Impact of Oviposition Sequence and Host Egg Density on Offspring Emergence and Interspecific Competition in Two Species of Trichogramma Parasitoids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitoids
2.2. Hosts
2.2.1. Asian Corn Borer (ACB), Ostrinia furnacalis
2.2.2. Rice Moth (RM), Corcyra cephalonica
2.3. Interspecific Competition Between Two Trichogramma Species Within the ACB and RM Eggs
2.4. Statistical Analysis
3. Results
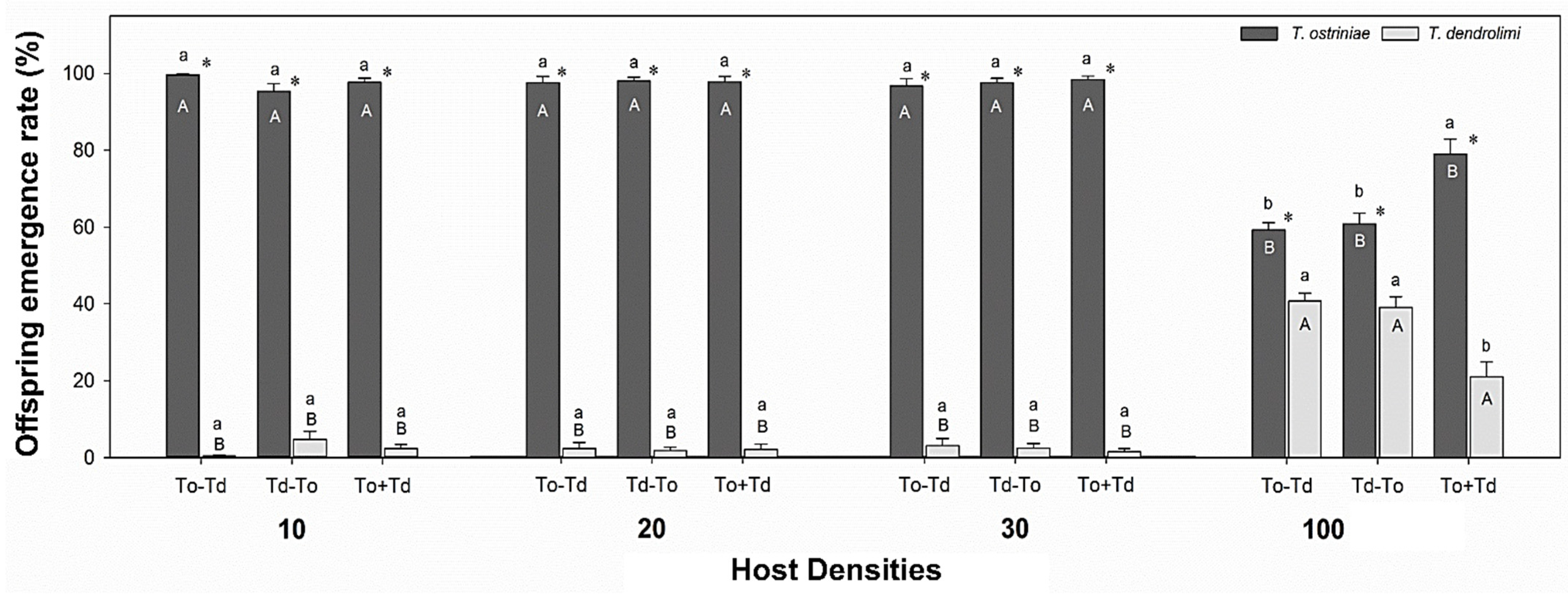
3.1. Influence of Wasp Oviposition Sequence and Host Densities on Offspring Emergence of Trichogramma Parasitoids from ACB Eggs
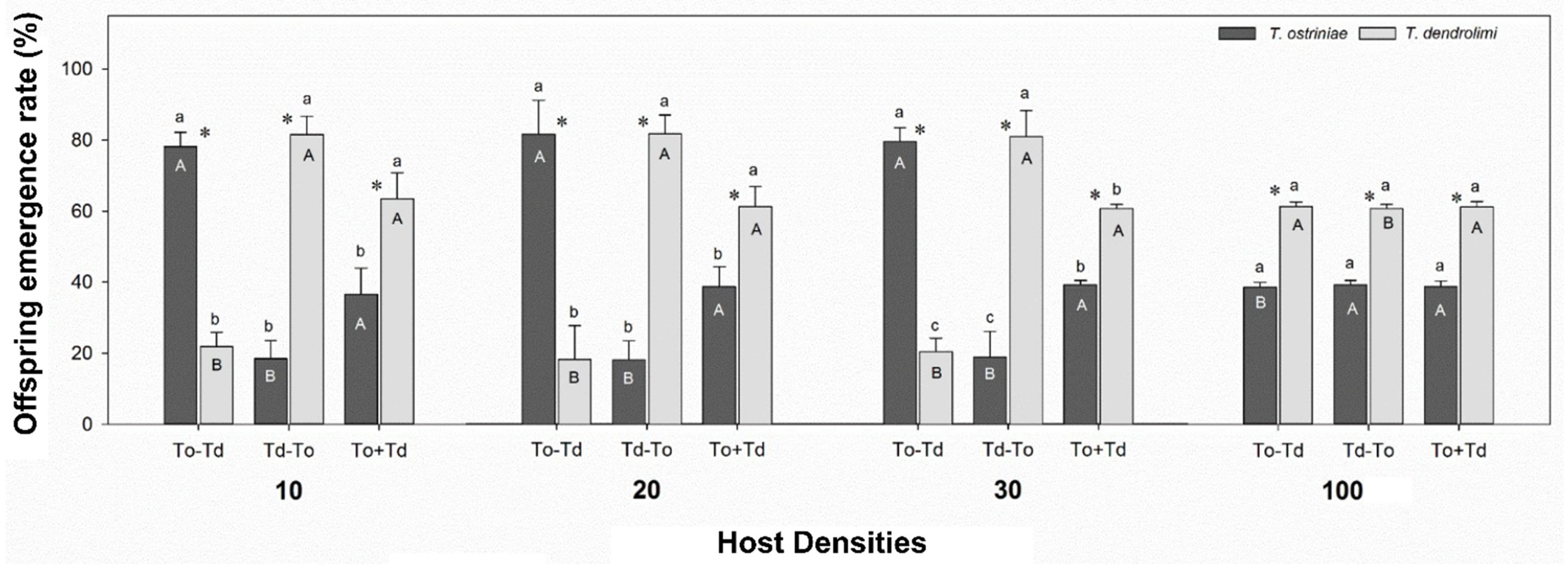
3.2. Influence of Wasp Oviposition Sequence and Host Densities on Offspring Emergence of Trichogramma Parasitoids from RM Eggs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; He, K.; Meng, Y.; Hellmich, R.L.; Chen, S.; Lopez, M.D.; Lauter, N.; Wang, Z. Asian corn borer damage is affected by rind penetration strength of corn stalks in a spatiotemporally dependent manner. Plant Direct. 2022, 6, e381. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Guo, J.F.; Wang, Z.; He, K.; Bai, S. Feeding preference of the Asia corn borer larvae for different host plants. Acta Phytophy. Sin 2013, 40, 205–209. [Google Scholar]
- Yuan, Z.H.; Wang, W.Q.; Wang, Z.Y.; He, K.L.; Bai, S.X. Host plants of the Asian corn borer, Ostrinia furnacalis (Guenee) (Lepidoptera: Crambidae). J. Plant Prot. 2015, 42, 957–964. [Google Scholar]
- Afidchao, M.M.; Musters, C.J.; de Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest. Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, T.; Trisyono, Y.A.; Witjaksono. Fitness of Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae) reared in an artificial diet. J. Asia Pac. Entomol. 2018, 21, 823–828. [Google Scholar] [CrossRef]
- Chen, R.Z.; Klein, M.G.; Li, Q.Y.; Li, L.B.; Li, P.P.; Sheng, C.F. Do second generation Asia corn borer (Lepidoptera: Crambidae) immigrate to corn fields from alternate habitats? J. Asia Pac. Entomol. 2015, 18, 687–693. [Google Scholar] [CrossRef]
- Wei, X.; Chen, R.Z. Effects of host plants on the development and protective enzyme activity of Ostrinia furnacalis. Chin. J. Appl. Entomol. 2020, 57, 355–362. [Google Scholar]
- Nafus, D.M.; Schreiner, I.H. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Trop. Pest. Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Vincent, A.; Singh, D.; Mathew, I.L. Corcyra cephalonica: A serious pest of stored products or a factitious host of biocontrol agents? J. Store. Prod. Res. 2021, 94, 101876. [Google Scholar] [CrossRef]
- Adarkwah, C.; Nyarko, G.; Opoku, N.; Badii, B.K.; Addai, I.K.; Prozell, S.; Ulrichs, C.; Schöller, M. Effectiveness of the egg parasitoid Trichogramma evanescens preventing rice moth from infesting stored bagged commodities. J. Store. Prod. Res. 2015, 61, 102–107. [Google Scholar] [CrossRef]
