Genetic Diversity and Connectivity of the Vulnerable Species Phengaris nausithous in Palencia (Northern Spain) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
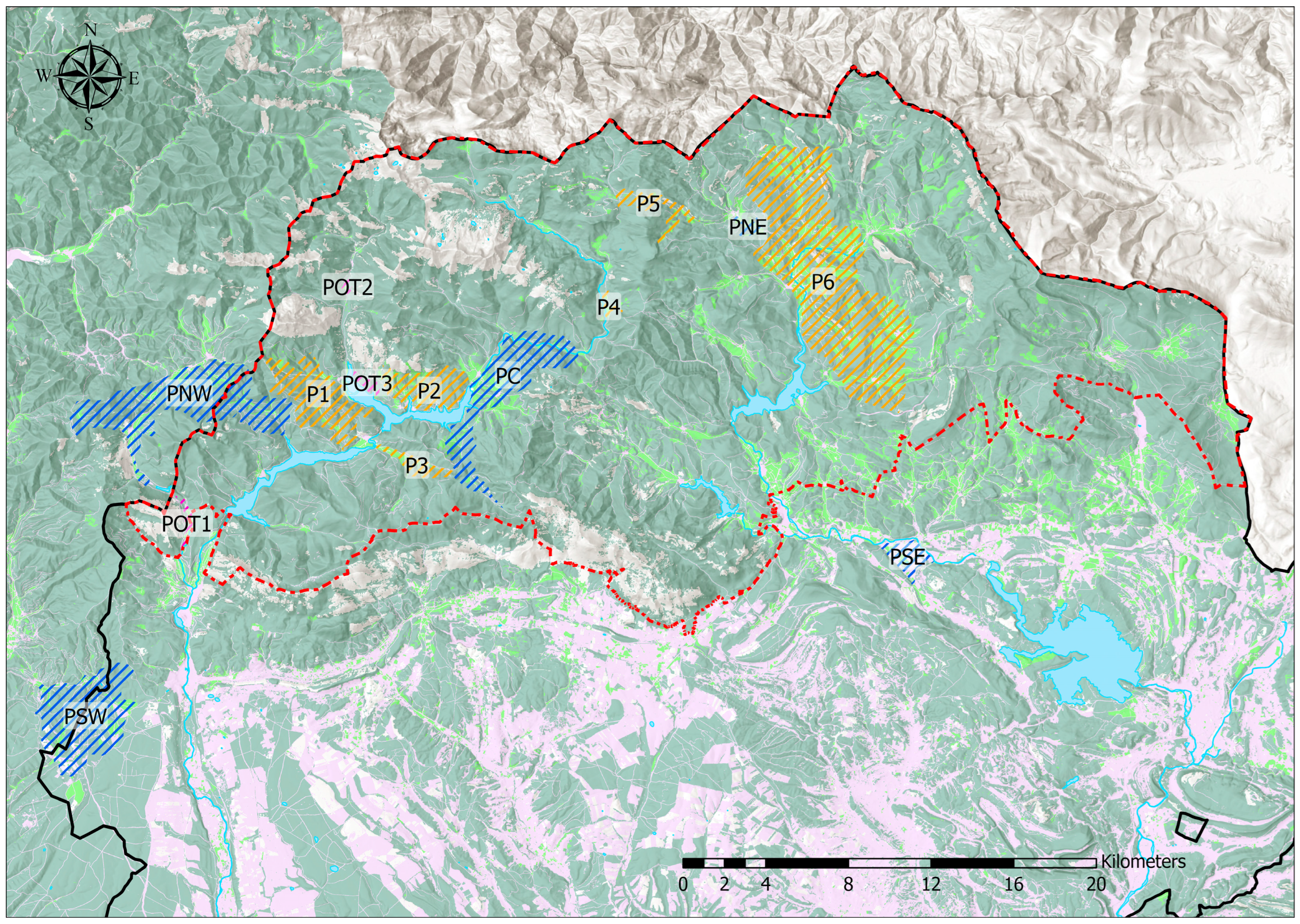
2.1. Study Area
2.2. Study Area Survey
2.3. Genetic Study
2.3.1. Sampling Point Selection
- PSW: Population southwest of the Natural Park. One patch was sampled;
- PNW: Population in the Carrión River watershed and part of the eastern Leonese mountains. Located west of Camporredondo and Compuerto reservoirs: three patches were sampled 2.3–10 km apart;
- PC: Population in the Carrión River watershed but east of Camporredondo and Compuerto reservoirs: two patches were sampled 8 km apart;
- PNE: Population in the Pisuerga River watershed: one patch was sampled 11 km east of the nearest PC patch;
- PSE: Reserve southeast of the Natural Park: one patch was sampled at a distance of 17.8 km south of PNE;
- Soria: Samples were taken from two patches 2.6 km apart in Soria province, approximately 200 km from the previous five.
