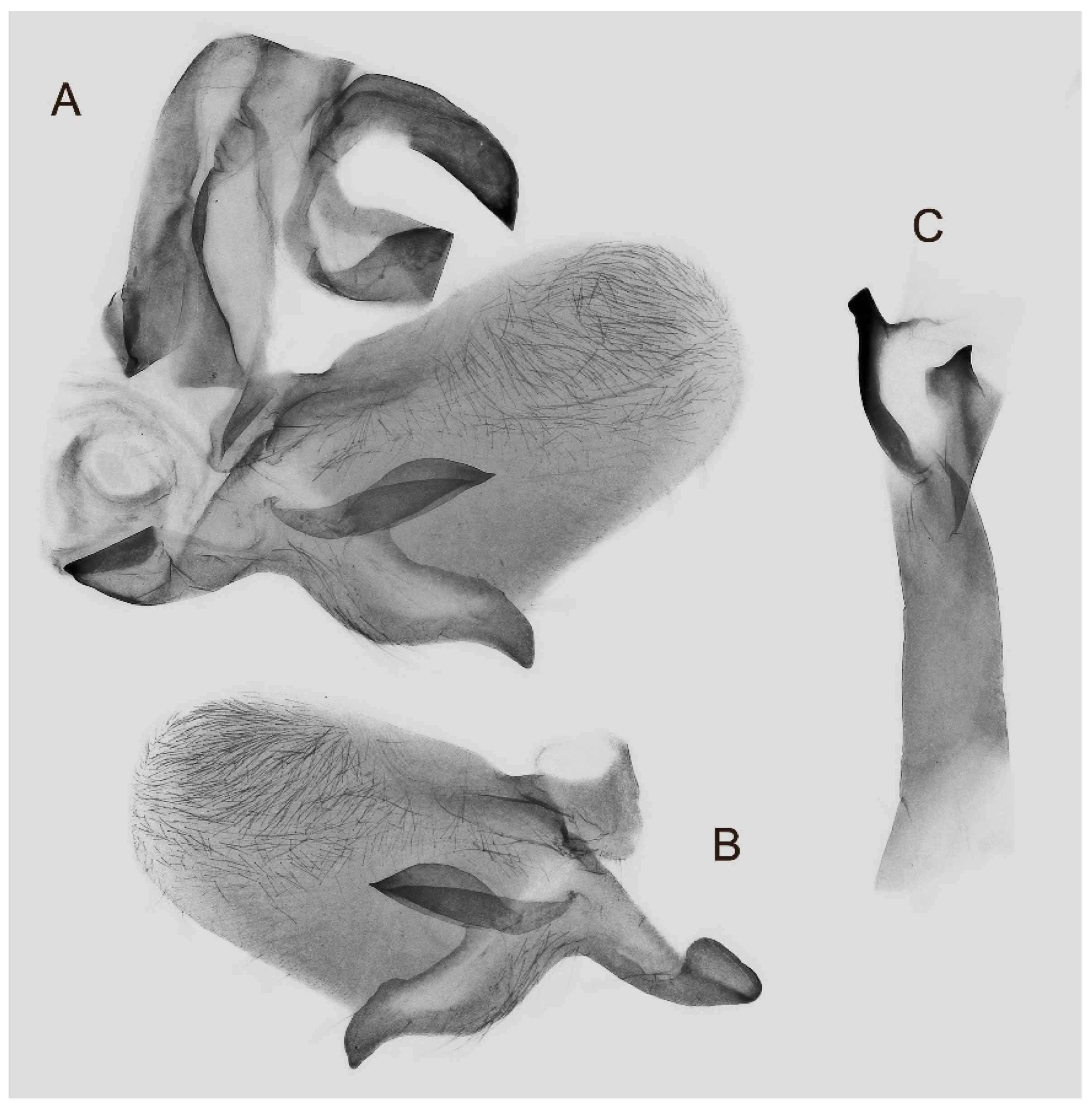
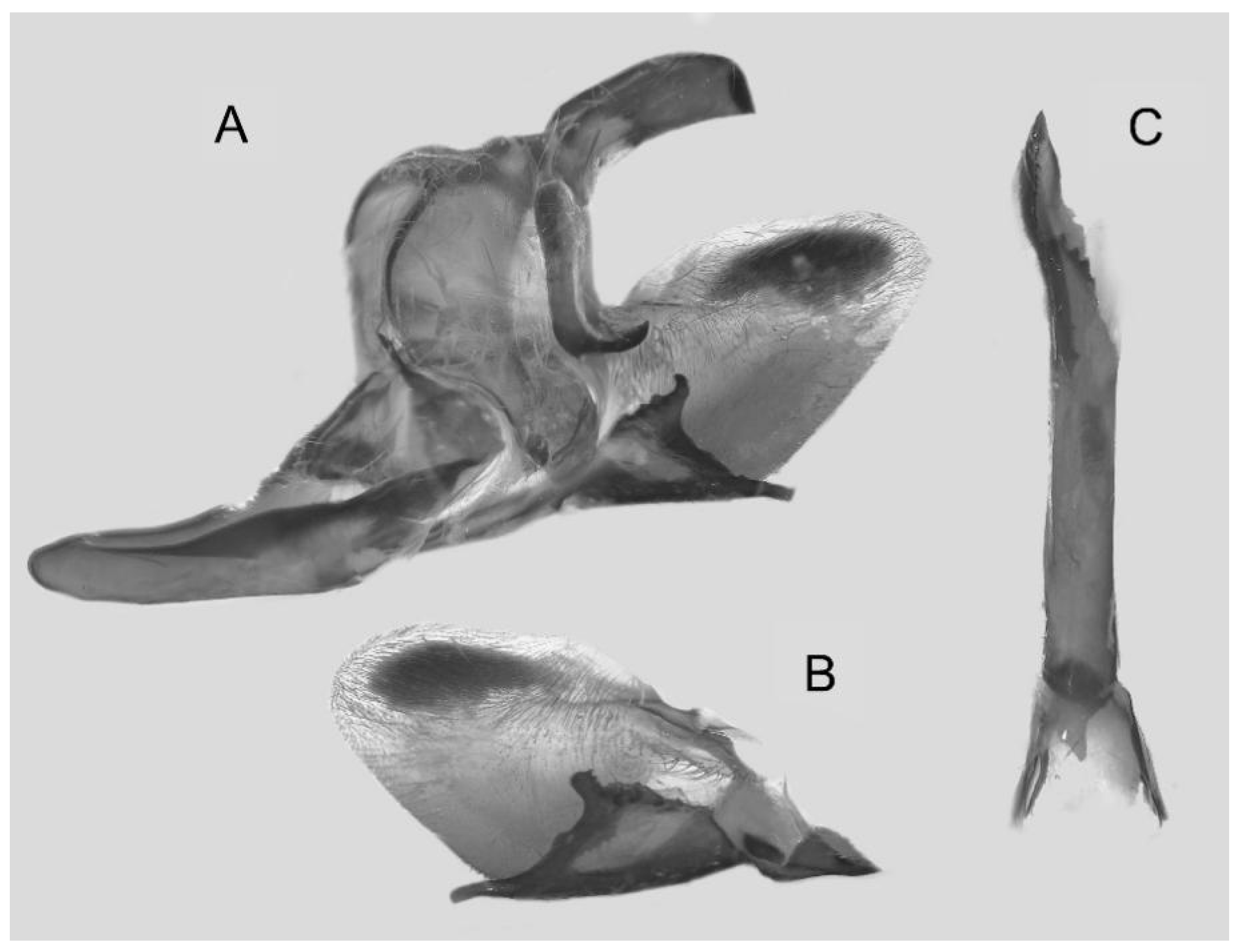
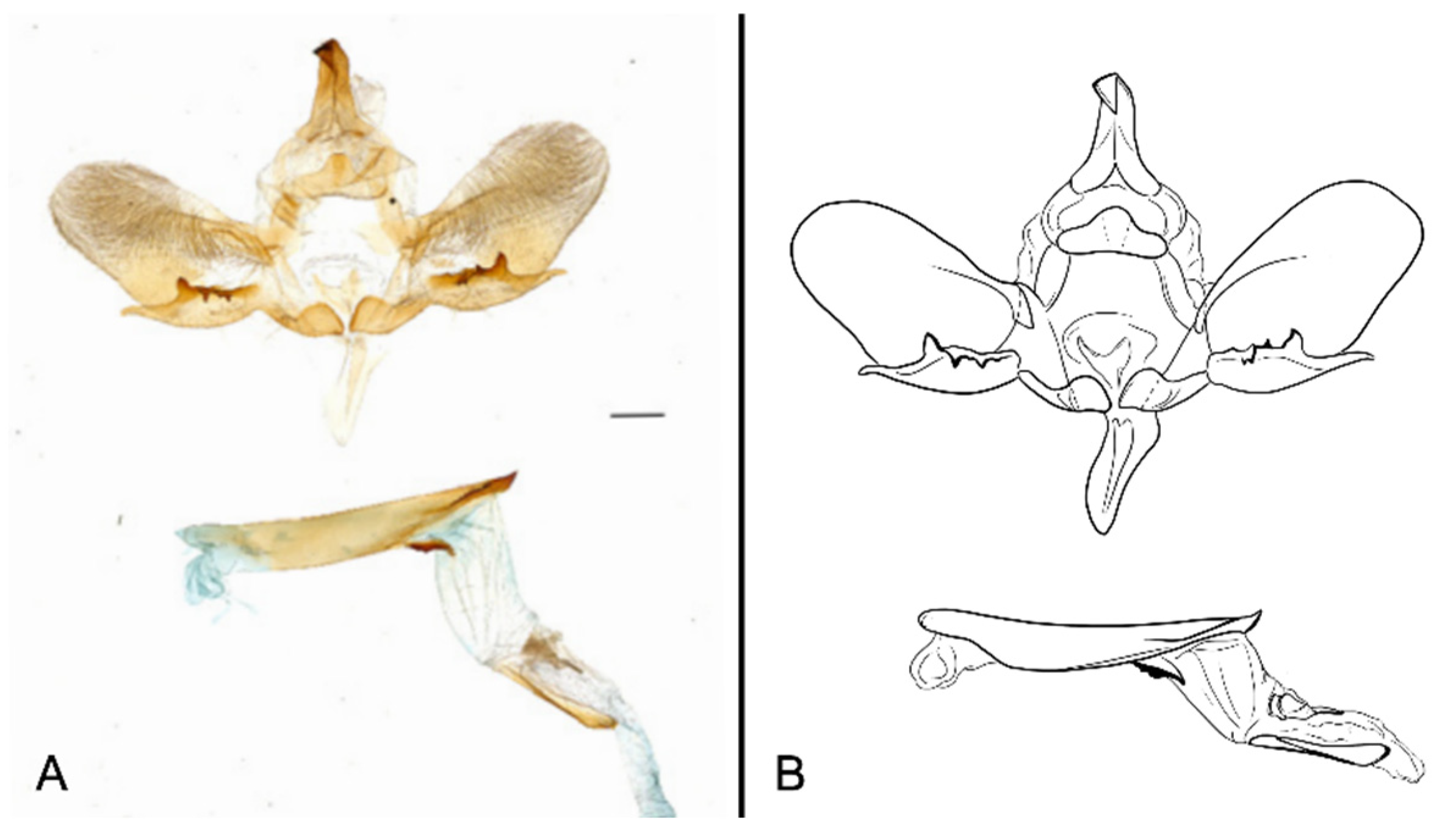
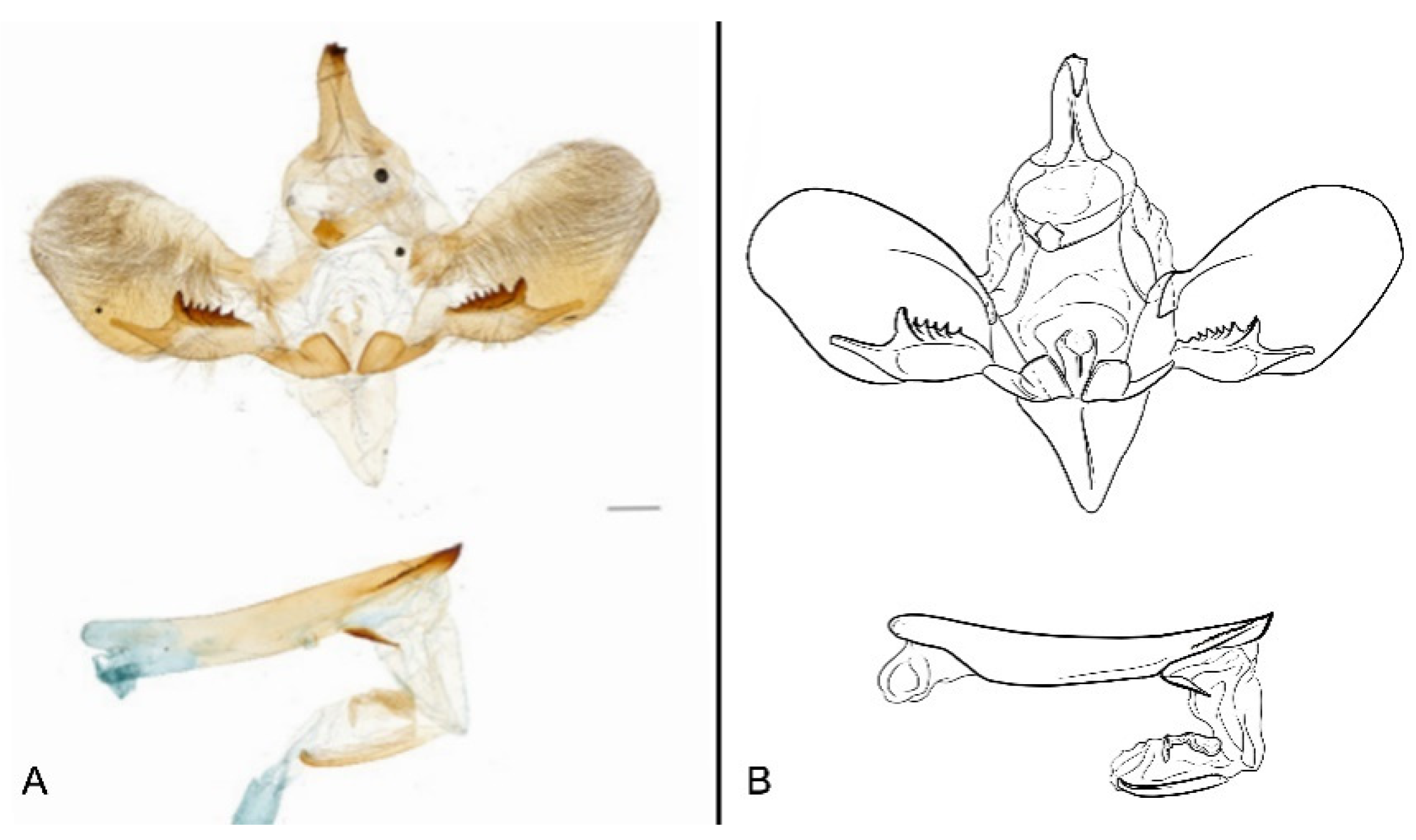
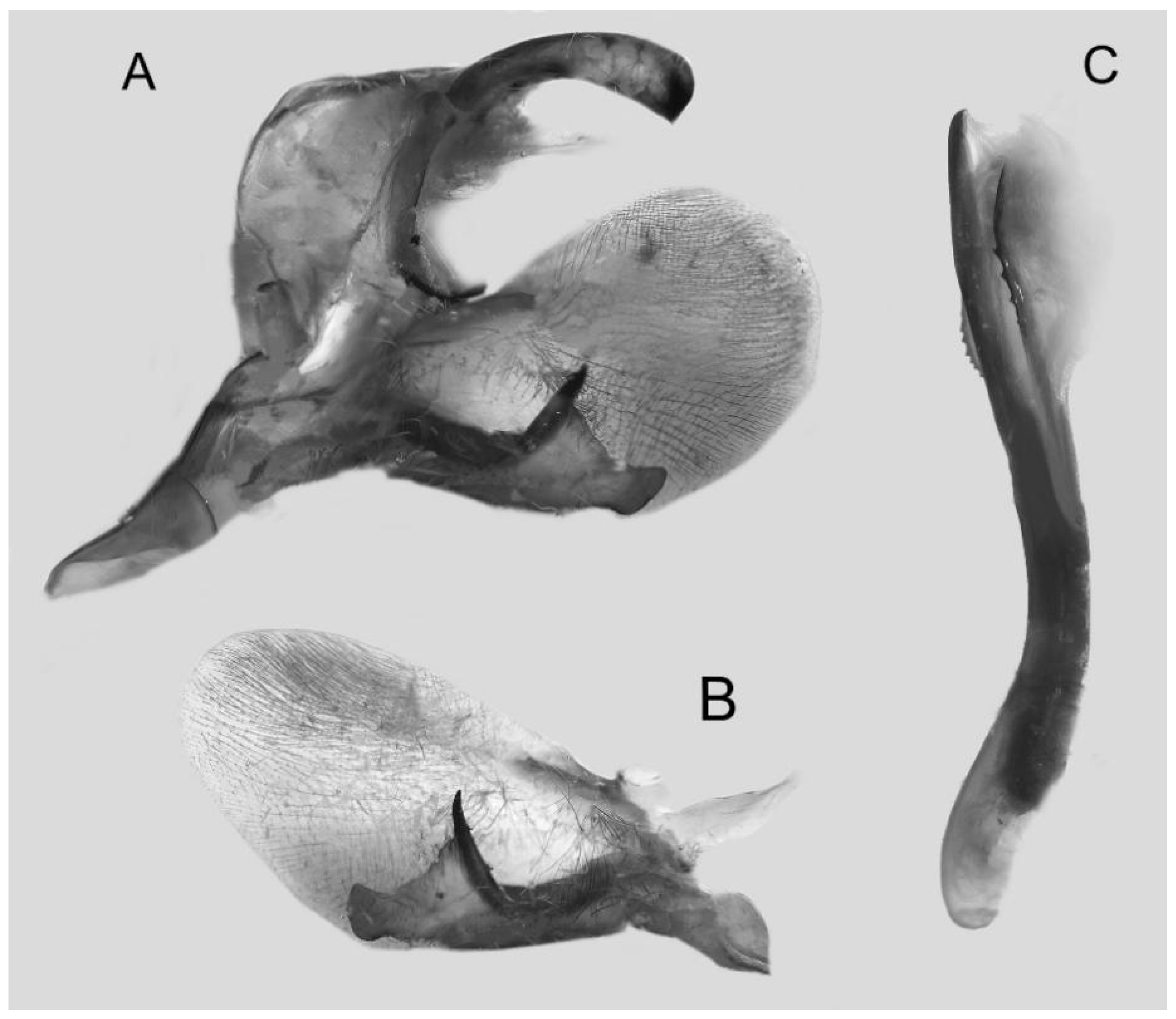
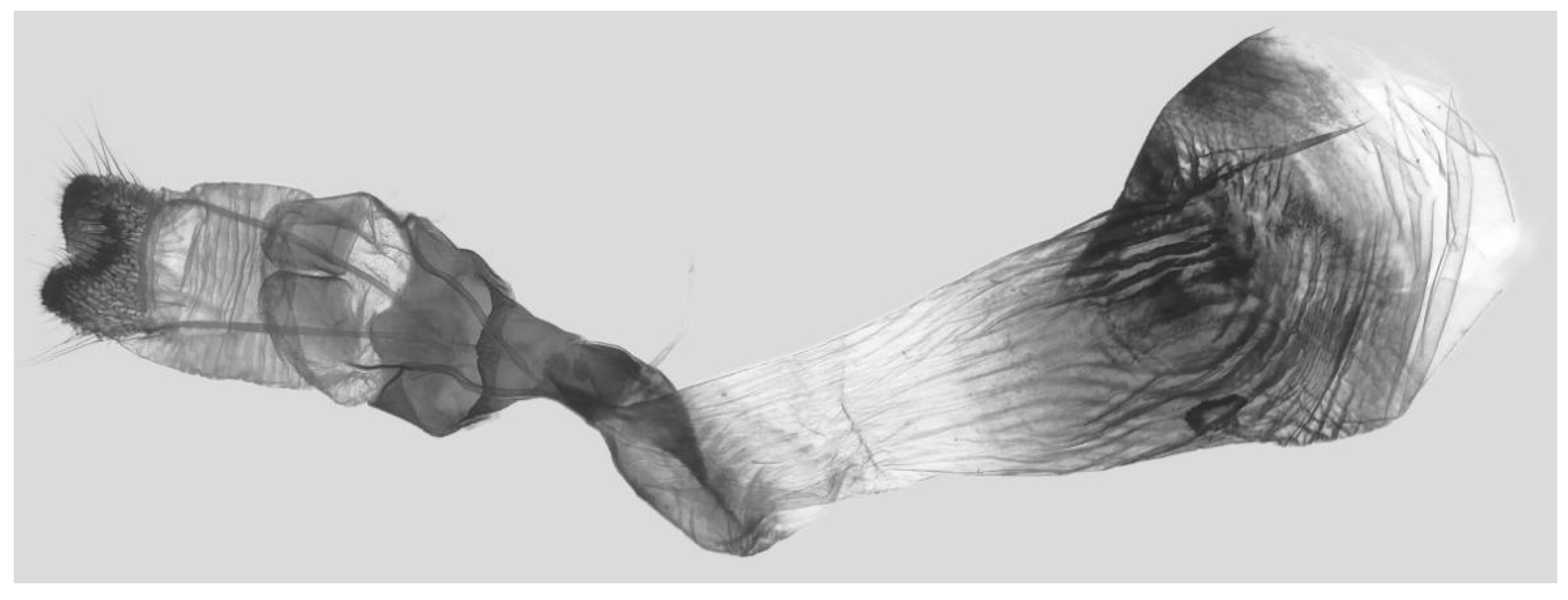
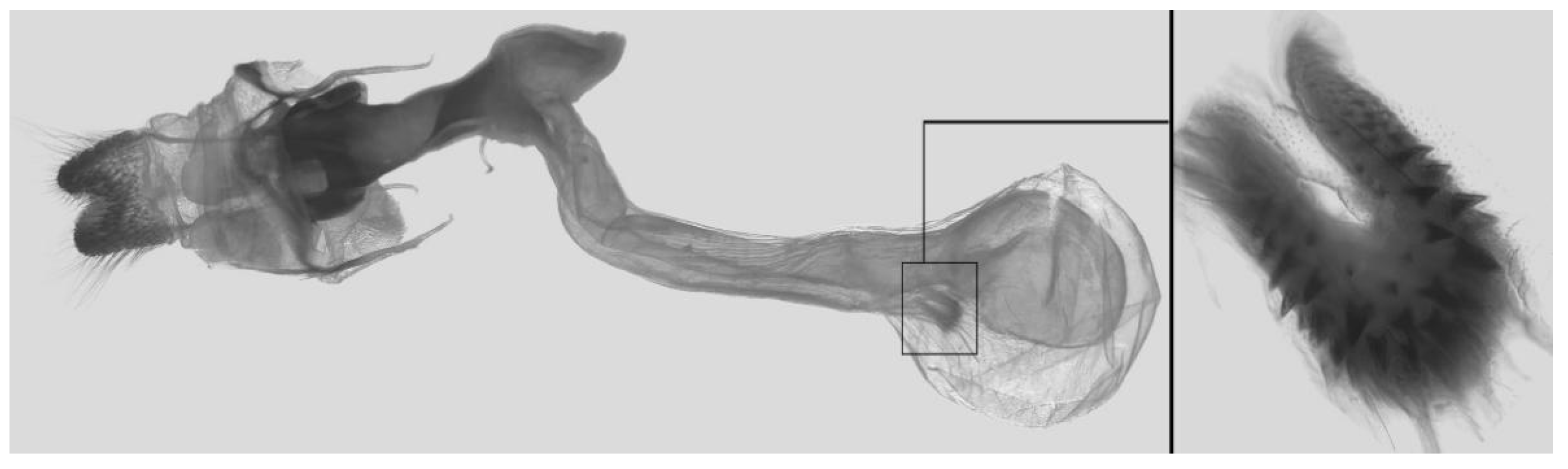
Figure 7.
Male genitalia of Ambulyx canescens, Yingjiang, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 7.
Male genitalia of Ambulyx canescens, Yingjiang, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
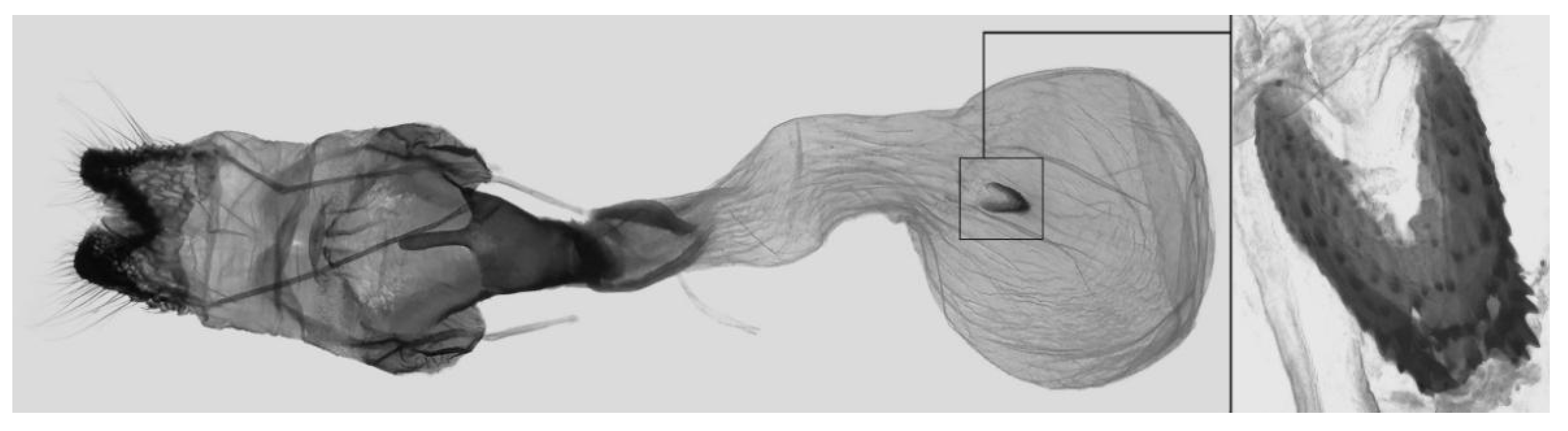
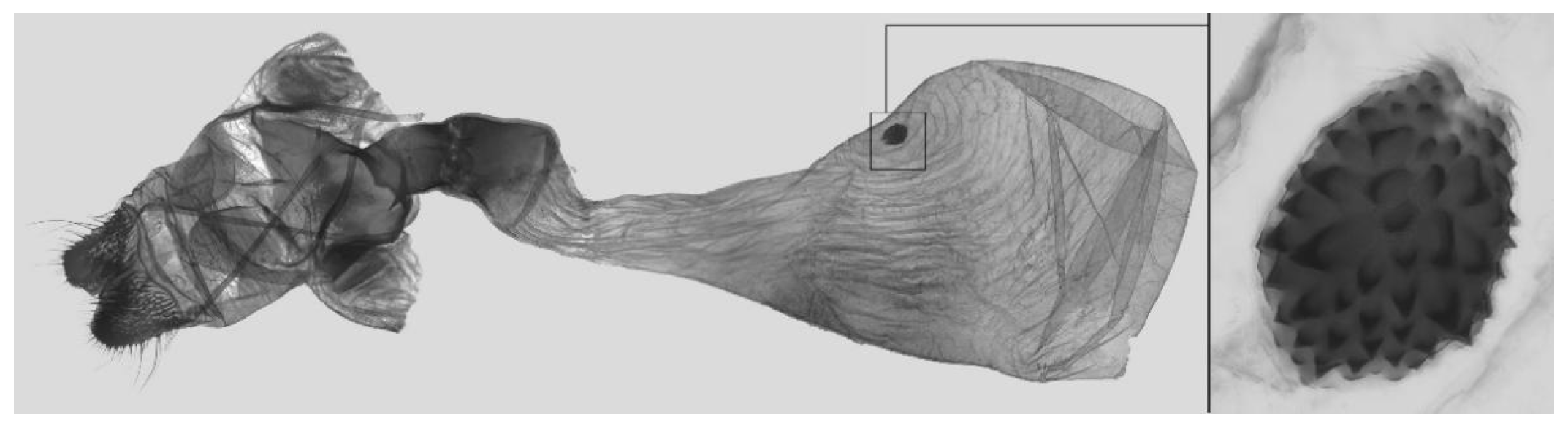
