Oviposition Deterrents from Extracts of Eryngium foetidum Against Potato Tuber Moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Preparation of Plant Minced Leaves, Extraction, and Chemicals
2.2.1. Minced Leaves
2.2.2. Plant Extracts
2.2.3. Chemicals
2.3. Effect of E. foetidum Minced Leaves on Oviposition of PTM in Non-Choice Assay
2.4. Effect of E. foetidum Minced Leaves on Oviposition of PTM in Choice Assays
2.5. Effect of E. foetidum Extracts on Oviposition of PTM in Non-Choice Assays
2.6. Effect of E. foetidum Extracts on Oviposition of PTM in Choice Assays
2.7. Coupled Gas Chromatography–Electroantennographic Detection (GC–EAD) Recording
2.8. Chemical Analyses
2.9. Effect of Individual EAD Active Compounds on Oviposition of PTM in Choice Assays
2.10. Effect of Mixtures of EAD Active Compounds on Oviposition Preference of PTM
2.11. Statistical Analysis
3. Results
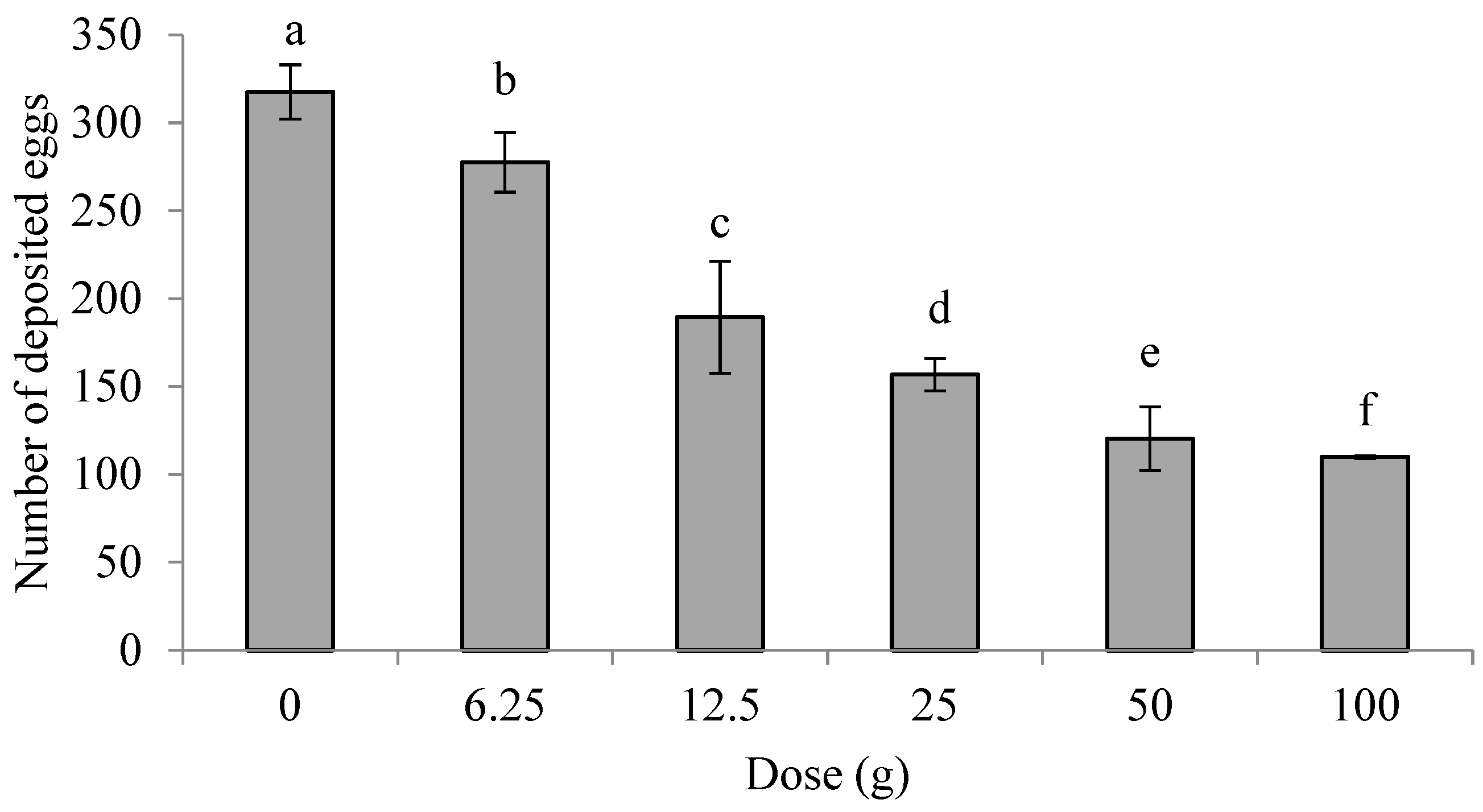
3.1. Effects of E. foetidum Minced Leaves on Oviposition Preferences of P. operculella in Non-Choice Assays
3.2. Effects of Minced Leaves of E. foetidum on Oviposition Preferences of P. operculella in Choice Assays
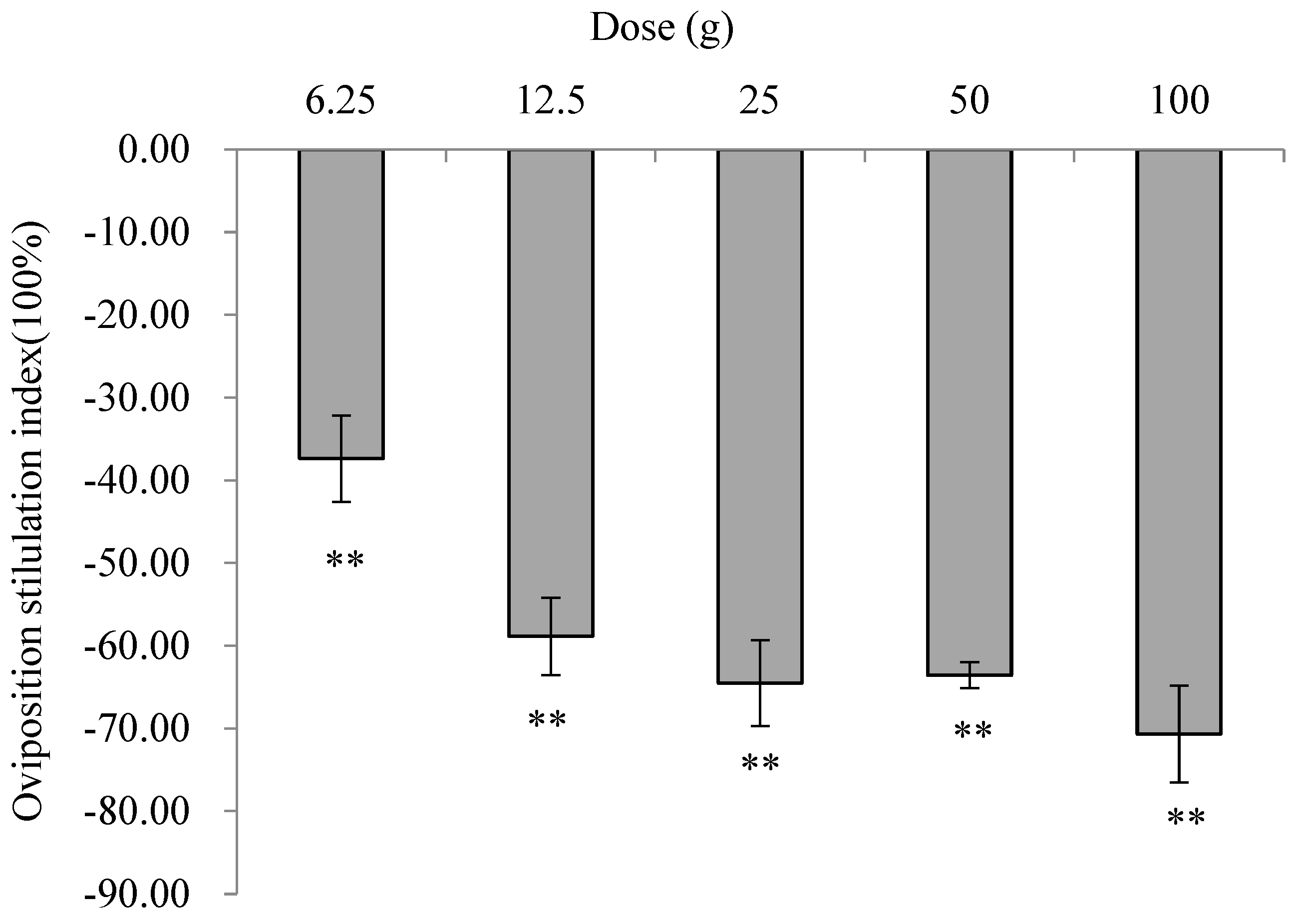