- P1: Groups two patches occupied by the butterfly and close to PNW;
- P2: Groups 4 nearby patches located between P1 and PC;
- P3: Patch extending along a valley bottom between P1 and PC, south of P2;
- P4: Small patch with few individuals on the northeastern border of the Carrión Valley;
- P5: Large patch located right at the watershed divide between Carrión and Pisuerga;
- P6: Zone grouping all patches occupied by P. nausithous in the Pisuerga River watershed, which would also include PNE.
2.3.2. DNA Sampling and Extraction
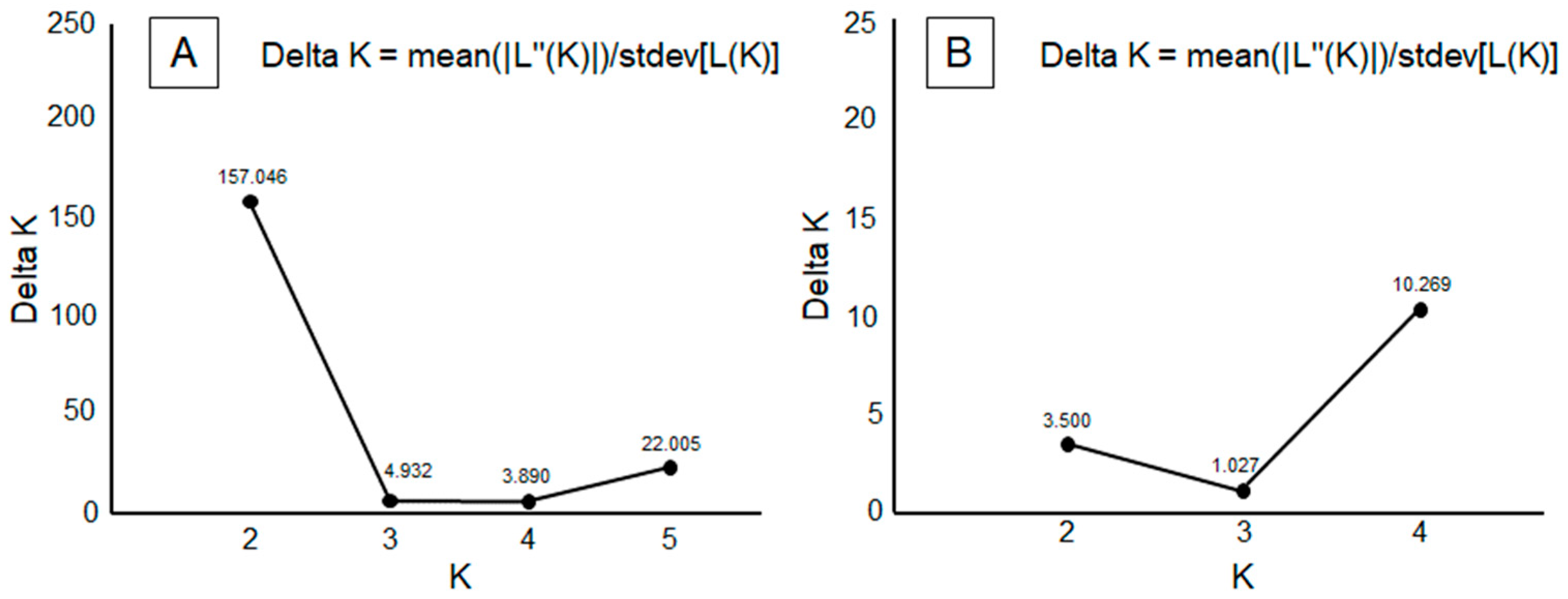
2.3.3. Statistical Analyses
3. Results
3.1. Study Area Survey
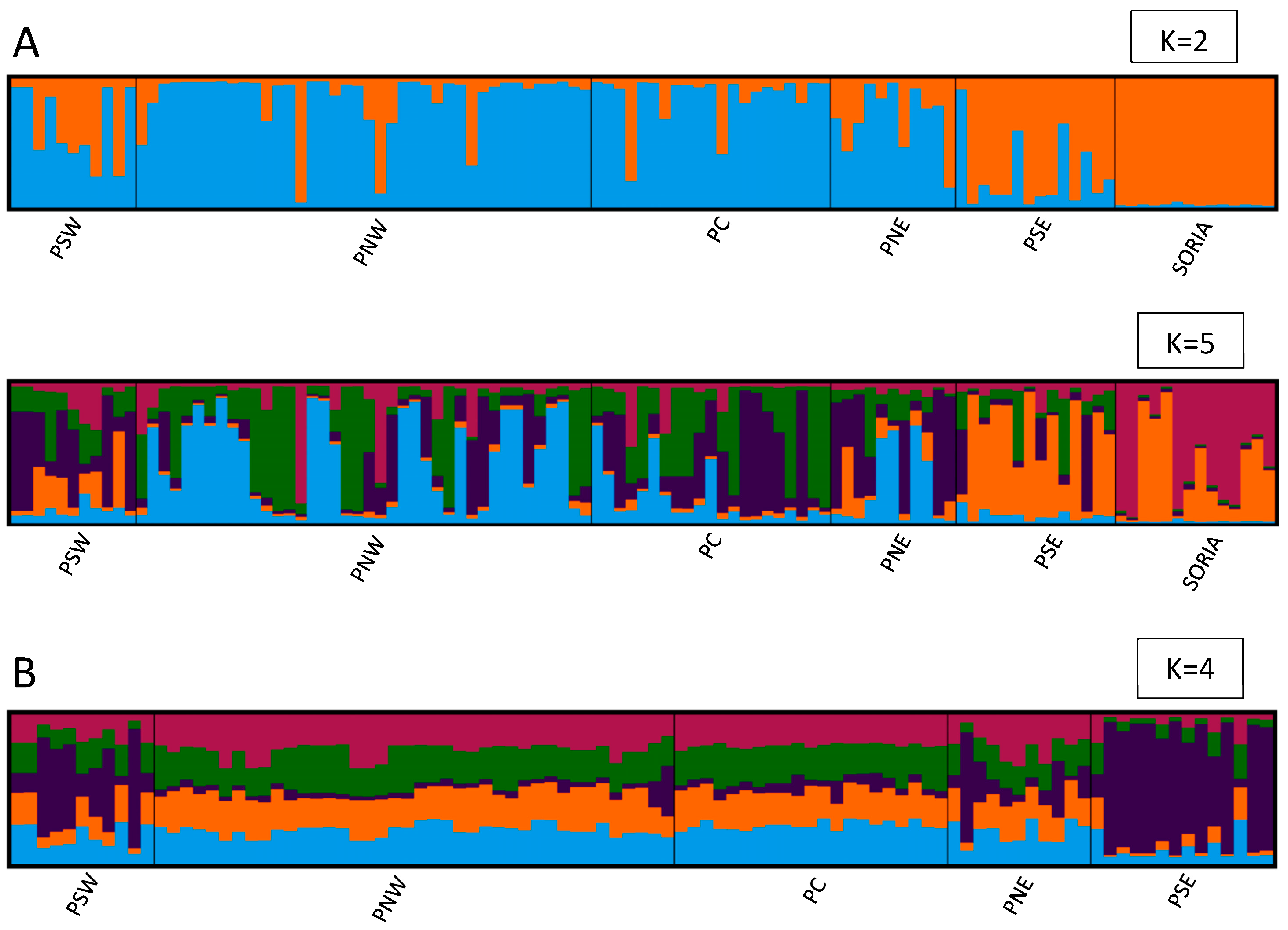
3.2. Genetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IUCN | International Union for Conservation of Nature |
| UTM | Universal Transverse Mercator |
| DNA | Deoxyribonucleic Acid |
| PCR | Polymerase Chain Reaction |
| HWE | Hardy–Weinberg Equilibrium |
| IBD | Isolation By Distance |
| IAM | Infinite Allele Model |
| SMM | Stepwise Mutation Model |
| Ne | Effective Population Size |
| MNA | Mean Number of Alleles |
| Ar | Allelic Richness |
| Fis | Inbreeding Coefficient |
| Nm | Gene Flow Between Populations |
| CMR | Capture–Mark–Recapture |
References
- Rogan, J.E.; Lacher, T.E. Impacts of habitat loss and fragmentation on terrestrial biodiversity. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Caughley, G. Directions in Conservation Biology. J. Anim. Ecol. 1994, 63, 215. [Google Scholar] [CrossRef]
- Hanski, I.; Gaggiotti, O. Ecology, Genetics and Evolution of Metapopulations; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar] [CrossRef]
- Fernández-Chacón, A.; Stefanescu, C.; Genovart, M.; Nichols, J.D.; Hines, J.E.; Páramo, F.; Turco, M.; Oro, D.; Childs, D. Determinants of extinction-colonization dynamics in Mediterranean butterflies: The role of landscape, climate and local habitat features. J. Anim. Ecol. 2013, 83, 276–285. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Maes, D.; Van Dyck, H. Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol. Conserv. 2001, 99, 263–276. [Google Scholar] [CrossRef]
- Thomas, J.A. Butterfly communities under threat. Science 2016, 353, 216–218. [Google Scholar] [CrossRef]
- Thomas, J.A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Thomas, J.A.; Bourn, N.A.D.; Clarke, R.T.; Stewart, K.E.; Simcox, D.J.; Pearman, G.S.; Curtis, R.; Goodger, B. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1791–1796. [Google Scholar] [CrossRef]
- Binzenhöfer, B.; Schröder, B.; Strauss, B.; Biedermann, R.; Settele, J. Habitat models and habitat connectivity analysis for butterflies and burnet moths—The example of Zygaena carniolica and Coenonympha arcania. Biol. Conserv. 2005, 126, 247–259. [Google Scholar] [CrossRef]
- Krauss, J.; Bommarco, R.; Guardiola, M.; Heikkinen, R.K.; Helm, A.; Kuussaari, M.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 2010, 13, 597–605. [Google Scholar] [CrossRef]
- Öckinger, E.; Lindborg, R.; Sjödin, N.E.; Bommarco, R. Landscape matrix modifies richness of plants and insects in grassland fragments. Ecography 2012, 35, 259–267. [Google Scholar] [CrossRef]
- Settele, J.; Kühn, E. Insect conservation. Science 2009, 325, 41–42. [Google Scholar] [CrossRef]