Figure 8.
Female genitalia of
Ambulyx canescens, Kao Yai, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on accessed 20 April 2024.
Figure 8.
Female genitalia of
Ambulyx canescens, Kao Yai, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on accessed 20 April 2024.
Figure 9.
Habitat and living adult of Ambulyx canescens. (A) Male; (B) Yingjiang County, Yunnan, China.
Figure 9.
Habitat and living adult of Ambulyx canescens. (A) Male; (B) Yingjiang County, Yunnan, China.
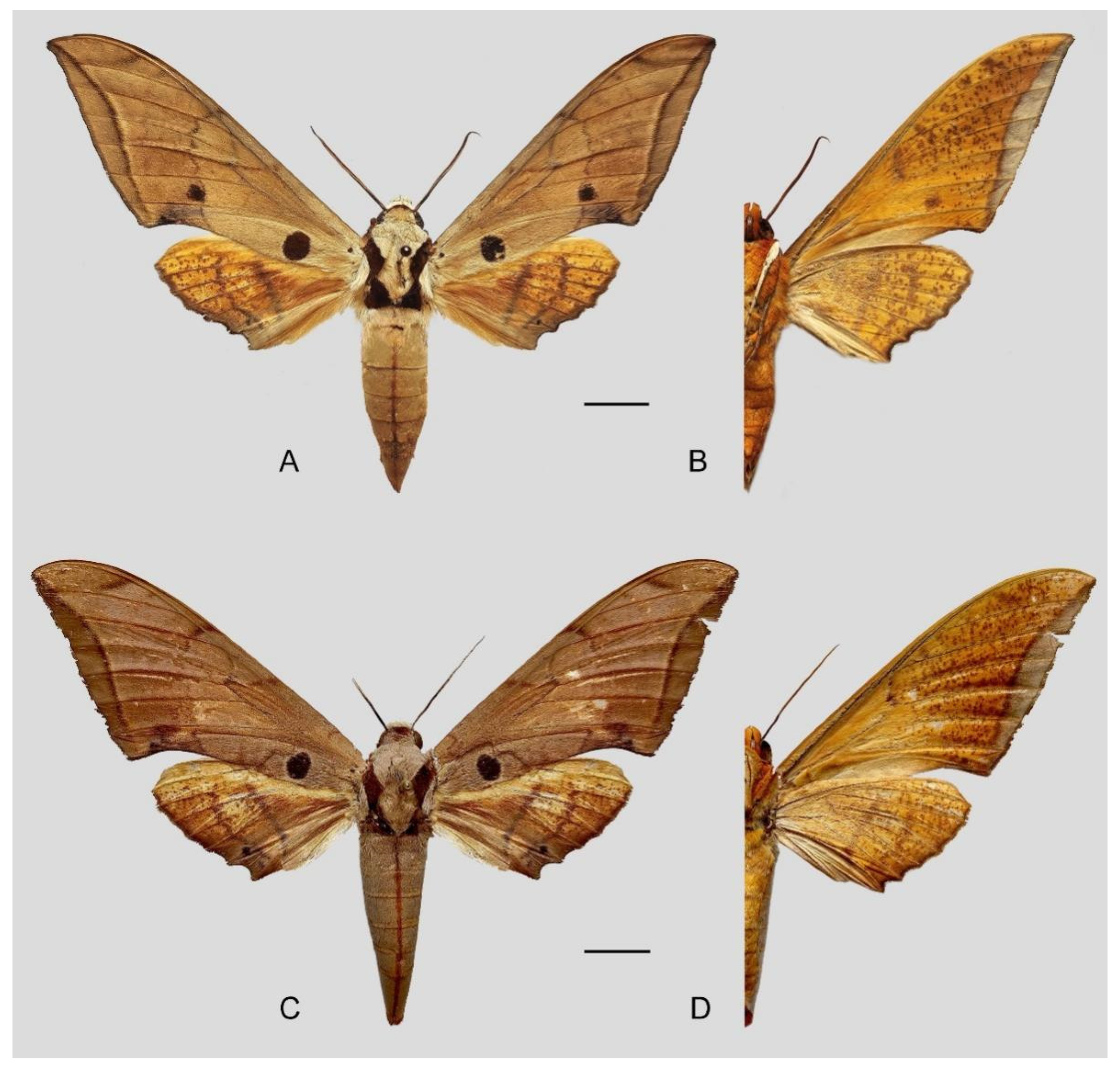
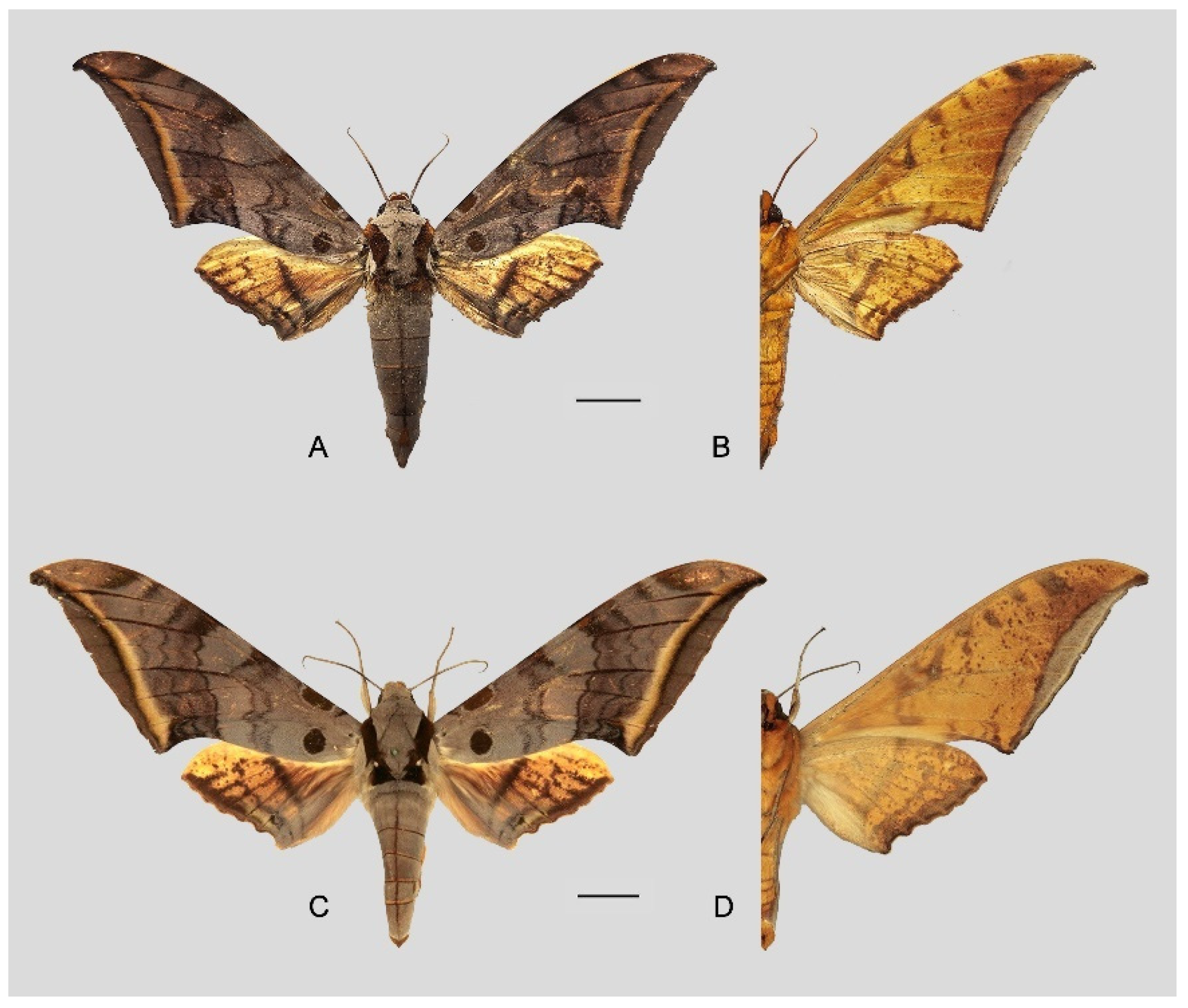
Figure 10.