- Manjunath, T.M. Rice moth, Corcyra cephalonica (Lepidoptera, Pyralidae)—A boon for biocontrol as a factitious host for mass production of parasitoids and predators. J. Biol. Cont. 2023, 37, 1–5. [Google Scholar] [CrossRef]
- Sun, C.; Li, S.; Wang, K.; Yin, X.; Wang, Y.; Du, M.; Wei, J.; An, S. Cyclosporin A as a potential insecticide to control the Asian corn borer Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae). Insects 2022, 13, 965. [Google Scholar] [PubMed]
- Liu, M.; Hao, Y.; Sun, Y.; Wang, J. Developing insect resistance with fusion gene transformation of chitinase and scorpion toxin gene in maize (Zea mays L). Maydica 2016, 61, 5. [Google Scholar]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Ann. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ruan, C.; Zang, L.; Shao, X.; Shi, S. Technological improvements for mass production of Trichogramma and current status of their applications for biological control on agricultural pests in China. Chi. J. Biol. Cont. 2015, 31, 638. [Google Scholar]
- Huang, N.X.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.Y.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Pinto, J.H.; Stouthamer, R. Systematics of the Trichogrammatidae with emphasis on Trichogramma. In Biological Control with Egg Parasitoids; WUR staff publications: Wageningen, The Netherlands, 1994; pp. 1–36. [Google Scholar]
- Vinson, S.B.; Greenberg, S.M.; Rao, A.; Volosciuk, L.F. (Eds.) Biological Control of Pests Using Trichogramma: Current Status and Perspectives; Northwest A&F University Press: Xianyang, China, 2015. [Google Scholar]
- Wang, Z.Y.; He, K.L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Cont. 2014, 1, 136–144. [Google Scholar] [CrossRef]
- Mills, N. Interspecific competition among natural enemies and single versus multiple introductions in biological control. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 191–220. [Google Scholar]
- Cusumano, A.; Peri, E.; Colazza, S. Interspecific competition/facilitation among insect parasitoids. Curr. Opin. Insect Sci. 2016, 14, 12–16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lü, L.; Chen, K. Interspecific competition between Trichogramma confusum and T. pretiosum on Corcyra cephaloica factitious eggs. Chin. J. Appl. Ecol. 2004, 12, 2401–2404. [Google Scholar]
- Al-Jalely, B.H.; Xu, W. Olfactory sensilla and olfactory genes in the parasitoid wasp Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Insects 2021, 12, 998. [Google Scholar] [CrossRef]
- Lin, L.; Ali, S.; Wu, J. Influences of varying host: Parasitoid ratios on parasitism of whitefly by three different parasitoid species. Egypt. J. Biol. Pest. Cont. 2018, 28, 59. [Google Scholar] [CrossRef]