- Dover, J.; Settele, J. The influences of landscape structure on butterfly distribution and movement: A review. J. Insect Conserv. 2008, 13, 3–27. [Google Scholar] [CrossRef]
- Ranius, T.; Nilsson, S.G.; Franzén, M. How frequent is metapopulation structure among butterflies in grasslands? Occurrence patterns in a forest-dominated landscape in southern Sweden. Écoscience 2011, 18, 138–144. [Google Scholar] [CrossRef]
- Thomas, C.D.; Hanski, I. Butterfly metapopulations. In Metapopulation Biology: Ecology, Genetics, and Evolution; Hanski, I., Gilpin, M.E., Eds.; Academic Press: Cambridge, MA, USA, 1997; pp. 359–386. [Google Scholar] [CrossRef]
- Levins, R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Am. 1969, 15, 237–240. [Google Scholar] [CrossRef]
- Hanski, I. A practical model of metapopulation dynamics. J. Anim. Ecol. 1994, 63, 151. [Google Scholar] [CrossRef]
- Skórka, P.; Nowicki, P.; Lenda, M.; Witek, M.; Śliwińska, E.B.; Settele, J.; Woyciechowski, M. Different flight behaviour of the endangered scarce large blue butterfly Phengaris teleius (Lepidoptera: Lycaenidae) within and outside its habitat patches. Landsc. Ecol. 2013, 28, 533–546. [Google Scholar] [CrossRef]
- Figurny-Puchalska, E.; Gadeberg, R.M.E.; Boomsma, J.J. Comparison of genetic population structure of the large blue butterflies Maculinea nausithous and M. teleius. Biodivers. Conserv. 2000, 9, 419–432. [Google Scholar] [CrossRef]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.E.; Brashares, J.S. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar] [CrossRef]
- Besold, J.; Schmitt, T.; Tammaru, T.; Cassel-Lundhagen, A. Strong genetic impoverishment from the centre of distribution in southern Europe to peripheral Baltic and isolated Scandinavian populations of the pearly heath butterfly. J. Biogeogr. 2008, 35, 2090–2101. [Google Scholar] [CrossRef]
- Lowe, A.; Harris, S.; Ashton, P. Ecological Genetics: Design, Analysis, and Application; Wiley-Blackwell: Malden, MA, USA, 2004; p. 352. [Google Scholar]
- Cobben, M.M.P.; Verboom, J.; Opdam, P.F.M.; Hoekstra, R.F.; Jochem, R.; Smulders, M.J.M. Landscape prerequisites for the survival of a modelled metapopulation and its neutral genetic diversity are affected by climate change. Landsc. Ecol. 2012, 27, 227–237. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Aguado, L.Ó. Las Mariposas Diurnas de Castilla y León (Lepidópteros Ropalóceros). Especies, Biología, Distribución y Conservación; León, J.D.C.Y., Ed.; Junta de Castilla y León, Consejería de Medio Ambiente; Fundación Patrimonio Natural: Valladolid, Spain, 2007; p. 1030. [Google Scholar]
- Munguira, M.L.; Martín, J.; Orueta, D.; Viejo, J.L.; García-Barros, E. Maculinea nausithous (Bergsträsser, 1779). In Los Invertebrados No Insectos de la “Directiva Hábitat” en España; Ramos, M., Bragado, D., Fernández, J., Eds.; Organismo Autónomo de Parques Nacionales—Ministerio de Medio Ambiente: Madrid, Spain, 2001; pp. 163–173. [Google Scholar]
- Witek, M.; Śliwińska, B.E.; Skórka, P.; Nowicki, P.; Wantuch, M.; Vrabec, V.; Settele, J.; Woyciechowski, M. Host ant specificity of large blue butterflies Phengaris (Maculinea) (Lepidoptera: Lycaenidae) inhabiting humid grasslands in East-central Europe. Eur. J. Entomol. 2008, 105, 871–877. [Google Scholar] [CrossRef]