Ambulyx japonica japonica. (
A,
B) Male, Mikoboyama, Gumma, Japan; (
C,
D) female, Iruma, Saitama, Japan. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info, accessed on 20 April 2024.
Figure 10.
Ambulyx japonica japonica. (
A,
B) Male, Mikoboyama, Gumma, Japan; (
C,
D) female, Iruma, Saitama, Japan. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info, accessed on 20 April 2024.
Figure 11.
Male genitalia of
Ambulyx japonica japonica, Namezawa Spa, Gumma, Japan. (
A) Lateral view; (
B) left valve; (
C) phallus. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 11.
Male genitalia of
Ambulyx japonica japonica, Namezawa Spa, Gumma, Japan. (
A) Lateral view; (
B) left valve; (
C) phallus. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 12.
Female genitalia of
Ambulyx japonica japonica, Iruma, Saitama, Japan. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info, accessed on 20 April 2024.
Figure 12.
Female genitalia of
Ambulyx japonica japonica, Iruma, Saitama, Japan. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info, accessed on 20 April 2024.
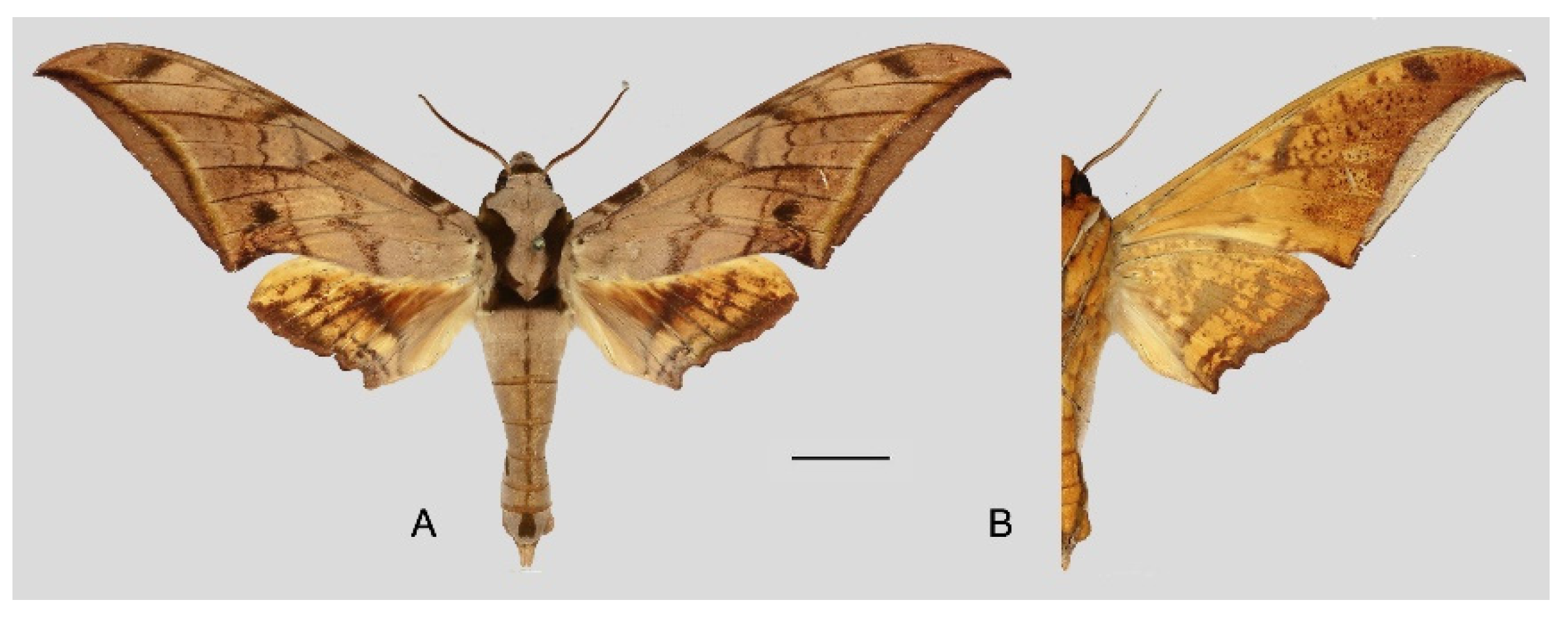
Figure 13.
Habitat of Ambulyx japonica japonica, Kaga, Ishikawa-ken, Japan.
Figure 13.
Habitat of Ambulyx japonica japonica, Kaga, Ishikawa-ken, Japan.
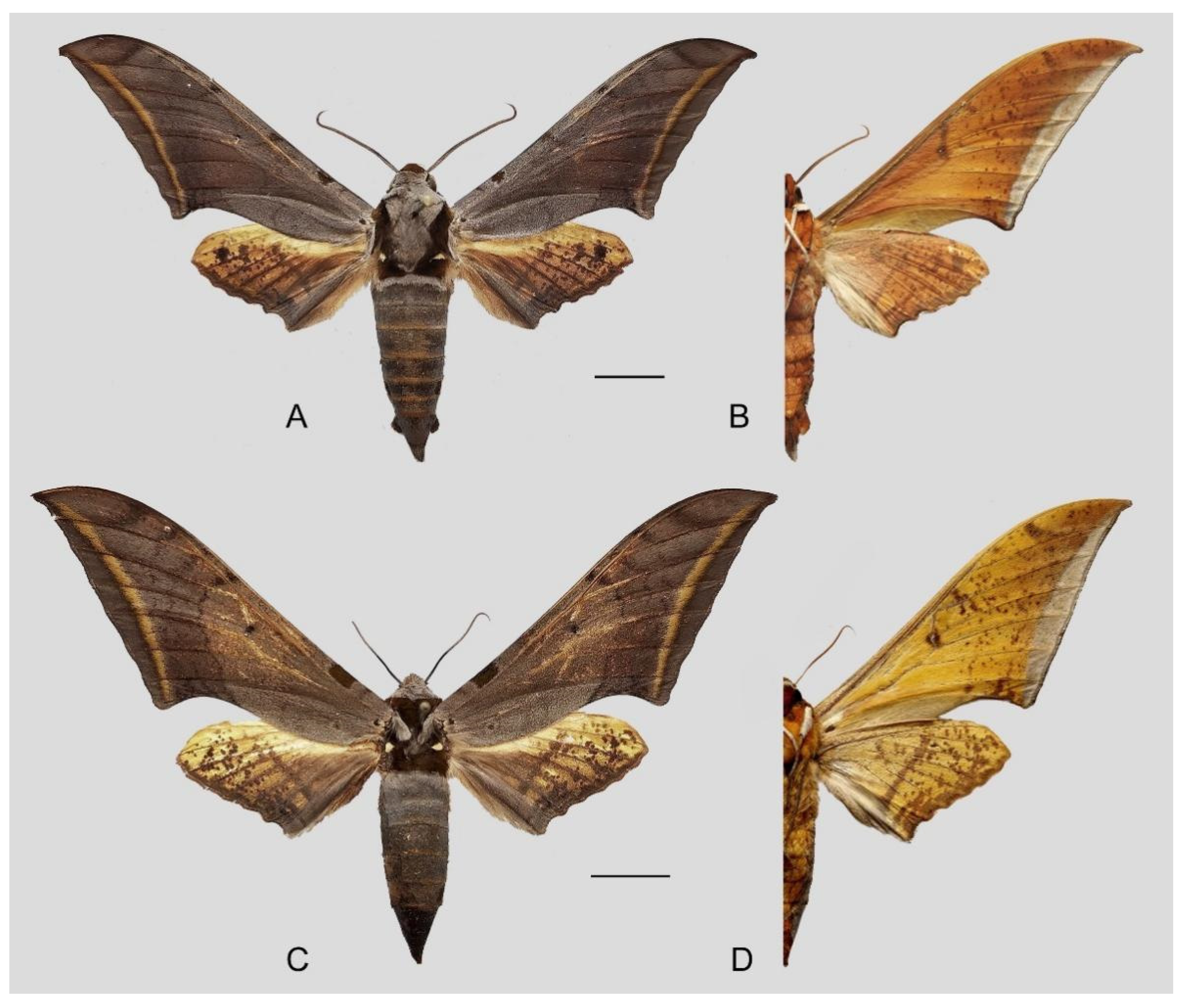
Figure 14.
Ambulyx japonica angustifasciata. (A,B) Male, Donghe Township, Taitung County, Taiwan, China; (C,D) female, Puli, Nantou County, Taiwan, China. © Sphingidae Museum, Czech Republic. Scale bar = 10 mm.
Figure 14.
Ambulyx japonica angustifasciata. (A,B) Male, Donghe Township, Taitung County, Taiwan, China; (C,D) female, Puli, Nantou County, Taiwan, China. © Sphingidae Museum, Czech Republic. Scale bar = 10 mm.
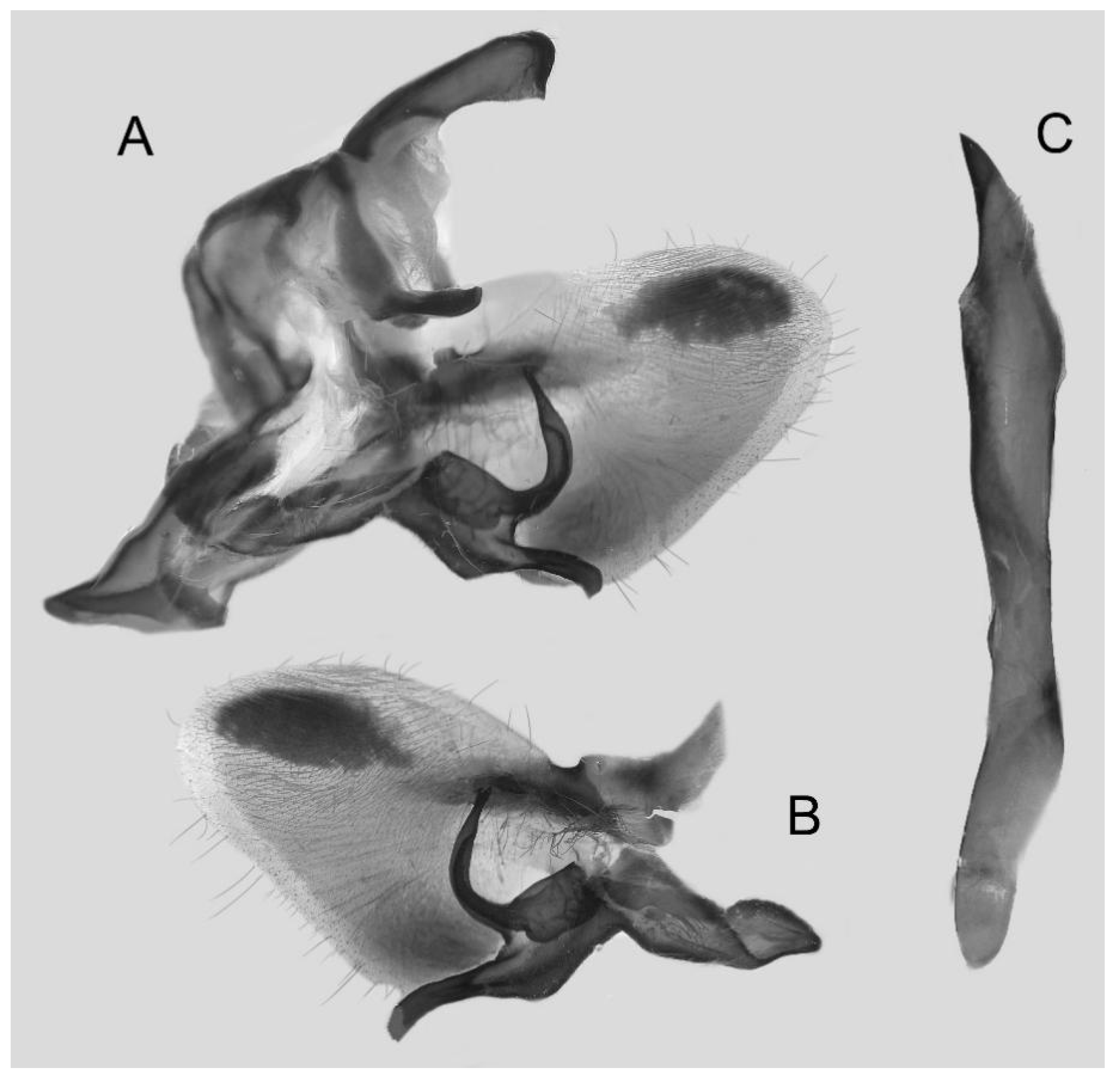
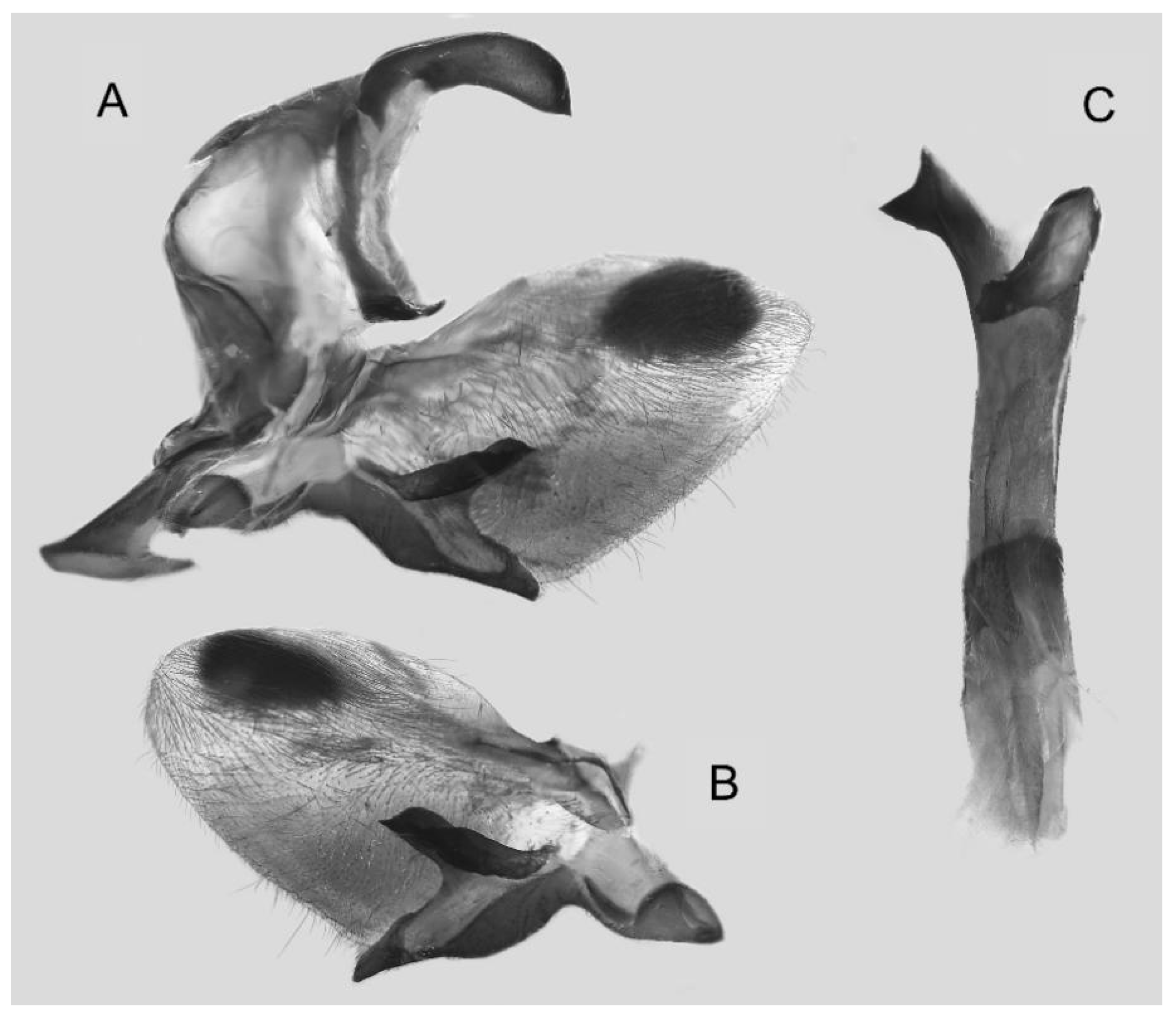
Figure 15.