3.3. Effects of E. foetidum Extracts on the Oviposition Preferences of P. operculella in Non-Choice Assays
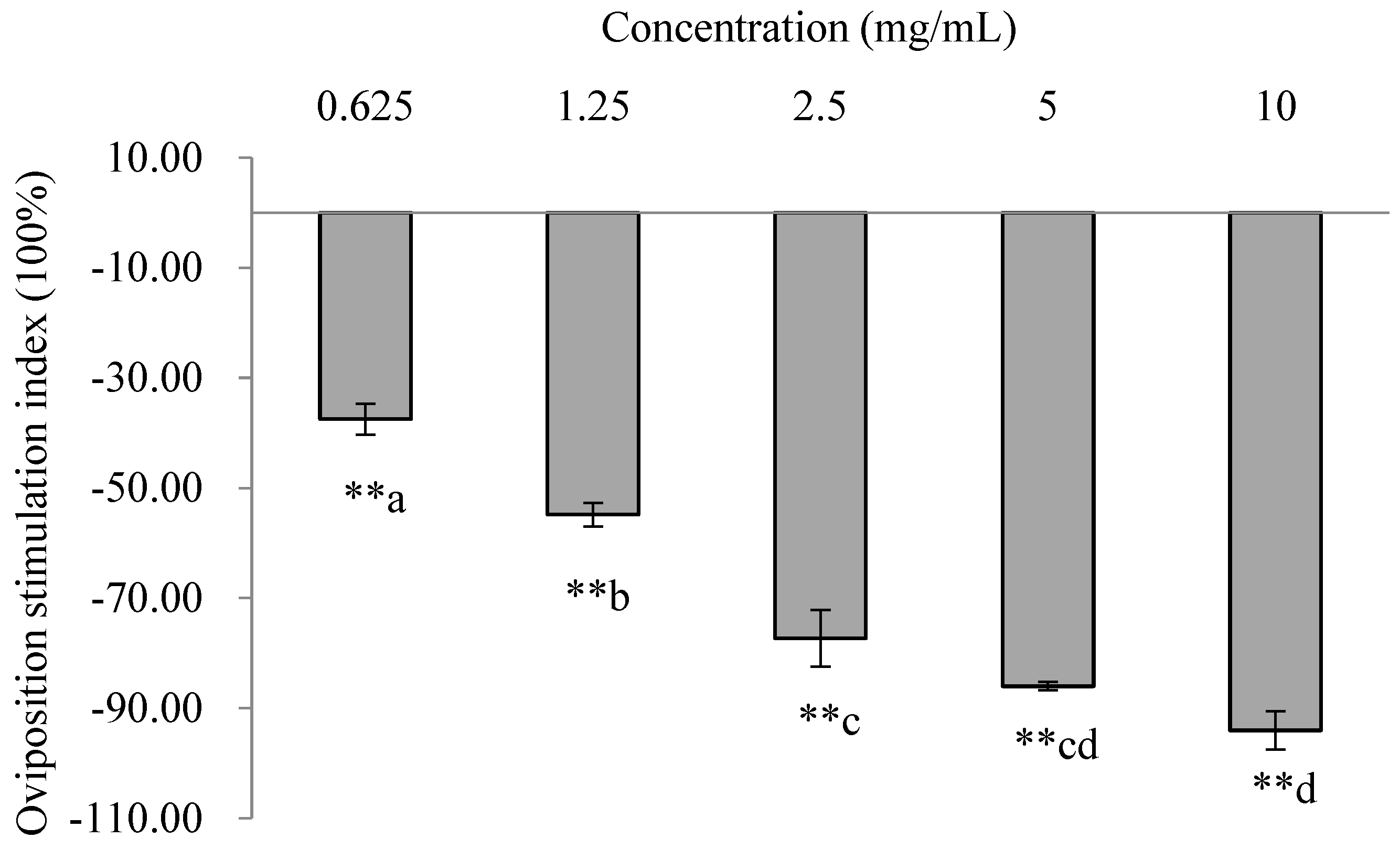
3.4. Effects of E. foetidum Extracts on Oviposition Preferences of P. operculella in Choice Assays
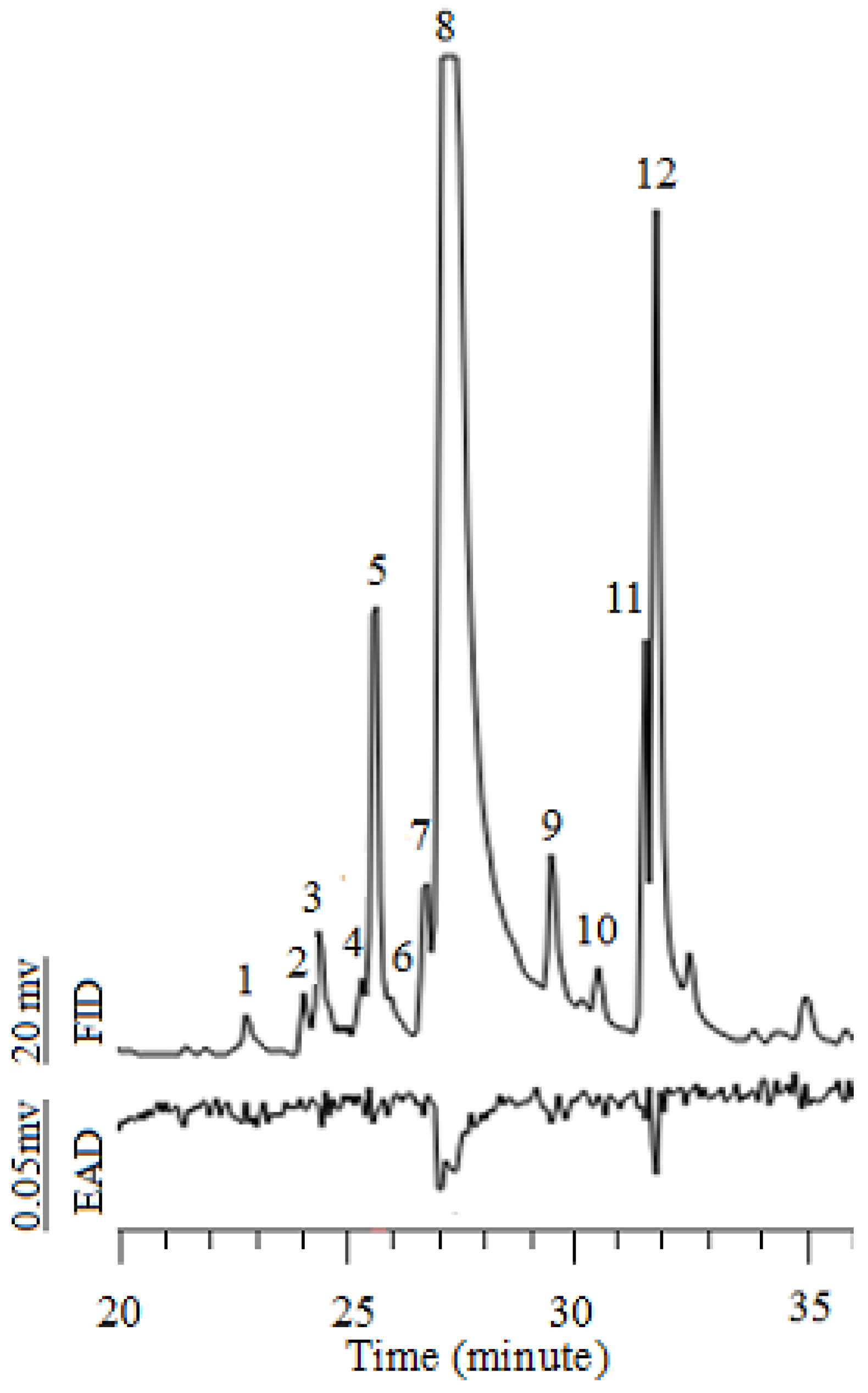
3.5. Chemical and Electrophysiological Analyses
3.6. Oviposition Responses of PTM Females to Individual EAD Active Compounds
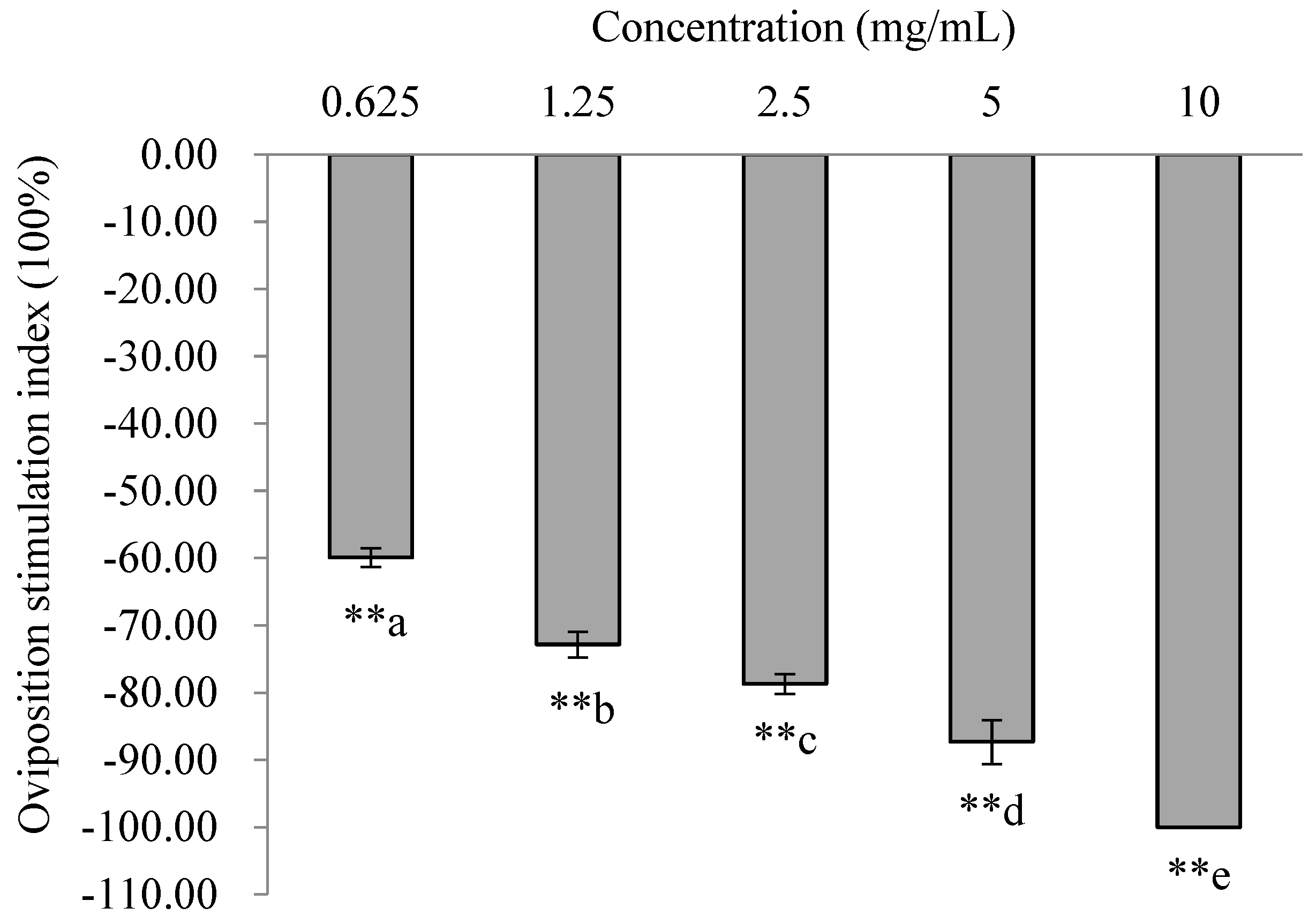
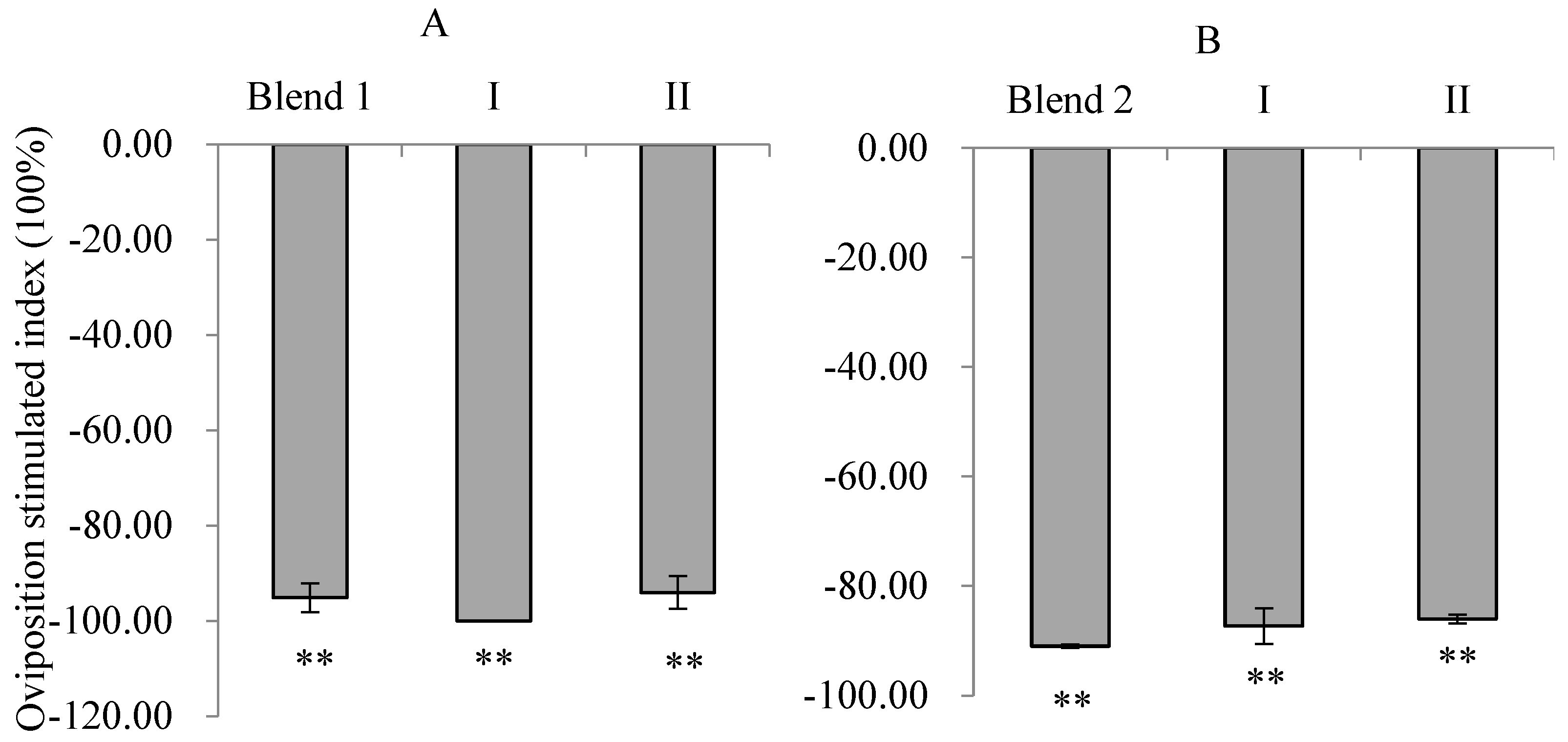
3.7. Oviposition Responses of PTM Females to Mixtures of EAD