- Tartally, A.; Thomas, J.A.; Anton, C.; Balletto, E.; Barbero, F.; Bonelli, S.; Bräu, M.; Casacci, L.P.; Csősz, S.; Czekes, Z.; et al. Patterns of host use by brood parasitic Maculinea butterflies across Europe. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180202. [Google Scholar] [CrossRef]
- Loritz, H.; Settele, J. Effects of human land-use on availability and quality of habitats of the Dusky Large Blue butterfly. In Studies on the Ecology and Conservation of Butterflies in Europe. Vol. 2. Species Ecology Along a European Gradient: Maculinea Butterflies as a Model; Thomas, J.A., Settele, J., Kühn, E., Eds.; Pensoft: Sofia, Bulgaria, 2005; pp. 225–227. [Google Scholar]
- Wynhoff, I. The recent distribution of the European Maculinea species. J. Insect Conserv. 1998, 2, 15–27. [Google Scholar] [CrossRef]
- Munguira, M.L.; Romo Benito, H.; Martín Cano, J.; García-Barros, E. Phengaris nausithous (Bergsträsser, 1779). In Atlas y Lista Roja de Invertebrados Amenazados de España (Especies Vulnerables); Verdú, J., Numa, E., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal; Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2011; Volume I: Artrópodos, pp. 1258–1264. [Google Scholar]
- Sanz Sanz, T.; Pomeda Maestre, M.Á.; Montoya Jiménez, M. Actualización de la distribución de Phengaris nausithous (Bergsträsser, 1779) (Lepidoptera: Lycaenidae) en la provincia de León (N. de España). Arq. Entomolóxicos 2017, 17, 225–232. [Google Scholar]
- Vicente Arranz, J.C.; Salvador Vilariño, V.; Alcalde de Miguel, J.; Parra-Arjona, B. Ampliación de la distribución de Phengaris nausithous (Bergstrasser, 1779) (Lepidoptera: Lycaenidae) en la Península Ibérica, y algunas consideraciones para su conservación. Boletín Soc. Entomológica Aragonesa 2013, 52, 249–258. [Google Scholar]
- Jubete, F. Actualización de la lista patrón y nuevos datos de distribución de mariposas diurnas de presencia escasa en la provincia de Palencia (Castilla y León, España) (Lepidoptera, Papilionoidea). Arq. Entomolóxicos 2021, 24, 83–98. [Google Scholar]
- Van Swaay, C.; Cuttelod, A.; Collins, S.; Maes, D.; López Munguira, M.; Šašic, M.; Settele, J.; Verovnik, R.; Verstrael, T.; Warren, M. European Red List of Butterflies; Publications Office of the European Union: Luxembourg, 2010; p. 60. [Google Scholar]
- Ministerio para la Transición Ecológica—Gobierno de España. Situación actual del Listado de Especies Silvestres en Régimen de Protección Especial y Catálogo Español de Especies Amenazadas; Ministerio para la Transición Ecológica—Gobierno de España: Madrid, Spain, 2019.
- Jubete, F.; Román, J. New large threatened populations of Phengaris nausithous discovered in the SW of Europe. J. Insect Conserv. 2016, 20, 155–158. [Google Scholar] [CrossRef]
- Romo, H.; Silvestre, M.; Munguira, M. Potential distribution models and the effect of climatic change on the distribution of Phengaris nausithous considering its food plant and host ants. J. Insect Conserv. 2015, 19, 1101–1118. [Google Scholar] [CrossRef]
- Als, T.D.; Vila, R.; Kandul, N.P.; Nash, D.R.; Yen, S.-H.; Hsu, Y.-F.; Mignault, A.A.; Boomsma, J.J.; Pierce, N.E. The evolution of alternative parasitic life histories in large blue butterflies. Nature 2004, 432, 386–390. [Google Scholar] [CrossRef]