Male genitalia of Ambulyx japonica angustifasciata, Donghe Township, Taitung County, Taiwan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 15.
Male genitalia of Ambulyx japonica angustifasciata, Donghe Township, Taitung County, Taiwan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 16.
Habitat and living adult of Ambulyx japonica angustifasciata. (A) Female; (B) Taitung County, Taiwan, China.
Figure 16.
Habitat and living adult of Ambulyx japonica angustifasciata. (A) Female; (B) Taitung County, Taiwan, China.
Figure 17.
Ambulyx japonica koreana. (A,B) Male, Wuxi County, Chongqing, China; (C,D) female, Mentougou, Beijing, China. Scale bar = 10 mm.
Figure 17.
Ambulyx japonica koreana. (A,B) Male, Wuxi County, Chongqing, China; (C,D) female, Mentougou, Beijing, China. Scale bar = 10 mm.
Figure 18.
Male genitalia of Ambulyx japonica koreana, Changyang County, Hubei, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 18.
Male genitalia of Ambulyx japonica koreana, Changyang County, Hubei, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 19.
Female genitalia of Ambulyx japonica koreana, Wuxi County, Chongqing, China.
Figure 19.
Female genitalia of Ambulyx japonica koreana, Wuxi County, Chongqing, China.
Figure 20.
Habitat and living adults of Ambulyx japonica koreana. (A) Male; (B) female; (C) Wuxi County, Chongqing, China.
Figure 20.
Habitat and living adults of Ambulyx japonica koreana. (A) Male; (B) female; (C) Wuxi County, Chongqing, China.
Figure 21.
Ambulyx wukong sp. nov. (A,B) Male, HOLOTYPE, Tacheng, Weixi County, Yunnan, China; (C,D) Female, PARATYPE, Tacheng, Weixi County, Yunnan, China. Scale bar = 10 mm.
Figure 21.
Ambulyx wukong sp. nov. (A,B) Male, HOLOTYPE, Tacheng, Weixi County, Yunnan, China; (C,D) Female, PARATYPE, Tacheng, Weixi County, Yunnan, China. Scale bar = 10 mm.
Figure 22.
PARATYPE, male genitalia of Ambulyx wukong sp. nov., Tacheng, Weixi County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 22.
PARATYPE, male genitalia of Ambulyx wukong sp. nov., Tacheng, Weixi County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 23.
PARATYPE, female genitalia of Ambulyx wukong sp. nov., Tacheng, Weixi County, Yunnan, China.
Figure 23.
PARATYPE, female genitalia of Ambulyx wukong sp. nov., Tacheng, Weixi County, Yunnan, China.
Figure 24.
Habitat and living adult of Ambulyx wukong sp. nov. (A) Male; (B) Weixi County, Yunnan, China.
Figure 24.
Habitat and living adult of Ambulyx wukong sp. nov. (A) Male; (B) Weixi County, Yunnan, China.
Figure 25.
Ambulyx kuangtungensis. (A,B) Male, Maolan Nature Reserve, Libo, Guizhou, China; (C,D) female, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China. Scale bar = 10 mm.
Figure 25.
Ambulyx kuangtungensis. (A,B) Male, Maolan Nature Reserve, Libo, Guizhou, China; (C,D) female, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China. Scale bar = 10 mm.
Figure 26.
Male genitalia of Ambulyx kuangtungensis, Mt. Tianmushan, Zhejiang, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 26.
Male genitalia of Ambulyx kuangtungensis, Mt. Tianmushan, Zhejiang, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 27.
Female genitalia of Ambulyx kuangtungensis, Yingshan County, Hubei, China.
Figure 27.
Female genitalia of Ambulyx kuangtungensis, Yingshan County, Hubei, China.
Figure 28.
Habitat and living adults of Ambulyx kuangtungensis. (A) Male; (B) female; (C) Wuxi County, Chongqing, China.
Figure 28.
Habitat and living adults of Ambulyx kuangtungensis. (A) Male; (B) female; (C) Wuxi County, Chongqing, China.
Figure 29.
Ambulyx latifascia. (A,B) Male, Hutiaoxia, Lijiang, Yunnan, China; (C,D) female, Hutiaoxia, Lijiang, Yunnan, China. Scale bar = 10 mm.
Figure 29.
Ambulyx latifascia. (A,B) Male, Hutiaoxia, Lijiang, Yunnan, China; (C,D) female, Hutiaoxia, Lijiang, Yunnan, China. Scale bar = 10 mm.
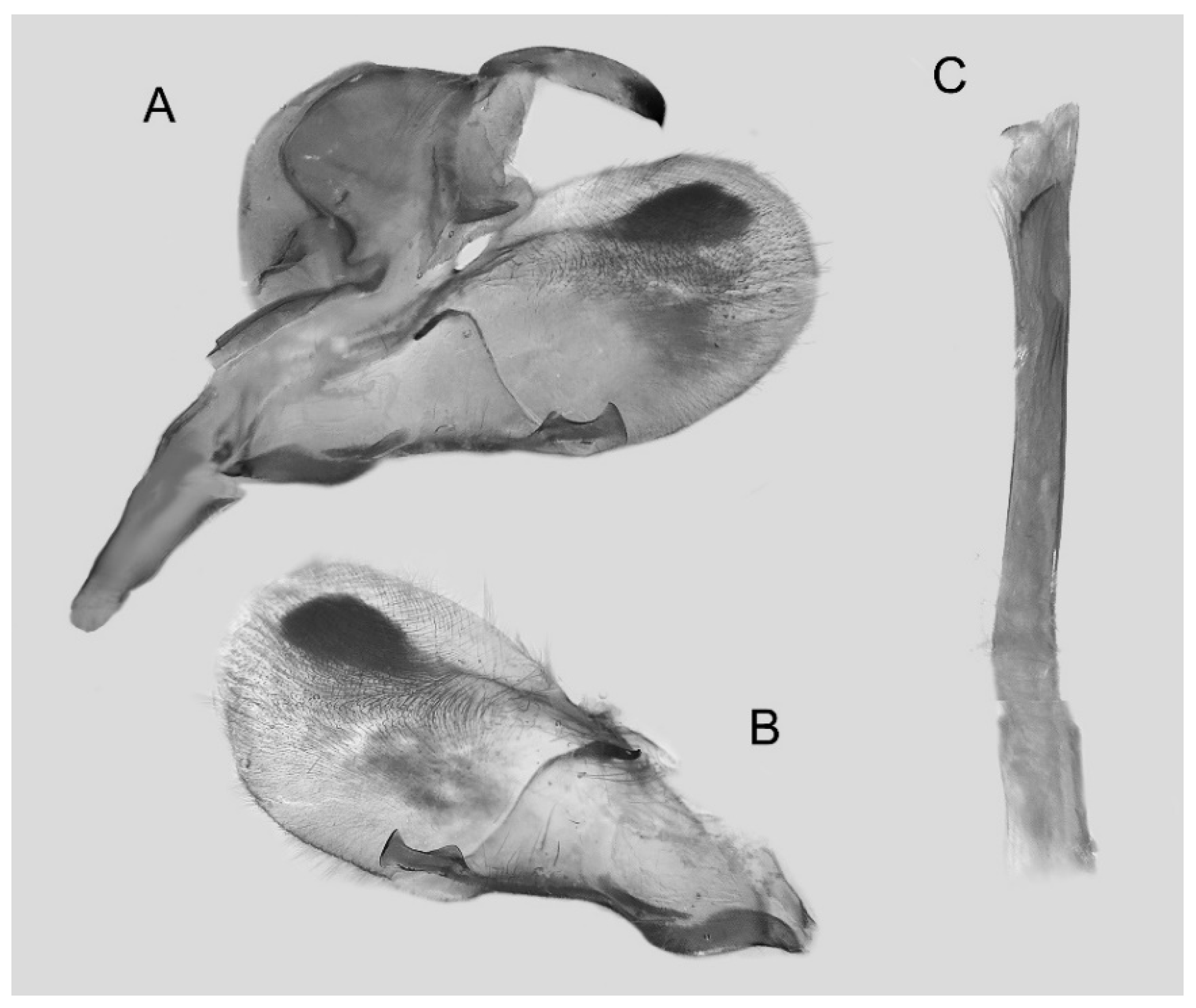
Figure 30.
Male genitalia of Ambulyx latifascia, Renhe Village, Lijiang, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 30.
Male genitalia of Ambulyx latifascia, Renhe Village, Lijiang, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 31.
Female genitalia of Ambulyx latifascia, Hutiaoxia, Lijiang, Yunnan, China.
Figure 31.
Female genitalia of Ambulyx latifascia, Hutiaoxia, Lijiang, Yunnan, China.
Figure 32.
Habitat and living adults of Ambulyx latifascia. (A) Male; (B) female; (C) Weixi County, Yunnan, China.
Figure 32.
Habitat and living adults of Ambulyx latifascia. (A) Male; (B) female; (C) Weixi County, Yunnan, China.
Figure 33.
Ambulyx liturata. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan; (C,D) female, Youxi County, Sanming, Fujian, China. Scale bar = 10 mm.
Figure 33.
Ambulyx liturata. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan; (C,D) female, Youxi County, Sanming, Fujian, China. Scale bar = 10 mm.
Figure 34.
Male genitalia of Ambulyx liturata, Mt. Tianmushan, Zhejiang, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 34.
Male genitalia of Ambulyx liturata, Mt. Tianmushan, Zhejiang, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 35.
Female genitalia of Ambulyx liturata, Youxi County, Sanming, Fujian, China.
Figure 35.
Female genitalia of Ambulyx liturata, Youxi County, Sanming, Fujian, China.
Figure 36.
Habitat and living adults of Ambulyx liturata. (A) Male; (B) female; (C) Lingshui, Hainan, China.
Figure 36.
Habitat and living adults of Ambulyx liturata. (A) Male; (B) female; (C) Lingshui, Hainan, China.
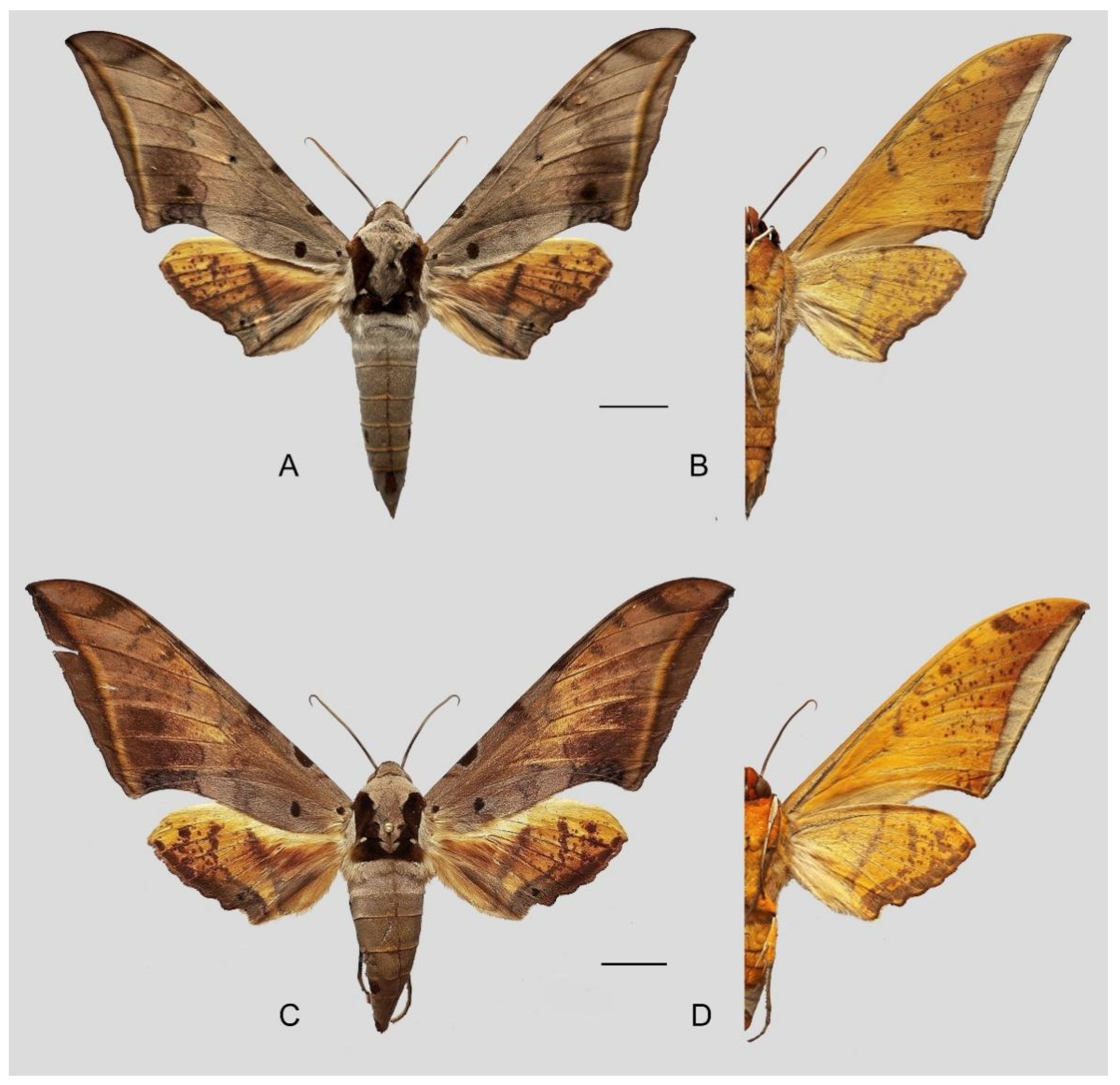
Figure 37.