Active Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekebo, A.; Aryal, S.; Jung, C. Suitability of tomato leaves for larval development of potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Entomol. Res. 2019, 49, 258–264. [Google Scholar] [CrossRef]
- Rondon, S.I. The potato tuberworm: A literature review of its biology, ecology, and control. Am. J. Potato Res. 2010, 87, 149–166. [Google Scholar] [CrossRef]
- Kroschel, J.; Schaub, B. Chapter 6—Biology and ecology of potato tuber moths as major pests of potato. In Insect Pests of Potato; Alyokhin, A., Vincent, C., Giordanengo, P., Eds.; Elsevier Inc.: New York, NY, USA, 2013; pp. 165–192. [Google Scholar]
- Rondon, S.I. Decoding Phthorimaea operculella (Lepidoptera: Gelechiidae) in the new age of change. J. Integr. Agric. 2020, 19, 316–324. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, X.; Gao, Y.L.; Liu, Y.; Dong, W.X.; Xiao, C. Oviposition deterrents in larval frass of Potato tuberworm Moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2019, 48, 496–502. [Google Scholar] [CrossRef]
- Sharaby, A.M.F.; Fallatah, S.B. Protection of stored potatoes from infestation with the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) using plant powders. Bull. Natl. Res. Cent. 2019, 43, 79. [Google Scholar] [CrossRef]