- Fric, Z.; Wahlberg, N.; Pech, P.; Zrzavý, J.A.N. Phylogeny and classification of the Phengaris–Maculinea clade (Lepidoptera: Lycaenidae): Total evidence and phylogenetic species concepts. Syst. Entomol. 2007, 32, 558–567. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Gómez, J.F.; Lobo, J.M.; Baselga, A.; Hortal, J. Challenging species distribution models: The case of Maculinea nausithous in the Iberian Peninsula. Ann. Zool. Fenn. 2008, 45, 200–210. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.J.; Schibalski, A.; Schröder, B.; Klimek, S.; Dauber, J. Disentangling the effects of host resources, local, and landscape variables on the occurrence pattern of the dusky large blue butterfly (Phengaris nausithous) in upland grasslands. J. Insect Conserv. 2020, 24, 327–341. [Google Scholar] [CrossRef]
- Hollós, A.; Pecsenye, K.; Bereczki, J.; Bátori, E.; Rákosy, L.; Varga, Z. Pattern of genetic and morphometric differentiation in Maculinea nausithous (Lepidoptera: Lycaenidae) in the Carpathian Basin. Acta Zool. Acad. Sci. Hung. 2012, 58, 87–103. [Google Scholar]
- Zeisset, I.; Damm Als, T.; Settele, J.; Boomsma, J.J. Microsatellite markers for the large blue butterflies Maculinea nausithous and Maculinea alcon (Lepidoptera: Lycaenidae) and their amplification in other Maculinea species. Mol. Ecol. Notes 2005, 5, 165–168. [Google Scholar] [CrossRef]
- Anton, C.; Zeisset, I.; Musche, M.; Durka, W.; Boomsma, J.J.; Settele, J. Population structure of a large blue butterfly and its specialist parasitoid in a fragmented landscape. Mol. Ecol. 2007, 16, 3828–3838. [Google Scholar] [CrossRef]
- Ritter, S.; Michalski, S.G.; Settele, J.; Wiemers, M.; Fric, Z.F.; Sielezniew, M.; Šašić, M.; Rozier, Y.; Durka, W. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 2013, 8, e78107. [Google Scholar] [CrossRef]
- Rutkowski, R.; Sielezniew, M.; Szostak, A. Contrasting levels of polymorphism in cross-amplified microsatellites in two endangered xerothermophilous, obligatorily myrmecophilous, butterflies of the genus Phengaris (Maculinea) (Lepidoptera: Lycaenidae). Eur. J. Entomol. 2009, 106, 457–469. [Google Scholar] [CrossRef]
- Sielezniew, M.; Rutkowski, R. Population isolation rather than ecological variation explains the genetic structure of endangered myrmecophilous butterfly Phengaris (=Maculinea) arion. J. Insect Conserv. 2012, 16, 39–50. [Google Scholar] [CrossRef]
- Ugelvig, L.V.; Andersen, A.; Boomsma, J.J.; Nash, D.R. Dispersal and gene flow in the rare, parasitic Large Blue butterfly Maculinea arion. Mol. Ecol. 2012, 21, 3224–3236. [Google Scholar] [CrossRef]
- Ugelvig, L.V.; Nielsen, P.S.; Boomsma, J.J.; Nash, D.R. Reconstructing eight decades of genetic variation in an isolated Danish population of the large blue butterfly Maculinea arion. BMC Evol. Biol. 2011, 11, 201. [Google Scholar] [CrossRef]
- Tartally, A.; Kelager, A.; Fürst, M.A.; Nash, D.R. Host plant use drives genetic differentiation in syntopic populations of Maculinea alcon. PeerJ 2016, 4, e1865. [Google Scholar] [CrossRef]
- Nowicki, P.; Deoniziak, K.; Dziekańska, I.; Kostro-Ambroziak, A.; Plazio, E.; Rutkowski, R.; Sielezniew, M. What keeps ‘living dead’ alive: Demography of a small and isolated population of Maculinea (=Phengaris) alcon. J. Insect Conserv. 2019, 23, 201–210. [Google Scholar] [CrossRef]