Ambulyx maculifera. (A,B) Male, Pu’er, Yunnan, China; (C,D) female, Xima Town, Yingjiang County, Yunnan, China. Scale bar = 10 mm.
Figure 37.
Ambulyx maculifera. (A,B) Male, Pu’er, Yunnan, China; (C,D) female, Xima Town, Yingjiang County, Yunnan, China. Scale bar = 10 mm.
Figure 38.
Male genitalia of Ambulyx maculifera, Motuo County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 38.
Male genitalia of Ambulyx maculifera, Motuo County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 39.
Female genitalia of Ambulyx maculifera, Xima Town, Yingjiang County, Yunnan, China.
Figure 39.
Female genitalia of Ambulyx maculifera, Xima Town, Yingjiang County, Yunnan, China.
Figure 40.
Habitat and living adults of Ambulyx maculifera. (A) Male; (B) female; (C) Dulongjiang, Yunnan, China.
Figure 40.
Habitat and living adults of Ambulyx maculifera. (A) Male; (B) female; (C) Dulongjiang, Yunnan, China.
Figure 41.
Ambulyx moorei. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan, China; (C,D) female, Xima Town, Yingjiang County, Yunnan, China. Scale bar = 10 mm.
Figure 41.
Ambulyx moorei. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan, China; (C,D) female, Xima Town, Yingjiang County, Yunnan, China. Scale bar = 10 mm.
Figure 42.
Male genitalia of Ambulyx moorei, Mengla County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 42.
Male genitalia of Ambulyx moorei, Mengla County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 43.
Female genitalia of Ambulyx moorei, Xima Town, Yingjiang County, Yunnan.
Figure 43.
Female genitalia of Ambulyx moorei, Xima Town, Yingjiang County, Yunnan.
Figure 44.
Habitat and living adults of Ambulyx moorei. (A) Male; (B) female; (C) Jinping, Yunnan, China.
Figure 44.
Habitat and living adults of Ambulyx moorei. (A) Male; (B) female; (C) Jinping, Yunnan, China.
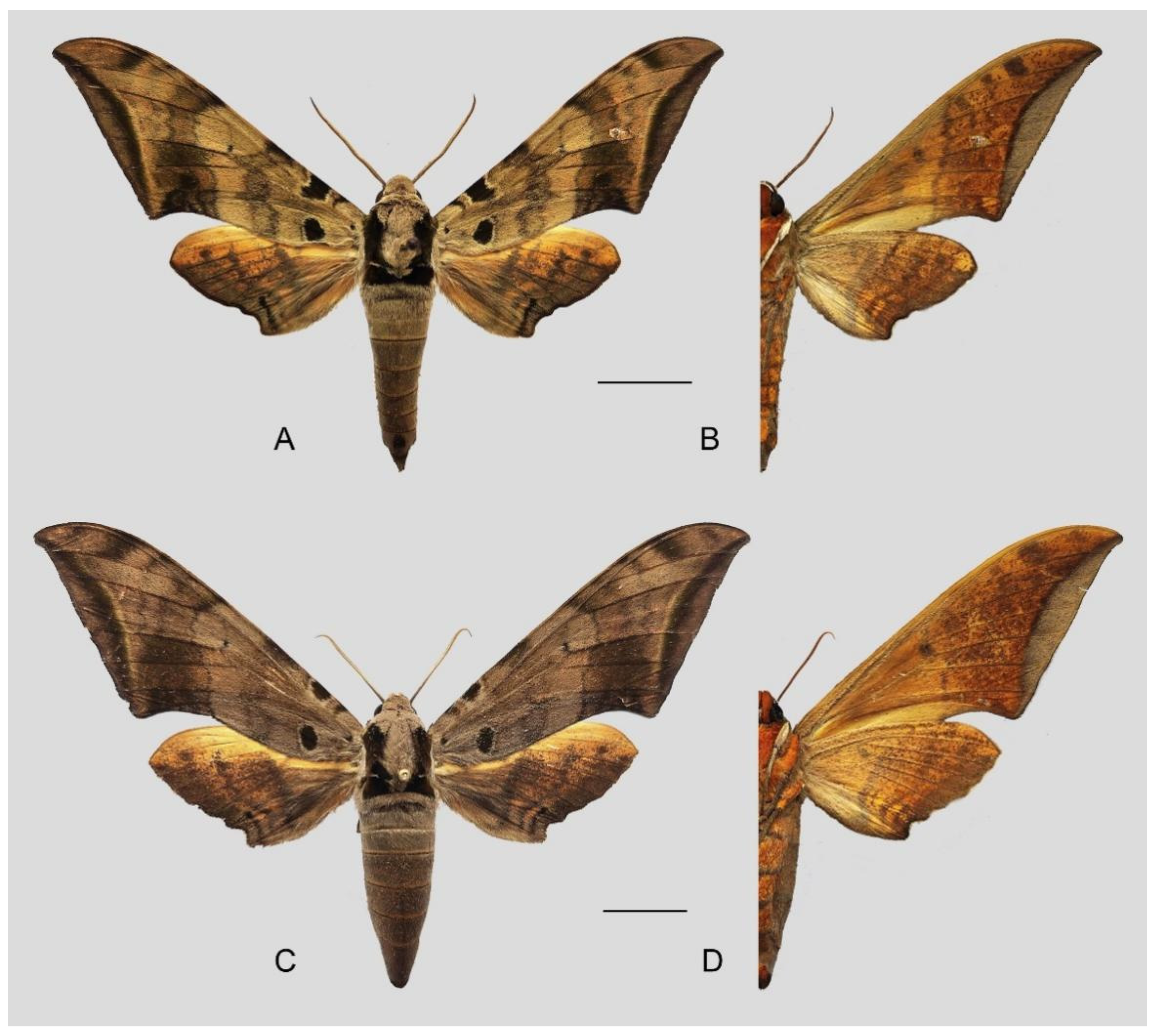
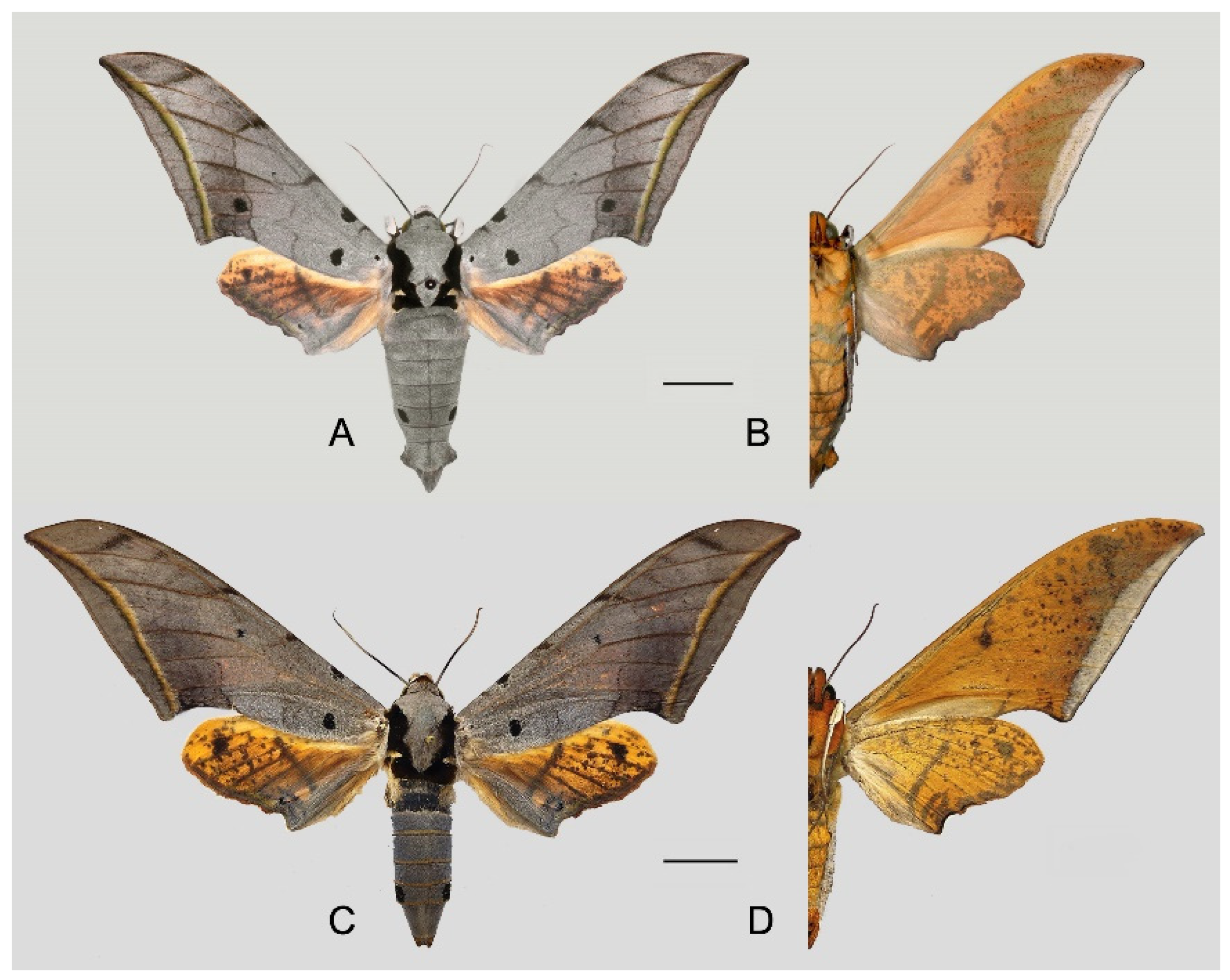
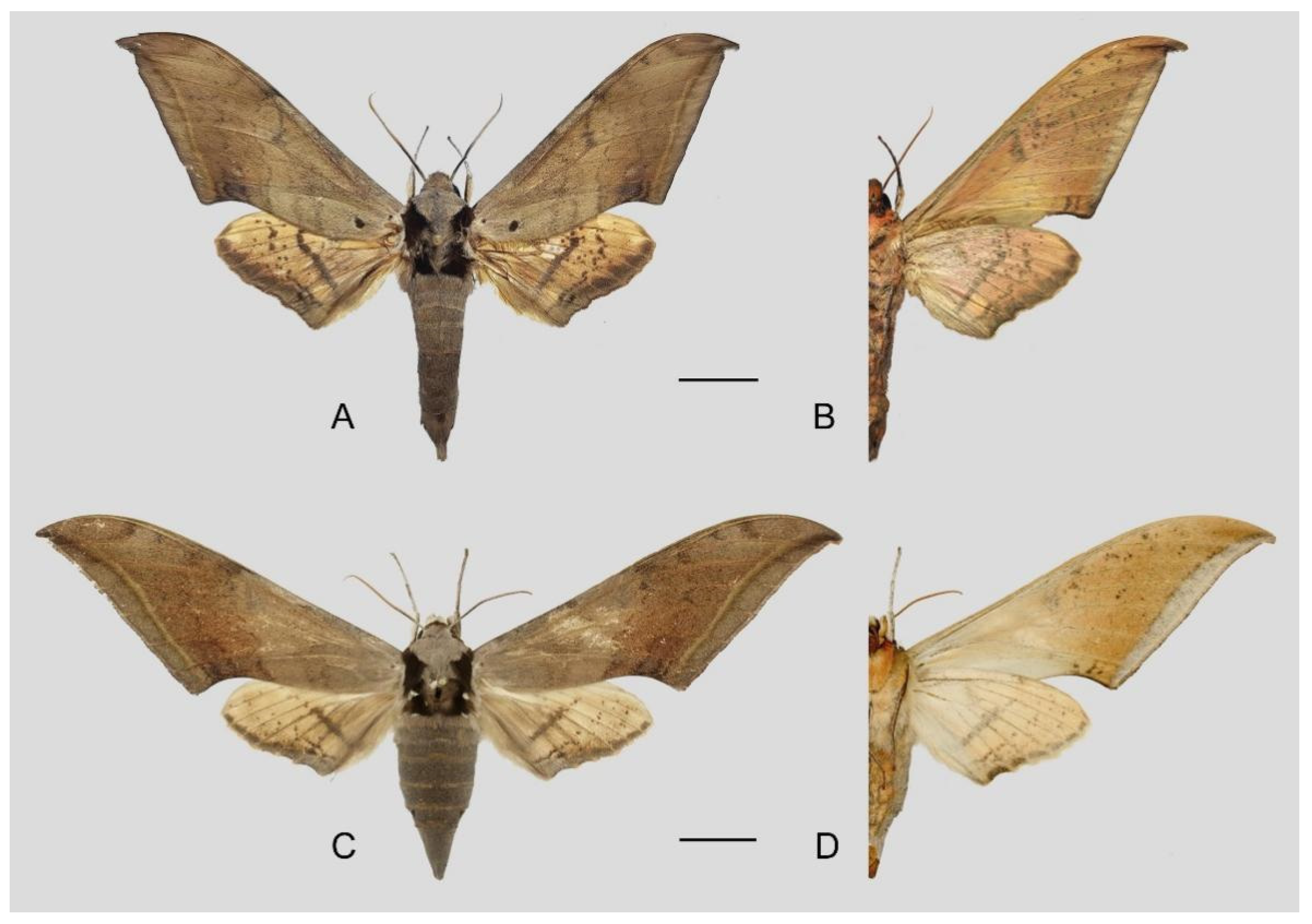
Figure 49.
Ambulyx placida. (A,B) Male, Nyingchi, Xizang, China; (C,D) female, S. Xizang, China. © Sphingidae Museum. Scale bar = 10 mm.
Figure 49.
Ambulyx placida. (A,B) Male, Nyingchi, Xizang, China; (C,D) female, S. Xizang, China. © Sphingidae Museum. Scale bar = 10 mm.
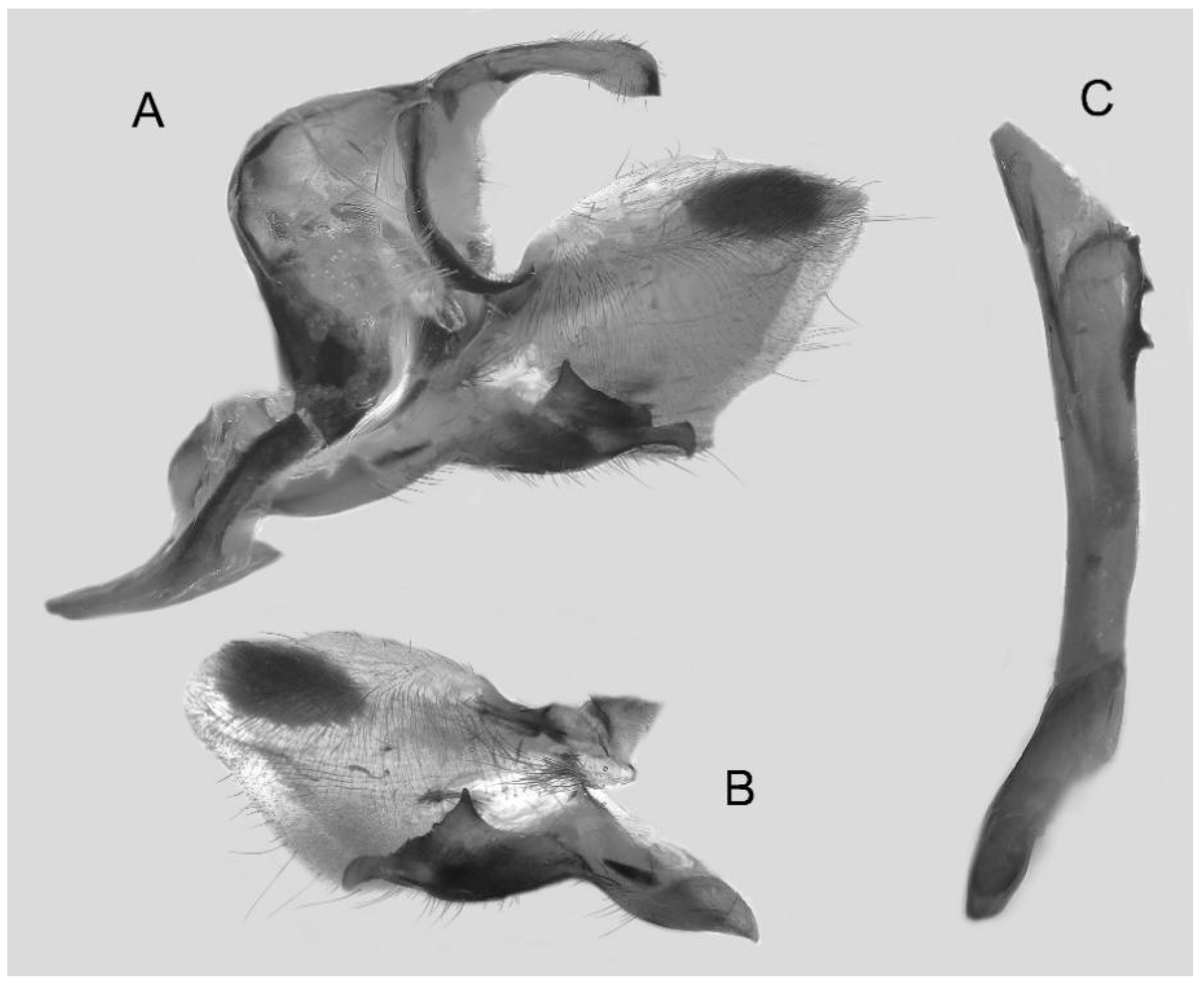
Figure 50.