- Saljoqi, A.U.; Salim, M.; Khalil, S.K.; Khurshid, I. Field application of Trichogramma chilonis (Ishii) for the management of sugarcane borers. Pak. J. Zool. 2015, 47, 283–291. [Google Scholar]
- Bigler, F.; Cerutti, F.; Laing, J. First draft of criteria for quality control (product control) of Trichogramma. In Fifth Workshop of the IOBC Global Working Group on Quality Control of Mass Reared Arthropods; Swiss Federal Research Station for Agronomy: Zurich, Switzerland, 1991; pp. 200–201. [Google Scholar]
- Sani, I.A.; Shabbir, S.; Zafar, M.; Ahmed, N.; Shahwani, N.A.; Yousafzai, A.; Irfan, S.; Ahmed, U.; Aziz, S.; Khan, A.G.; et al. Biological control of insect pests using Trichogramma minutum as biological control agent in the vicinity of buitems. COMU J. Agric. Fac. 2016, 4, 95–103. [Google Scholar]
- Luo, S.; Zhang, F.; Wu, K. Interspecific competition between Peristenus spretus and Peristenus relictus (Hymenoptera: Braconidae), larval parasitoids of Apolygus lucorum (Hemiptera: Miridae). Biol. Cont. 2018, 117, 115–122. [Google Scholar] [CrossRef]
- Harvey, J.A.; Poelman, E.H.; Tanaka, T. Intrinsic inter- and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 2013, 58, 333–351. [Google Scholar] [CrossRef]
- Pinto, J.D. Novel taxa of Trichogramma from the New World Tropics and Australia (Hymenoptera: Trichogrammatidae). J. N. Y. Entomol. Soc. 1992, 100, 621–633. [Google Scholar]
- Stouthamer, R.; Hu, J.; van Kan, F.J.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Pinto, J.R.; Torres, A.F.; Truzi, C.C.; Vieira, N.F.; Vacari, A.M.; De Bortoli, S.A. Artificial corn-based diet for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Bolter, C.J.; Laing, L.E. Competition between Diadegma insulare (Hymenoptera: Ichneumonidae) and Microplitis plutellae (Hymenoptera: Braconidae) for larvae of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Proc. Entomol. Soc. Ont. 1983, 114, 1–10. [Google Scholar]
- Hawkins, B.A. Species diversity in the third and fourth trophic levels: Patterns and mechanisms. J. Ani. Ecol. 1988, 57, 137–162. [Google Scholar] [CrossRef]
- Hågvar, E.B. Interspecific competition in parasitoids, with implications for biological control. Acta Entomol. Bohemoslov. 1989, 86, 321–335. [Google Scholar]
- Bai, S.; Wang, Z.; He, K.; Wen, L.; Zhou, D. Olfactory responses of Trichogramma ostriniae Pang et Chen to kairomones from eggs and different stages of female adults of Ostrinia furnacalis (Guenee). Kun Chong Xue Bao Acta Entomol. Sin. 2004, 1, 48–54. [Google Scholar]
- Liu, S.S.; Zhang, G.M.; Zhang, F. Factors influencing parasitism of Trichogramma dendrolimi on eggs of the Asian corn borer, Ostrinia furnacalis. BioControl 1998, 43, 273–287. [Google Scholar] [CrossRef]