- Bereczki, J.; Sielezniew, M.; Verovnik, R.; Beshkov, S.; Kuznetsov, G.; Bonelli, S.; Tóth, J.P. Phylogeography reveals the origin of the two phenological forms of large blue, Phengaris arion (Lepidoptera: Lycaenidae). Biol. J. Linn. Soc. 2022, 137, 359–373. [Google Scholar] [CrossRef]
- Binzenhöfer, B.; Settele, J. Vergleichende autökologische Untersuchungen an Maculinea nausithous (BERGSTR., [1779]) und Maculinea teleius (BERGSTR., [1779]) (Lep.: Lycaenidae) im nördlichen Steigerwald. UFZ-Ber. 2000, 2, 1–98. [Google Scholar]
- Sánchez-Sastre, L.F. Informe de la Asistencia Técnica para la Definición de Medidas de Gestión y Conservación de las Poblaciones de Hormiguera Oscura (Phengaris nausithous) y Especies de Interés del Orden Odonata (TSA0069221); Junta de Castilla y León: Valladolid, Spain, 2020; p. 130. [Google Scholar]
- Sánchez-Sastre, L.F.; Casanueva, P.; Campos, F.; Ramírez del Palacio, Ó. Nuevas citas de Aeshna juncea, Sympetrum flaveolum y Coenagrion mercuriale (Odonata: Aeshnidae, Libellulidae, Coenagrionidae) de la provincia de Palencia (norte de España). Boletín Soc. Entomológica Aragonesa 2020, 67, 391–395. [Google Scholar]
- Hamm, C.A.; Aggarwal, D.; Landis, D.A. Evaluating the impact of non-lethal DNA sampling on two butterflies, Vanessa cardui and Satyrodes eurydice. J. Insect Conserv. 2010, 14, 11–18. [Google Scholar] [CrossRef]
- Rose, O.C.; Brookes, M.I.; Mallet, J.L.B. A quick and simple nonlethal method for extracting DNA from butterfly wings. Mol. Ecol. 1994, 3, 275. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Maes, D.; Kelager, A.; Wynhoff, I.; WallisDeVries, M.F.; Nash, D.R.; Oostermeijer, J.G.B.; Van Dyck, H.; Mergeay, J. Gene flow and effective population sizes of the butterfly Maculinea alcon in a highly fragmented, anthropogenic landscape. Biol. Conserv. 2017, 209, 89–97. [Google Scholar] [CrossRef]
- Nowicki, P.; Settele, J.; Thomas, J.A.; Woyciechowski, M. A review of population structure of Maculinea butterflies. In Studies on the Ecology and Conservation of Butterflies in Europe. Vol. 2: Species Ecology along a European Gradient: Maculinea Butterflies as a Model; Settele, J., Kühn, E., Thomas, J.A., Eds.; Pensoft: Sofia, Bulgaria, 2005; pp. 144–149. [Google Scholar]
- Nowicki, P.; Witek, M.; Skórka, P.; Settele, J.; Woyciechowski, M. Population ecology of the endangered butterflies Maculinea teleius and M. nausithous and the implications for conservation. Popul. Ecol. 2005, 47, 193–202. [Google Scholar] [CrossRef]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, N.; Bonhomme, F. GENETIX 4.05, Logiciel Sous Windows TM Pour la Génétique des Populations; Laboratoire Génome, Populations, Interactions, CNRS UMR 5171, Université de Montpellier II: Montpellier, France, 2004. [Google Scholar]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the Genetics of Populations, Volume 2: Theory of Gene Frequencies; University of Chicago Press: Chicago, IL, USA, 1984; Volume 2, p. 520. [Google Scholar]