Male genitalia of Ambulyx placida, Nyingchi, Xizang, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 50.
Male genitalia of Ambulyx placida, Nyingchi, Xizang, China. (A) Lateral view; (B) left valve; (C) phallus.
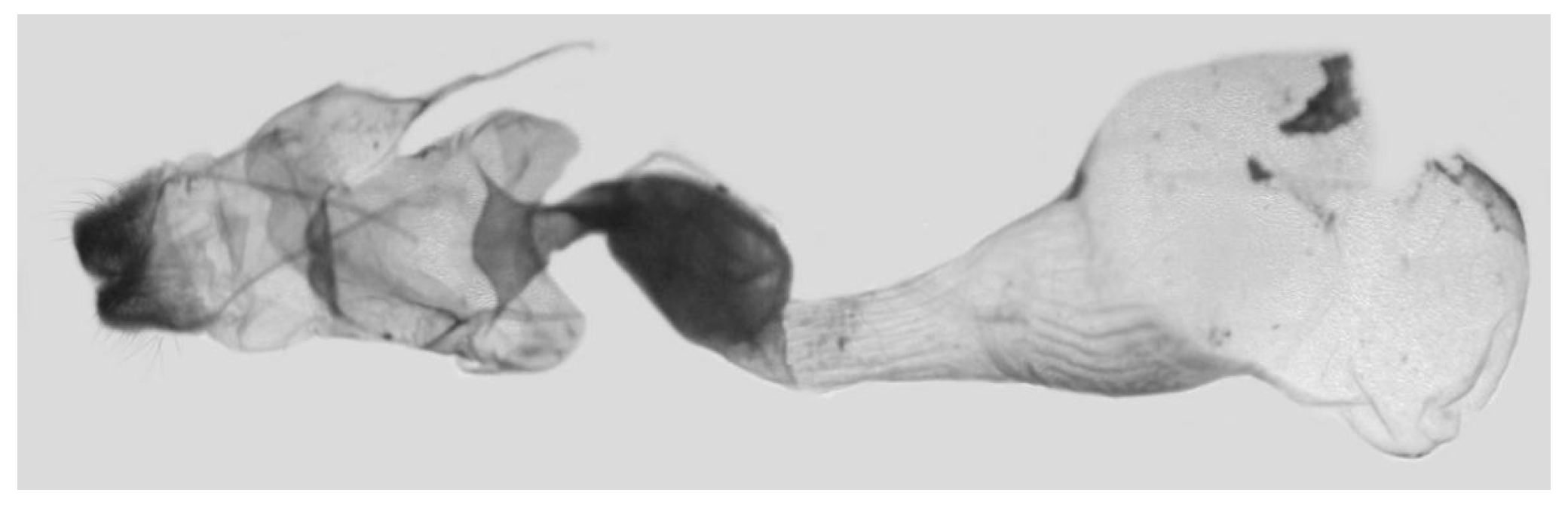
Figure 51.
Female genitalia of
Ambulyx placida, © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 51.
Female genitalia of
Ambulyx placida, © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 52.
Habitat and living adult of Ambulyx placida. (A) Male; (B) Nyingchi, Xizang, China.
Figure 52.
Habitat and living adult of Ambulyx placida. (A) Male; (B) Nyingchi, Xizang, China.
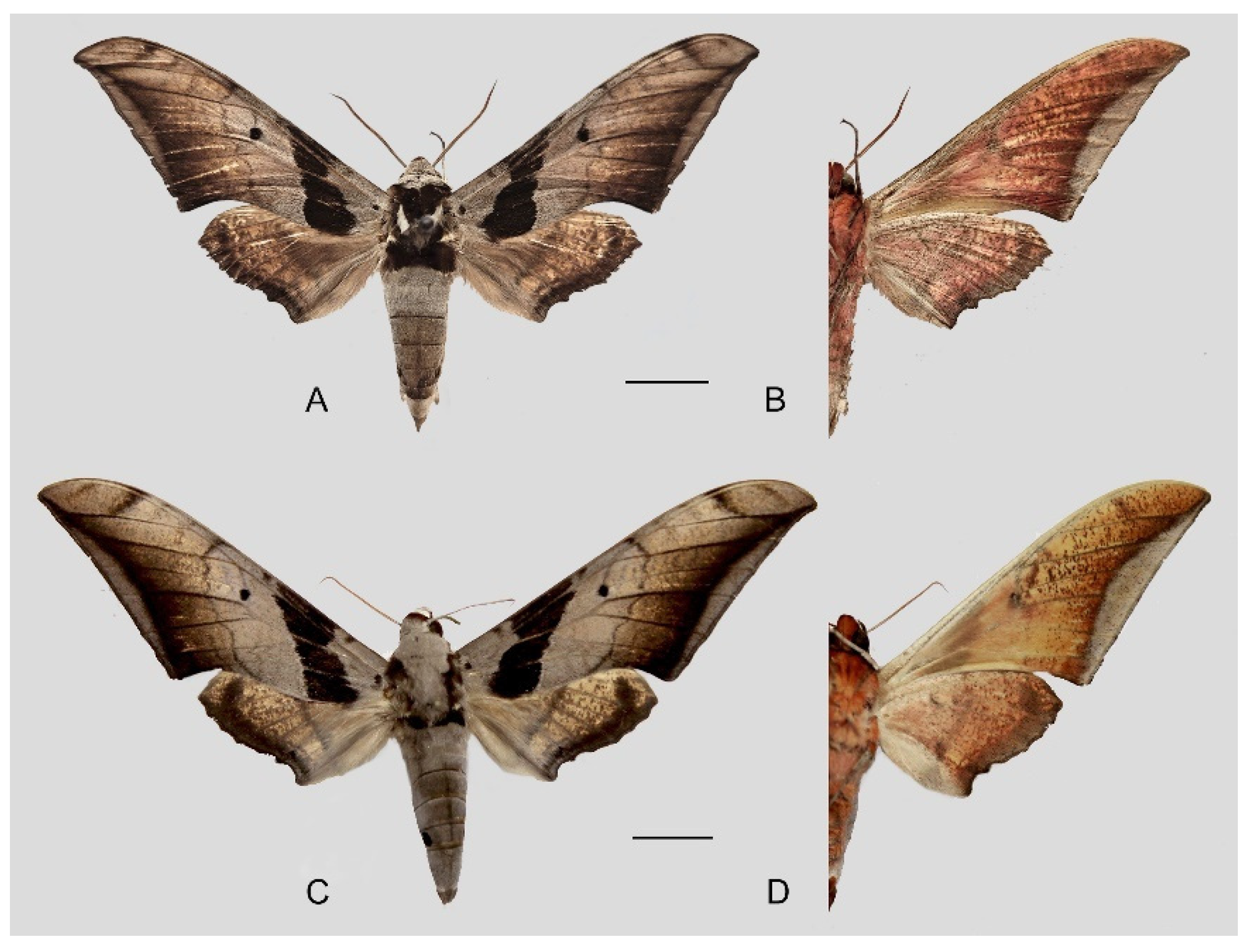
Figure 53.
Ambulyx schauffelbergeri. (A,B) Male, Mt. Laoshan, Nanjing, Jiangsu, China; (C,D) female, Zhenping County, Shaanxi, China. Scale bar = 10 mm.
Figure 53.
Ambulyx schauffelbergeri. (A,B) Male, Mt. Laoshan, Nanjing, Jiangsu, China; (C,D) female, Zhenping County, Shaanxi, China. Scale bar = 10 mm.
Figure 54.
Male genitalia of Ambulyx schauffelbergeri, Menghai County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 54.
Male genitalia of Ambulyx schauffelbergeri, Menghai County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 55.
Female genitalia of Ambulyx schauffelbergeri, Yingshan County, Hubei, China.
Figure 55.
Female genitalia of Ambulyx schauffelbergeri, Yingshan County, Hubei, China.
Figure 56.
Habitat and living adults of Ambulyx schauffelbergeri. (A) Male; (B) female; (C) Yuexi County, Anhui, China.
Figure 56.
Habitat and living adults of Ambulyx schauffelbergeri. (A) Male; (B) female; (C) Yuexi County, Anhui, China.
Figure 57.
Ambulyx semiplacida semiplacida. (A,B) Male, Mt. Alishan, Taiwan, China; (C,D) female, Nantou County, Taiwan, China. Scale bar = 10 mm.
Figure 57.
Ambulyx semiplacida semiplacida. (A,B) Male, Mt. Alishan, Taiwan, China; (C,D) female, Nantou County, Taiwan, China. Scale bar = 10 mm.
Figure 58.
Male genitalia of Ambulyx semiplacida semiplacida, Mt. Alishan, Taiwan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 58.
Male genitalia of Ambulyx semiplacida semiplacida, Mt. Alishan, Taiwan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 59.
Female genitalia of Ambulyx semiplacida semiplacida, Nantou County, Taiwan, China.
Figure 59.
Female genitalia of Ambulyx semiplacida semiplacida, Nantou County, Taiwan, China.
Figure 60.
Habitat and living adult of Ambulyx semiplacida semiplacida. (A) Male; (B) Alishan, Taiwan, China.
Figure 60.
Habitat and living adult of Ambulyx semiplacida semiplacida. (A) Male; (B) Alishan, Taiwan, China.
Figure 61.
Ambulyx semiplacida bhutana. (A,B) HOLOTYPE, male, Trongsa Dzong, Bhutan; (C,D) ALLOTYPE, female, Jongkhar, Morong, Bhutan. Scale bar = 10 mm. © Ronald Brechlin.
Figure 61.
Ambulyx semiplacida bhutana. (A,B) HOLOTYPE, male, Trongsa Dzong, Bhutan; (C,D) ALLOTYPE, female, Jongkhar, Morong, Bhutan. Scale bar = 10 mm. © Ronald Brechlin.
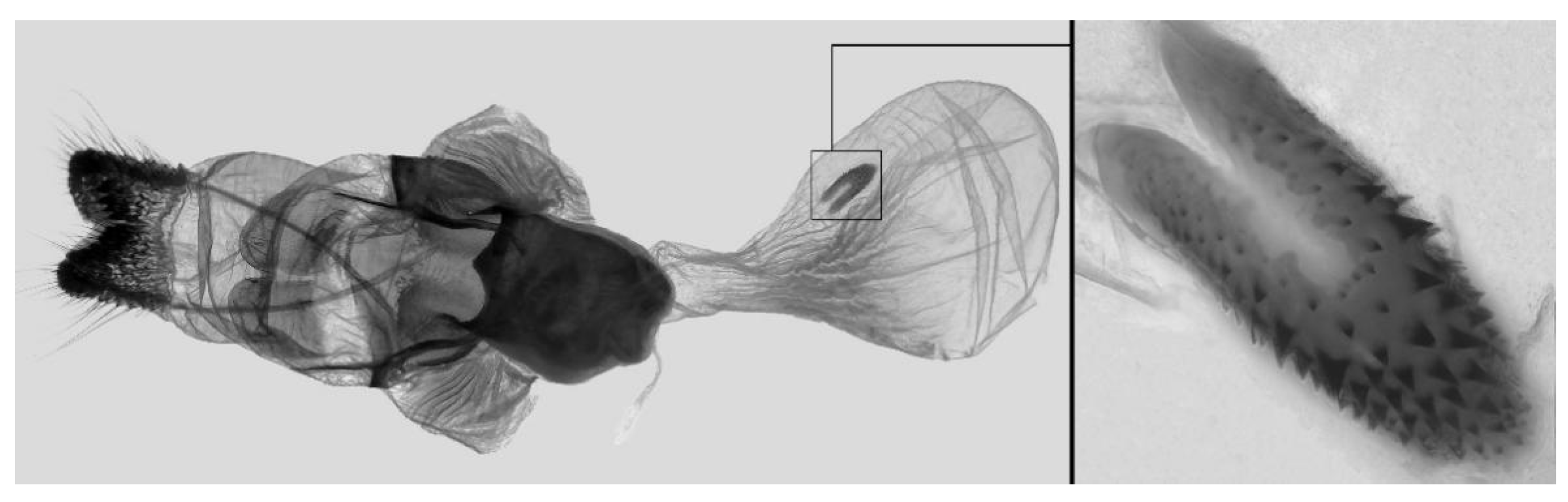
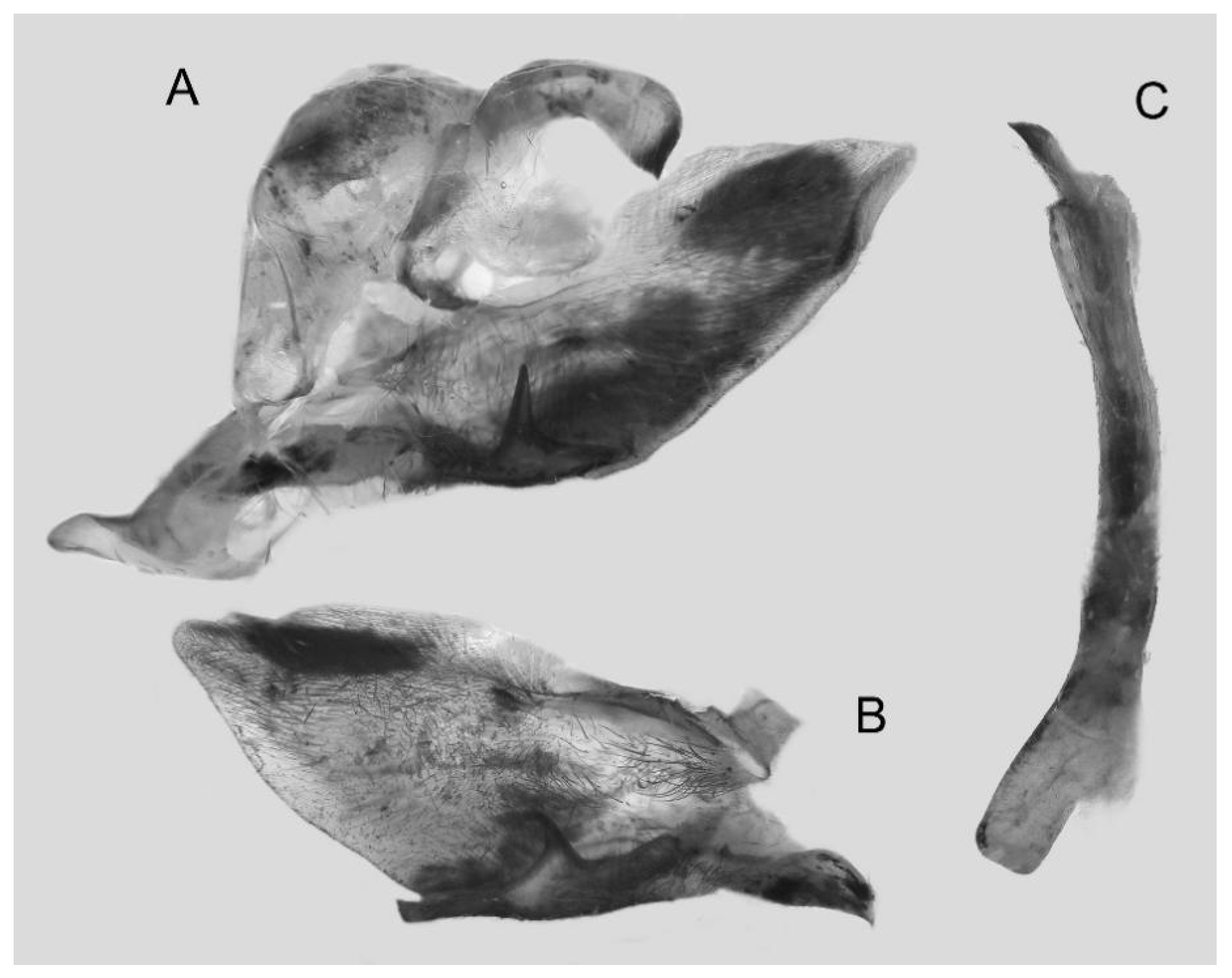
Figure 62.