- Boo, K.S.; Yang, J.P. Kairomones used by Trichogramma chilonis to find Helicoverpa assulta eggs. J. Chem. Ecol. 2000, 26, 359–375. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.Y.; Benelli, G.; Desneux, N.; Ali, A.; Zang, L.S. Trichogramma ostriniae is more effective than Trichogramma dendrolimi as a biocontrol agent of the Asian corn borer, Ostrinia furnacalis. Insects 2022, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Subandi, M.; Setiati, Y.; Mutmainah, N.H. Suitability of Corcyra cephalonica eggs parasitized with Trichogramma japonicum as intermediate host against sugarcane borer Chilo auricilius. Bulg. J. Agric. Sci. 2017, 23, 779–786. [Google Scholar]
- Myint, Y.Y.; Bai, S.; Zhang, T.; Babendreier, D.; He, K.; Wang, Z. Ovipositional preference of Trichogramma dedrolimi and Trichogramma ostriniae strains from Myanmar on different host egg ages of Asian corn borer, Otrinia furnacalis (Lepidoptera: Crambidae). Biocont. Sci. Technol. 2022, 3, 700–714. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, X.; Monticelli, L.S.; Zhang, F.; Desneux, N.; Huijie, D.; Ramirez-Romero, R.; Wang, S. Parasitism performance of the parasitoid Trichogramma dendrolimi on the plum fruit moth Grapholitha funebrana. Entomol. Gen. 2020, 40, 385–395. [Google Scholar] [CrossRef]
- Tang, L.D.; Sun, J.W.; Dai, P.; Mu, M.Y.; Nkunika, P.O.; Desneux, N.; Zang, L.S. Performance of two dominant trichogrammatid species of fall armyworm from China and Africa under contrasted temperature and humidity regimes. Biol. Cont. 2023, 1, 105179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Iqbal, A.; Ahmed, K.S.; Zhang, Z.-K.; Cui, J.; Zhang, C. Impact of Oviposition Sequence and Host Egg Density on Offspring Emergence and Interspecific Competition in Two Species of Trichogramma Parasitoids. Insects 2025, 16, 214. https://doi.org/10.3390/insects16020214
Wang Y, Iqbal A, Ahmed KS, Zhang Z-K, Cui J, Zhang C. Impact of Oviposition Sequence and Host Egg Density on Offspring Emergence and Interspecific Competition in Two Species of Trichogramma Parasitoids. Insects. 2025; 16(2):214. https://doi.org/10.3390/insects16020214
Chicago/Turabian StyleWang, Yu, Asim Iqbal, Kanwer Shahzad Ahmed, Zheng-Kun Zhang, Juan Cui, and Chen Zhang. 2025. "Impact of Oviposition Sequence and Host Egg Density on Offspring Emergence and Interspecific Competition in Two Species of Trichogramma Parasitoids" Insects 16, no. 2: 214. https://doi.org/10.3390/insects16020214
APA StyleWang, Y., Iqbal, A., Ahmed, K. S., Zhang, Z.-K., Cui, J., & Zhang, C. (2025). Impact of Oviposition Sequence and Host Egg Density on Offspring Emergence and Interspecific Competition in Two Species of Trichogramma Parasitoids. Insects, 16(2), 214. https://doi.org/10.3390/insects16020214