- Galacatos, K.; Cognato, A.I.; Sperling, F.A.H. Population genetic structure of two water strider species in the Ecuadorian Amazon. Freshw. Biol. 2002, 47, 391–399. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. ARLEQUIN suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Kalinowski, S.T. HP-RARE 1.0: A computer program for performing rarefaction on measures of allelic richness. Mol. Ecol. Notes 2004, 5, 187–189. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2017, 18, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT (Version 1.2): A computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 1996, 144, 2001–2014. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J.M. Computer note. BOTTLENECK: A computer program for detecting recent reductions in the effective size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Waples, R.S.; Do, C.H.I. LDNE: A program for estimating effective population size from data on linkage disequilibrium. Mol. Ecol. Resour. 2008, 8, 753–756. [Google Scholar] [CrossRef]
- Piry, S.; Alapetite, A.; Cornuet, J.M.; Paetkau, D.; Baudouin, L.; Estoup, A. GENECLASS2: A software for genetic assignment and first-generation migrant detection. J. Hered. 2004, 95, 536–539. [Google Scholar] [CrossRef]
- Paetkau, D.; Slade, R.; Burden, M.; Estoup, A. Genetic assignment methods for the direct, real-time estimation of migration rate: A simulation-based exploration of accuracy and power. Mol. Ecol. 2004, 13, 55–65. [Google Scholar] [CrossRef]
- Moran, P.A.P. The interpretation of statistical maps. J. R. Stat. Soc. Ser. B Stat. Methodol. 1948, 10, 243–251. [Google Scholar] [CrossRef]
- Anselin, L.; Li, X.; Koschinsky, J. GeoDa, from the desktop to an ecosystem for exploring spatial data. Geogr. Anal. 2022, 54, 439–466. [Google Scholar] [CrossRef]
- Sánchez-Sastre, L.F. Informe de la Asistencia Técnica para la Elaboración de las Afecciones Ambientales de las Obras del Proyecto de Infraestructura Rural y Restauración del Medio Natural de la Zona de Concentración Parcelaria “Camporredondo—Los Cardaños” (Palencia) y Análisis de Medidas de Restauración del Medio Natural; Junta de Castilla y León: Valladolid, Spain, 2019; p. 81. [Google Scholar]
- Nowicki, P.; Vrabec, V.; Binzenhöfer, B.; Feil, J.; Zakšek, B.; Hovestadt, T.; Settele, J. Butterfly dispersal in inhospitable matrix: Rare, risky, but long-distance. Landsc. Ecol. 2014, 29, 401–412. [Google Scholar] [CrossRef]
- Kajzer-Bonk, J.; Skórka, P.; Nowicki, P.; Bonk, M.; Król, W.; Szpiłyk, D.; Woyciechowski, M. Relative contribution of matrix structure, patch resources and management to the local densities of two large blue butterfly species. PLoS ONE 2016, 11, e0168679. [Google Scholar] [CrossRef] [PubMed]
- Villemey, A.; van Halder, I.; Ouin, A.; Barbaro, L.; Chenot, J.; Tessier, P.; Calatayud, F.; Martin, H.; Roche, P.; Archaux, F. Mosaic of grasslands and woodlands is more effective than habitat connectivity to conserve butterflies in French farmland. Biol. Conserv. 2015, 191, 206–215. [Google Scholar] [CrossRef]
- Batáry, P.; Kőrösi, Á.; Örvössy, N.; Kövér, S.; Peregovits, L. Species-specific distribution of two sympatric Maculinea butterflies across different meadow edges. J. Insect Conserv. 2009, 13, 223–230. [Google Scholar] [CrossRef]
- Popović, M.; Nowicki, P. Movements of a specialist butterfly in relation to mowing management of its habitat patches. Biology 2023, 12, 344. [Google Scholar] [CrossRef]
- Schmitt, T.; Hewitt, G.M. The genetic pattern of population threat and loss: A case study of butterflies. Mol. Ecol. 2003, 13, 21–31. [Google Scholar] [CrossRef]
- Hanski, I.; Moilanen, A.; Gyllenberg, M. Minimum viable metapopulation size. Am. Nat. 1996, 147, 527–541. [Google Scholar] [CrossRef]