PARATYPE, Male genitalia of Ambulyx semiplacida bhutana, Trongsa, Bhutan. (A) Genital capsule; (B) phallus. © Ronald Brechlin.
Figure 62.
PARATYPE, Male genitalia of Ambulyx semiplacida bhutana, Trongsa, Bhutan. (A) Genital capsule; (B) phallus. © Ronald Brechlin.
Figure 63.
Ambulyx semiplacida interplacida. (A,B) Male, Shaoguan, Guangdong, China; (C,D) female, Fengtongzhai, Baoxing County, Sichuan, China. Scale bar = 10 mm.
Figure 63.
Ambulyx semiplacida interplacida. (A,B) Male, Shaoguan, Guangdong, China; (C,D) female, Fengtongzhai, Baoxing County, Sichuan, China. Scale bar = 10 mm.
Figure 64.
Male genitalia of Ambulyx semiplacida interplacida, Mt. Leigongshan, Suiyang County, Guizhou, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 64.
Male genitalia of Ambulyx semiplacida interplacida, Mt. Leigongshan, Suiyang County, Guizhou, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 65.
Female genitalia of Ambulyx semiplacida interplacida, Fengtongzhai, Baoxing County, Sichuan, China.
Figure 65.
Female genitalia of Ambulyx semiplacida interplacida, Fengtongzhai, Baoxing County, Sichuan, China.
Figure 66.
Habitat and living adult of Ambulyx semiplacida interplacida. (A) Male; (B) Nanling, Guangdong, China.
Figure 66.
Habitat and living adult of Ambulyx semiplacida interplacida. (A) Male; (B) Nanling, Guangdong, China.
Figure 67.
Ambulyx semiplacida montana. (A,B) Male, Pingbian, Yunnan, China; (C,D) female, Pingbian, Yunnan, China. Scale bar = 10 mm.
Figure 67.
Ambulyx semiplacida montana. (A,B) Male, Pingbian, Yunnan, China; (C,D) female, Pingbian, Yunnan, China. Scale bar = 10 mm.
Figure 68.
Male genitalia of Ambulyx semiplacida montana, Pingbian, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 68.
Male genitalia of Ambulyx semiplacida montana, Pingbian, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 69.
Female genitalia of Ambulyx semiplacida montana, Pingbian, Yunnan, China.
Figure 69.
Female genitalia of Ambulyx semiplacida montana, Pingbian, Yunnan, China.
Figure 70.
Habitat and living adults of Ambulyx semiplacida montana. (A) Male; (B) female; (C) Pingbian County, Yunnan, China.
Figure 70.
Habitat and living adults of Ambulyx semiplacida montana. (A) Male; (B) female; (C) Pingbian County, Yunnan, China.
Figure 71.
Ambulyx sericeipennis sericeipennis. (A,B) Male, Maolan Nature Reserve, Libo, Guizhou, China. (C,D) Female, Mt. Alishan, Taiwan, China. Scale bar = 10 mm.
Figure 71.
Ambulyx sericeipennis sericeipennis. (A,B) Male, Maolan Nature Reserve, Libo, Guizhou, China. (C,D) Female, Mt. Alishan, Taiwan, China. Scale bar = 10 mm.
Figure 72.
Male genitalia of Ambulyx sericeipennis sericeipennis, Mengla County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 72.
Male genitalia of Ambulyx sericeipennis sericeipennis, Mengla County, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 73.
Female genitalia of Ambulyx sericeipennis sericeipennis, Gongshan County, Yunnan, China.
Figure 73.
Female genitalia of Ambulyx sericeipennis sericeipennis, Gongshan County, Yunnan, China.
Figure 74.
Habitat and living adults of Ambulyx sericeipennis sericeipennis. (A) Male; (B) female; (C) Yuanjiang, Yunnan, China.
Figure 74.
Habitat and living adults of Ambulyx sericeipennis sericeipennis. (A) Male; (B) female; (C) Yuanjiang, Yunnan, China.
Figure 75.
Ambulyx sericeipennis joiceyi. (A,B) Male, Sabah, Borneo, Malaysia. (C,D) Female, Sumatra, Indonesia. Scale bar = 10 mm.
Figure 75.
Ambulyx sericeipennis joiceyi. (A,B) Male, Sabah, Borneo, Malaysia. (C,D) Female, Sumatra, Indonesia. Scale bar = 10 mm.
Figure 76.
Male genitalia of Ambulyx sericeipennis joiceyi, Sabah, Borneo, Malaysia. (A) Genital capsule; (B) phallus.
Figure 76.
Male genitalia of Ambulyx sericeipennis joiceyi, Sabah, Borneo, Malaysia. (A) Genital capsule; (B) phallus.
Figure 77.
Female genitalia of Ambulyx sericeipennis joiceyi, Sumatra, Indonesia.
Figure 77.
Female genitalia of Ambulyx sericeipennis joiceyi, Sumatra, Indonesia.
Figure 78.
Habitat and living adults of Ambulyx sericeipennis joiceyi. (A) Male; (B) female; (C) Sabah, Borneo, Malaysia.
Figure 78.
Habitat and living adults of Ambulyx sericeipennis joiceyi. (A) Male; (B) female; (C) Sabah, Borneo, Malaysia.
Figure 79.
Ambulyx sericeipennis javanica. (
A,
B) Male, Mt. Halimun, Java, Indonesia. (
C,
D) Female, Mt. Mt. Halimun, Java, Indonesia. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 79.
Ambulyx sericeipennis javanica. (
A,
B) Male, Mt. Halimun, Java, Indonesia. (
C,
D) Female, Mt. Mt. Halimun, Java, Indonesia. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 80.
Male genitalia of Ambulyx sericeipennis javanica, Mt. Halimun, Java, Indonesia. (A) Genital capsule; (B) phallus.
Figure 80.
Male genitalia of Ambulyx sericeipennis javanica, Mt. Halimun, Java, Indonesia. (A) Genital capsule; (B) phallus.
Figure 81.
Habitat of Ambulyx sericeipennis javanica, Mt. Tangkuban Perabu, Java, Indonesia.
Figure 81.
Habitat of Ambulyx sericeipennis javanica, Mt. Tangkuban Perabu, Java, Indonesia.
Figure 82.
Ambulyx sericeipennis luzoni. (A,B) Male, Mt. Polis, Luzon, Philippines. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK.
Figure 82.
Ambulyx sericeipennis luzoni. (A,B) Male, Mt. Polis, Luzon, Philippines. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK.
Figure 83.
Male genitalia of
Ambulyx sericeipennis luzoni, Luzon, Philippines. (
A) Original genitalia slide photos [
16]; (
B) line drawing by the first author.
Figure 83.
Male genitalia of
Ambulyx sericeipennis luzoni, Luzon, Philippines. (
A) Original genitalia slide photos [
16]; (
B) line drawing by the first author.
Figure 84.
Ambulyx sericeipennis palawanica. (
A) HOLOTYPE, male, Palawan, Philippines. (
B) PARATYPE, female, Palawan, Philippines. Scale bar = 10 mm. [
16].
Figure 84.
Ambulyx sericeipennis palawanica. (
A) HOLOTYPE, male, Palawan, Philippines. (
B) PARATYPE, female, Palawan, Philippines. Scale bar = 10 mm. [
16].
Figure 85.
Ambulyx sericeipennis palawanica. Male, Mt. Brooks, Palawan, Philippines. (A) upperside; (B) underside. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK.
Figure 85.
Ambulyx sericeipennis palawanica. Male, Mt. Brooks, Palawan, Philippines. (A) upperside; (B) underside. Scale bar = 10 mm. © The Trustees of the Natural History Museum, London, UK.
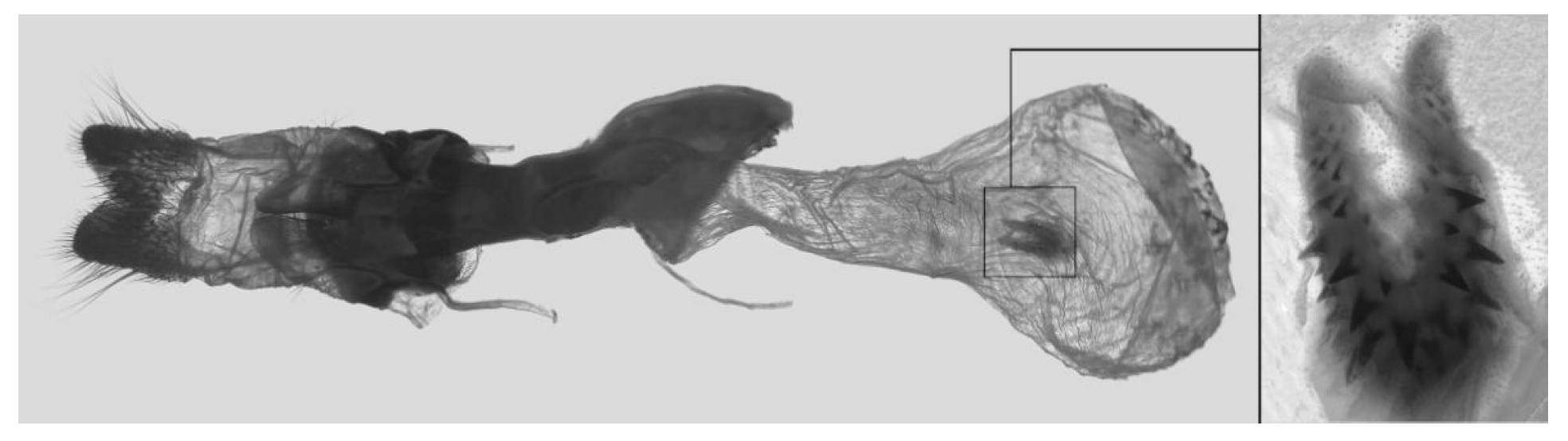
Figure 86.
PARATYPE, male genitalia of
Ambulyx sericeipennis palawanica, Palawan, Philippines. (
A) Original genitalia slide photo [
16]; (
B) line drawing from the photo by the first author.
Figure 86.
PARATYPE, male genitalia of
Ambulyx sericeipennis palawanica, Palawan, Philippines. (
A) Original genitalia slide photo [
16]; (
B) line drawing from the photo by the first author.
Figure 87.
Ambulyx siamensis. (
A,
B) Male, Nabanhe Nature Reserve (1093 m), Xishuangbanna, Yunnan, China. (
C,
D) Female, Nan, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 87.
Ambulyx siamensis. (
A,
B) Male, Nabanhe Nature Reserve (1093 m), Xishuangbanna, Yunnan, China. (
C,
D) Female, Nan, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed on 20 April 2024.
Figure 88.
Male genitalia of Ambulyx siamensis, Xima Town, Yingjiang County, Yunnan. (A) Genital capsule with left valva removed; (B) left valva; (C) phallus.
Figure 88.
Male genitalia of Ambulyx siamensis, Xima Town, Yingjiang County, Yunnan. (A) Genital capsule with left valva removed; (B) left valva; (C) phallus.
Figure 89.
Female genitalia of
Ambulyx siamensis, Chiang Mai, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed 20 April 2024.
Figure 89.
Female genitalia of
Ambulyx siamensis, Chiang Mai, Thailand. © The Trustees of the Natural History Museum, London, UK (downloaded from Kitching, I.J. Sphingidae Taxonomic Inventory.
http://sphingidae.myspecies.info/, accessed 20 April 2024.
Figure 90.
Habitat and living adults of Ambulyx siamensis. (A) Male; (B) female; (C) Mengla Couny, Yunnan, China.
Figure 90.
Habitat and living adults of Ambulyx siamensis. (A) Male; (B) female; (C) Mengla Couny, Yunnan, China.
Figure 91.
Ambulyx substrigilis. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan; (C,D) female, Xiajinchang Township, Malipo County, Yunnan, China. Scale bar = 10 mm.
Figure 91.
Ambulyx substrigilis. (A,B) Male, Mt. Jianfengling, Ledong County, Hainan; (C,D) female, Xiajinchang Township, Malipo County, Yunnan, China. Scale bar = 10 mm.
Figure 92.
Male genitalia of Ambulyx substrigilis, Nabanhe Nature Reserve, Xishuangbanna, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 92.
Male genitalia of Ambulyx substrigilis, Nabanhe Nature Reserve, Xishuangbanna, Yunnan, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 93.
Female genitalia of Ambulyx substrigilis, Malipo County, Yunnan, China.
Figure 93.
Female genitalia of Ambulyx substrigilis, Malipo County, Yunnan, China.
Figure 94.
Habitat and living adult of Ambulyx substrigilis. (A) Male; (B) Jianfengling, Hainan, China.
Figure 94.
Habitat and living adult of Ambulyx substrigilis. (A) Male; (B) Jianfengling, Hainan, China.
Figure 95.
Ambulyx tattina tattina. (A,B) Male, Menghai County, Xishuangbanna, Yunnan. (C,D) Female, Menghai County, Xishuangbanna, Yunnan, China. Scale bar = 10 mm.
Figure 95.
Ambulyx tattina tattina. (A,B) Male, Menghai County, Xishuangbanna, Yunnan. (C,D) Female, Menghai County, Xishuangbanna, Yunnan, China. Scale bar = 10 mm.
Figure 96.
Male genitalia of Ambulyx tattina tattina, Menghai County, Xishuangbanna, Yunnan. (A) Lateral view; (B) left valve; (C) phallus.
Figure 96.
Male genitalia of Ambulyx tattina tattina, Menghai County, Xishuangbanna, Yunnan. (A) Lateral view; (B) left valve; (C) phallus.
Figure 97.
Feale genitalia of Ambulyx tattina tattina, Menghai County, Xishuangbanna, Yunnan.
Figure 97.
Feale genitalia of Ambulyx tattina tattina, Menghai County, Xishuangbanna, Yunnan.
Figure 98.
Habitat and living adults of Ambulyx tattina tattina. (A) Male; (B) female; (C) Pu’er, Yunnan, China.
Figure 98.
Habitat and living adults of Ambulyx tattina tattina. (A) Male; (B) female; (C) Pu’er, Yunnan, China.
Figure 99.
Ambulyx tobii. (A,B) Male, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China; (C,D) female, Huairou County, Beijing. Scale bar = 10 mm.
Figure 99.
Ambulyx tobii. (A,B) Male, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China; (C,D) female, Huairou County, Beijing. Scale bar = 10 mm.
Figure 100.
Male genitalia of Ambulyx tobii, Mt. Leigongshan, Suiyang County, Guizhou, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 100.
Male genitalia of Ambulyx tobii, Mt. Leigongshan, Suiyang County, Guizhou, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 101.
Female genitalia of Ambulyx tobii, Yingshan County, Hubei, China.
Figure 101.
Female genitalia of Ambulyx tobii, Yingshan County, Hubei, China.
Figure 102.
Habitat and living adults of Ambulyx tobii. (A) Male; (B) female; (C) Huairou County, Beijing, China.
Figure 102.
Habitat and living adults of Ambulyx tobii. (A) Male; (B) female; (C) Huairou County, Beijing, China.
Figure 103.
Ambulyx zhejiangensis. (A,B) Male, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China; (C,D) female, Tapa Shan, China. © Sphingidae Museum, Czech Republic. Scale bar = 10 mm.
Figure 103.
Ambulyx zhejiangensis. (A,B) Male, Yintiaoling Nature Reserve, Wuxi County, Chongqing, China; (C,D) female, Tapa Shan, China. © Sphingidae Museum, Czech Republic. Scale bar = 10 mm.
Figure 104.
Male genitalia of Ambulyx zhejiangensis, Wuxi County, Chongqing, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 104.
Male genitalia of Ambulyx zhejiangensis, Wuxi County, Chongqing, China. (A) Lateral view; (B) left valve; (C) phallus.
Figure 105.
Habitat and living adult of Ambulyx zhejiangensis. (A) Male; (B) Wuxi County, Chongqing, China.
Figure 105.
Habitat and living adult of Ambulyx zhejiangensis. (A) Male; (B) Wuxi County, Chongqing, China.
Figure 106.
Larvae of Ambulyx species from China. (A) A. moorei, Foshan, Guangdong, China; (B) A. sericeipennis sericeipennis, Hongkong, China; (C) A. ochracea, Guangzhou, Guangdong, China; (D) A. kuangtungensis, Guangzhou, Guangdong, China; (E) A. semiplacida montana, Pingbian County, Yunnan, China; (F) A. schauffelbergeri, Chongqing City, China; (G) A. siamensis, Mengla County, Yunnan, China; (H) A. siamensis, Mengla County, Yunnan, China; (I) A. liturata, Mt. Maoershan, Guangxi, China; (J) A. tobii, Kunming, Yunnan, China.
Figure 106.
Larvae of Ambulyx species from China. (A) A. moorei, Foshan, Guangdong, China; (B) A. sericeipennis sericeipennis, Hongkong, China; (C) A. ochracea, Guangzhou, Guangdong, China; (D) A. kuangtungensis, Guangzhou, Guangdong, China; (E) A. semiplacida montana, Pingbian County, Yunnan, China; (F) A. schauffelbergeri, Chongqing City, China; (G) A. siamensis, Mengla County, Yunnan, China; (H) A. siamensis, Mengla County, Yunnan, China; (I) A. liturata, Mt. Maoershan, Guangxi, China; (J) A. tobii, Kunming, Yunnan, China.
Figure 107.
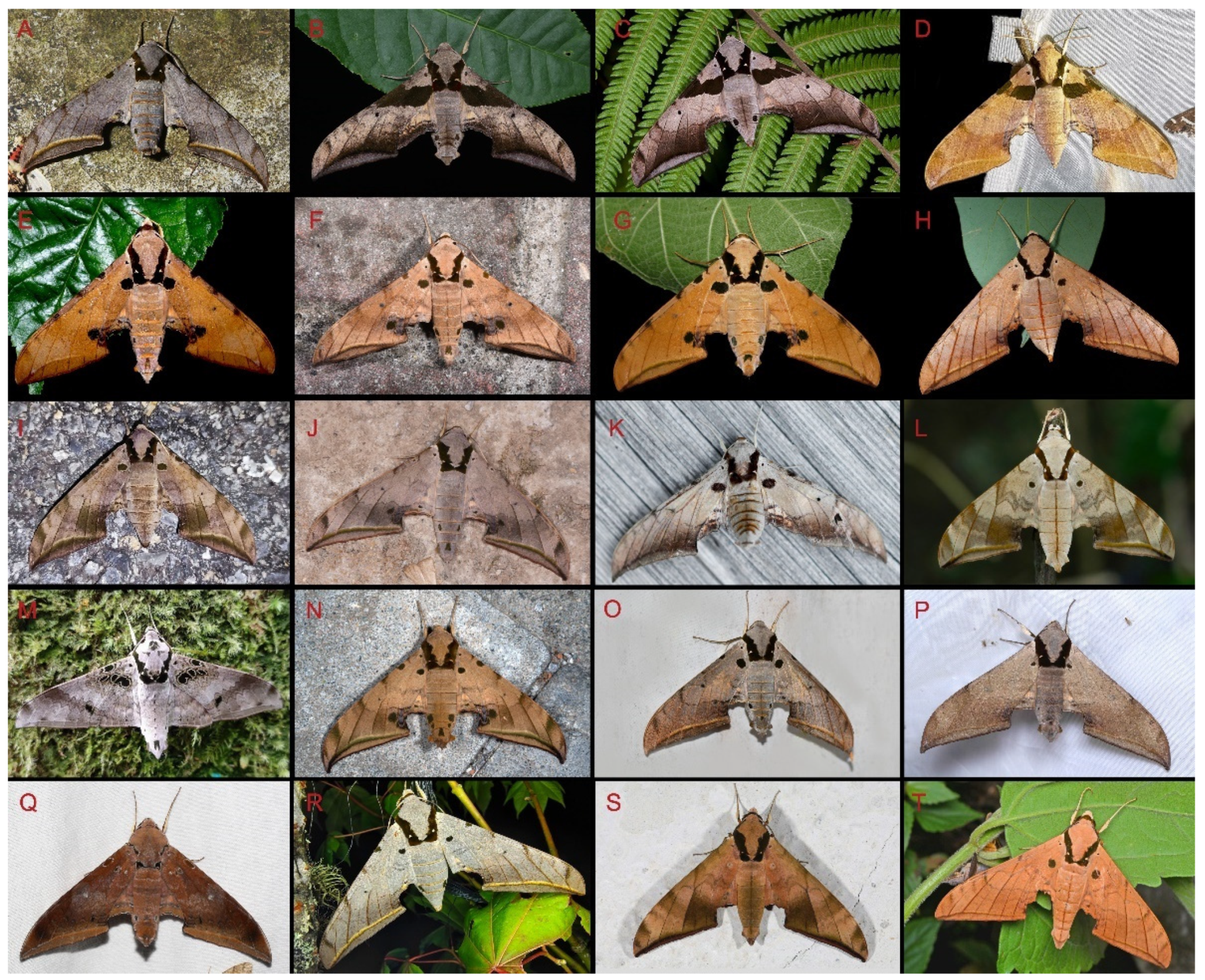
Morphological comparison of living adults of Ambulyx species from China. (A) Male A. semiplacida interplacida, Mt. Leigongshan, Guizhou, China; (B) male A. japonica koreana, Wuxi County, Chongqing, China; (C) female A. japonica angustifasciata, Nantou County, Taiwan, China; (D) female A. latifascia, Hutiaoxia, Yunnan, China; (E) Male A. kuangtungensis, Wuxi County, Chongqing, China; (F) male A. maculifera, Motuo, Xizang, China; (G) male A. ochracea, Wuxi County, Chongaqing, China; (H) female A. liturata, Shenzhen, Guangdong, China; (I) Female A. schauffelbergeri, Yuexi, Anhui, China; (J) male A. sericeipennis sericeipennis, Zunyi, Guizhou, China; (K) male wukong Jiang and Kitching sp. nov., Weixi, Yunnan, China; (L) male A. substrigilis, Haikou, Hainan, China; (M) male A. canescens, Yingjiang, Yunnan, China; (N) male A. tobii, Huairou County, Beijing, China; (O) male A. placida, Motuo, Xizang, China; (P) male A. zhejiangensis, Wuxi County, Chongqing, China; (Q) male A. moori, Hezhou, Guangxi, China; (R) female A. semiplacida montana, Pingbian County, Yunnan, China; (S) male A. tattina tattina, Mengla County, Yunnan, China; (T) male A. siamensis, Menglun, Xishuangbanna, Yunnan, China.
Figure 107.
Morphological comparison of living adults of Ambulyx species from China. (A) Male A. semiplacida interplacida, Mt. Leigongshan, Guizhou, China; (B) male A. japonica koreana, Wuxi County, Chongqing, China; (C) female A. japonica angustifasciata, Nantou County, Taiwan, China; (D) female A. latifascia, Hutiaoxia, Yunnan, China; (E) Male A. kuangtungensis, Wuxi County, Chongqing, China; (F) male A. maculifera, Motuo, Xizang, China; (G) male A. ochracea, Wuxi County, Chongaqing, China; (H) female A. liturata, Shenzhen, Guangdong, China; (I) Female A. schauffelbergeri, Yuexi, Anhui, China; (J) male A. sericeipennis sericeipennis, Zunyi, Guizhou, China; (K) male wukong Jiang and Kitching sp. nov., Weixi, Yunnan, China; (L) male A. substrigilis, Haikou, Hainan, China; (M) male A. canescens, Yingjiang, Yunnan, China; (N) male A. tobii, Huairou County, Beijing, China; (O) male A. placida, Motuo, Xizang, China; (P) male A. zhejiangensis, Wuxi County, Chongqing, China; (Q) male A. moori, Hezhou, Guangxi, China; (R) female A. semiplacida montana, Pingbian County, Yunnan, China; (S) male A. tattina tattina, Mengla County, Yunnan, China; (T) male A. siamensis, Menglun, Xishuangbanna, Yunnan, China.
![Insects 16 00223 g107]()
Figure 108.
(A) A. tattina tattina resting after emerging in the rainforest in Mengla County, Yunnan, China; (B) an A. kuangtungensis at rest by day in the forest in Yuanjiang County, Yunnan, China; (C,D) A. semiplacida montana resting after emerging in the forest in Pingbian County, Yunnan, China; (E) Pupa of the A. semiplacida montana from Pingbian County, Yunnan, China.
Figure 108.
(A) A. tattina tattina resting after emerging in the rainforest in Mengla County, Yunnan, China; (B) an A. kuangtungensis at rest by day in the forest in Yuanjiang County, Yunnan, China; (C,D) A. semiplacida montana resting after emerging in the forest in Pingbian County, Yunnan, China; (E) Pupa of the A. semiplacida montana from Pingbian County, Yunnan, China.
Figure 109.
Some species of Ambulyx from China visiting flowers at night. (A,B) Female A. tobii visiting Lilium sulphureum, Yanhe County, Guizhou, China; (C,D) female A. ochracea visiting Lilium primulinum var. ochraceum, Guiyang City, Guizhou, China.
Figure 109.
Some species of Ambulyx from China visiting flowers at night. (A,B) Female A. tobii visiting Lilium sulphureum, Yanhe County, Guizhou, China; (C,D) female A. ochracea visiting Lilium primulinum var. ochraceum, Guiyang City, Guizhou, China.
Figure 110.
Ambulyx liturata infested by Akanthomyces sp. in the rainforest of Mengla County, Xishuangbanna, Yunnan, China (the red line indicate its concealed position in rainforest).
Figure 110.
Ambulyx liturata infested by Akanthomyces sp. in the rainforest of Mengla County, Xishuangbanna, Yunnan, China (the red line indicate its concealed position in rainforest).
Figure 111.
Morphological comparison of species of the Ambulyx placida-group (A) A. wukong sp. nov., Wexi, Yunnan, China, male; (B) A. placida, Nyalam County, Xizang, China, male; (C) A. semiplacida semiplacida, Pingtung, Taiwan, China, male; (D) A. semiplacida interplacida, Shaoguan, Guangdong, China, male; (E) A. semiplacida montana, Pingbian, Yunnan, China, male; (F) A. semiplacida bhutana, Trongsa Dzong, Bhutan, male. © Ronald Brechlin. Uppersides in the top row, undersides in the bottom row.
Figure 111.
Morphological comparison of species of the Ambulyx placida-group (A) A. wukong sp. nov., Wexi, Yunnan, China, male; (B) A. placida, Nyalam County, Xizang, China, male; (C) A. semiplacida semiplacida, Pingtung, Taiwan, China, male; (D) A. semiplacida interplacida, Shaoguan, Guangdong, China, male; (E) A. semiplacida montana, Pingbian, Yunnan, China, male; (F) A. semiplacida bhutana, Trongsa Dzong, Bhutan, male. © Ronald Brechlin. Uppersides in the top row, undersides in the bottom row.
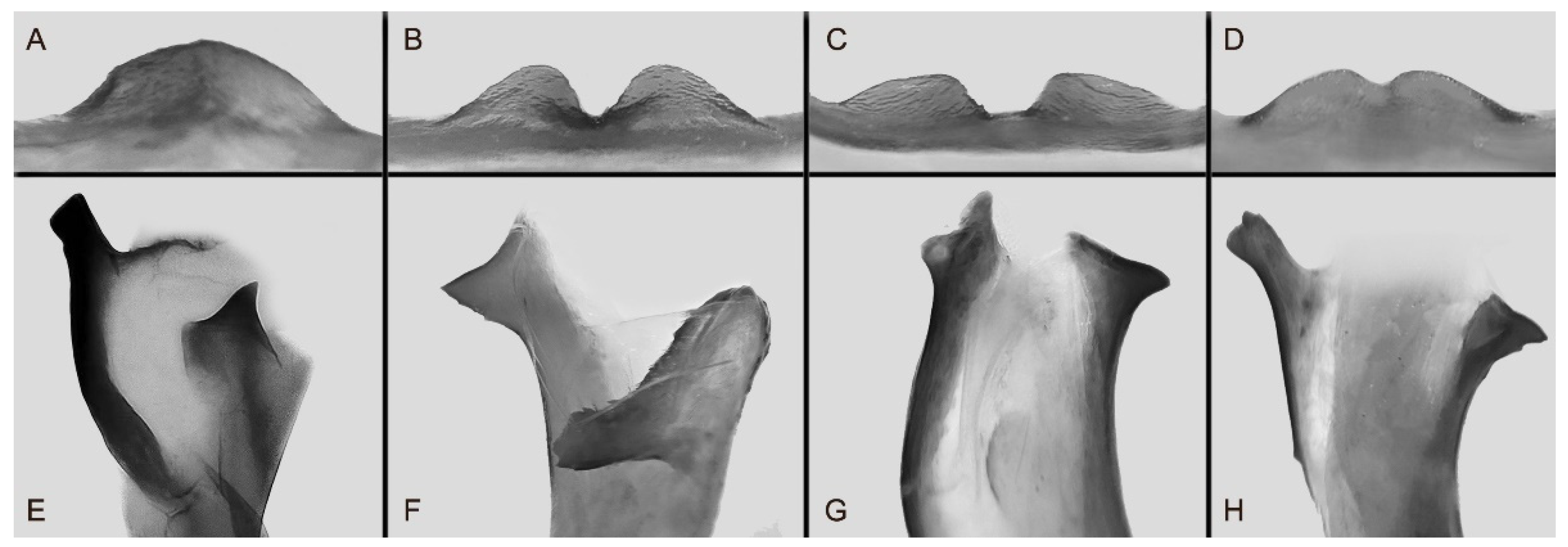
Figure 112.
Male genitalia of the Ambulyx placida-group. (A) Gnathos of A. placida, Nyingchi, Xizang, China; (B) Gnathos of A. s. semiplacida, Taitung, Taiwan, China; (C) Gnathos of A. wukong sp. nov., Wexi, Yunnan, China; (D) Gnathos of A. japonica koreana, Wuxi, Chongqing, China; (E) Phallus lobe of A. placida, Nyingchi, Xizang, China; (F) phallus lobe of A. s. semiplacida, Taitung, Taiwan, China; (G) phallus lobe of A. wukong sp. nov., Wexi, Yunnan, China, China; (H) phallus lobe of A. japonica koreana, Wuxi, Chongqing, China.
Figure 112.
Male genitalia of the Ambulyx placida-group. (A) Gnathos of A. placida, Nyingchi, Xizang, China; (B) Gnathos of A. s. semiplacida, Taitung, Taiwan, China; (C) Gnathos of A. wukong sp. nov., Wexi, Yunnan, China; (D) Gnathos of A. japonica koreana, Wuxi, Chongqing, China; (E) Phallus lobe of A. placida, Nyingchi, Xizang, China; (F) phallus lobe of A. s. semiplacida, Taitung, Taiwan, China; (G) phallus lobe of A. wukong sp. nov., Wexi, Yunnan, China, China; (H) phallus lobe of A. japonica koreana, Wuxi, Chongqing, China.
Figure 113.
Morphological comparison of similar species of Ambulyx of China (A) A. kuangtungensis, Libo, Guizhou, China, male; (B) A. kuangtungensis f. adhemariusa, Yuexi, Anhui, China, male; (C) A. latifascia, Panzhihua, Sichuan, China, male; (D) A. ochracea, Hangzhou, Zhejiang, China, male; (E) A. schauffelbergeri, Nanjing, Jiangsu, China, male; (F) A. maculifera, Yingjiang, Yunnan, China, male. Uppersides in the top row, undersides in the bottom row.
Figure 113.
Morphological comparison of similar species of Ambulyx of China (A) A. kuangtungensis, Libo, Guizhou, China, male; (B) A. kuangtungensis f. adhemariusa, Yuexi, Anhui, China, male; (C) A. latifascia, Panzhihua, Sichuan, China, male; (D) A. ochracea, Hangzhou, Zhejiang, China, male; (E) A. schauffelbergeri, Nanjing, Jiangsu, China, male; (F) A. maculifera, Yingjiang, Yunnan, China, male. Uppersides in the top row, undersides in the bottom row.
Figure 114.
Morphological comparison of similar species of Ambulyx of China (A) A. tobii, Wuxi, Chongqing, China, male; (B) A. sericeipennis sericeipennis, Libo, Guizhou, China, male; (C) A. liturata, Sanming, Fujian, China, male; (D) A. substrigilis, Mengla, Yunnan, China, male; (E) A. siamensis, Mengla, Yunnan, China, male. Uppersides in the top row, undersides in the bottom row.
Figure 114.
Morphological comparison of similar species of Ambulyx of China (A) A. tobii, Wuxi, Chongqing, China, male; (B) A. sericeipennis sericeipennis, Libo, Guizhou, China, male; (C) A. liturata, Sanming, Fujian, China, male; (D) A. substrigilis, Mengla, Yunnan, China, male; (E) A. siamensis, Mengla, Yunnan, China, male. Uppersides in the top row, undersides in the bottom row.