| Population | Total Number of Patches | Sampled Patches | Area of Sampled Patches (ha) | Area of All Patches (ha) | Area of Matrix + Patches (ha) | Maximum Distance Between Patches (km) |
|---|---|---|---|---|---|---|
| PNW | 6 | 3 | 2.96, 8.03, 10.2 | 23.78 | 2295 | 10 |
| PC | 8 | 2 | 3.78, 0.56 | 27.24 | 1487 | 8 |
| PNE | 1 | 1 | 1.96 | 1.96 | ||
| PSW | 3 | 1 | 12.95 | 70.35 | 1668 | 3.8 |
| PSE | 1 | 1 | 3.20 | 3.20 | ||
| Soria | 2 | 2 | 1.5, 6.8 | 8.3 | 2.6 |
| Population | Total Number of Patches | Area of All Patches (ha) | Area of Matrix + Patches (ha) | Maximum Distance Between Patches (km) |
|---|---|---|---|---|
| P1 | 2 | 8.96 | 1059 | 2 |
| P2 | 4 | 14.99 | 670 | 2.8 |
| P3 | 1 | 18.88 | ||
| P4 | 1 | 1 | ||
| P5 | 1 | 6 | ||
| P6 | 25 | 186 | 5077 | 13 |
| Locations | N | He | Ho | MNA | Ar | Fis (1000 Bootstraps) |
|---|---|---|---|---|---|---|
| Soria | 14 | 0.53 | 0.44 | 5.40 | 5.08 | 0.202 |
| PSW | 11 | 0.25 | 0.24 | 2.60 | 2.60 | 0.085 |
| PNW | 40 | 0.43 | 0.32 | 3.80 | 3.26 | 0.281 |
| PC | 21 | 0.29 | 0.24 | 2.40 | 2.24 | 0.204 |
| PNE | 11 | 0.37 | 0.27 | 2.80 | 2.80 | 0.312 |
| PSE | 14 | 0.40 | 0.46 | 2.80 | 2.75 | −0.117 |
| Locations | Soria | PSW | PNW | PC | PNE | PSE |
|---|---|---|---|---|---|---|
| Soria | - | 0.27 | 0.24 | 0.31 | 0.20 | 0.10 |
| PSW | 0.69 | - | 0.14 | 0.05 | 0.09 | 0.10 |
| PNW | 0.82 | 1.58 | - | 0.07 | 0.10 | 0.14 |
| PC | 0.56 | 3.51 | 4.60 | - | 0.10 | 0.15 |
| PNE | 1.00 | 2.30 | 2.44 | 2.27 | - | 0.07 |
| PSE | 2.29 | 1.59 | 2.28 | 1.37 | 3.18 | - |
| PNW 1 | PNW 2 | PNW 3 | PC 1 | PC 2 | PSW | PNE | PSE | |
|---|---|---|---|---|---|---|---|---|
| PNW 1 | - | 2.5 * | 10 | 9.2 | 14.3 | 18.8 | 25.1 | 30.7 |
| PNW 2 | 2.5 * | - | 8.1 | 11.5 | 15.9 | 19.2 | 26.4 | 33 |
| PNW 3 | 10 | 8.1 | - | 19.2 | 23.9 | 16.2 | 34.3 | 40.8 |
| PC 1 | 9.2 | 11.5 | 19.2 | - | 7.7 | 24 | 18.5 | 21.6 |
| PC 2 | 14.3 | 15.9 | 23.9 | 7.7 | - | 31.4 | 11 | 19.5 |
| PSW | 18.8 | 19.2 | 16.2 | 24 | 31.4 | - | 42.3 † | 42 |
| PNE | 25.1 | 26.4 | 34.3 | 18.5 | 11 | 42.3 † | - | 17.8 |
| PSE | 30.7 | 33 | 40.8 | 21.6 | 19.5 | 42 | 17.8 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Sastre, L.F.; Ramírez-del-Palacio, Ó.; Martín-Ramos, P.; Hernández-Minguillón, M.-Á. Genetic Diversity and Connectivity of the Vulnerable Species Phengaris nausithous in Palencia (Northern Spain). Insects 2025, 16, 193. https://doi.org/10.3390/insects16020193
Sánchez-Sastre LF, Ramírez-del-Palacio Ó, Martín-Ramos P, Hernández-Minguillón M-Á. Genetic Diversity and Connectivity of the Vulnerable Species Phengaris nausithous in Palencia (Northern Spain). Insects. 2025; 16(2):193. https://doi.org/10.3390/insects16020193
Chicago/Turabian StyleSánchez-Sastre, Luis Fernando, Óscar Ramírez-del-Palacio, Pablo Martín-Ramos, and María-Ángeles Hernández-Minguillón. 2025. "Genetic Diversity and Connectivity of the Vulnerable Species Phengaris nausithous in Palencia (Northern Spain)" Insects 16, no. 2: 193. https://doi.org/10.3390/insects16020193
APA StyleSánchez-Sastre, L. F., Ramírez-del-Palacio, Ó., Martín-Ramos, P., & Hernández-Minguillón, M.-Á. (2025). Genetic Diversity and Connectivity of the Vulnerable Species Phengaris nausithous in Palencia (Northern Spain). Insects, 16(2), 193. https://doi.org/10.3390/insects16020193