- Gao, Y.L. Potato tuberworm: A threat for China potatoes. Entomol. Ornithol. Herpetol. Curr. Res. 2018, 7, 2. [Google Scholar] [CrossRef]
- Gao, Y.L. Potato tuberworm: Impact and methods for control-MiniReview. CAB Rev. 2018, 13, 1–3. [Google Scholar]
- Meabed, H.A.A.; Rizk, A.M.; Elhefnawy, N.N.; Elhusseini, M.M. Biocontrol agents compared to chemical insecticide for controlling the potato tuber moth, Phthorimaea operculella (Zeller) in the newly reclaimed land in Egypt. Egypt. J. Biol. Pest Control 2011, 21, 97–100. [Google Scholar]
- Das, P.D.; Raina, R.; Prasad, A.R.; Sen, A. Electroantennogram responses of the potato tuber moth, Phthorimaea operculella (Lepidoptera; Gelichiidae) to plant volatiles. J. Biosci. 2007, 32, 339–349. [Google Scholar] [CrossRef]
- Kroschel, J.; Zegarra, O. Attract-and-kill: A new strategy for the management of the potato tuber moths Phthorimaea operculella (Zeller) and Symmetrischema tangolias (Gyen) in potato: Laboratory experiments towards optimizing pheromone and insecticide concentration. Pest Manag. Sci. 2010, 66, 490–496. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.H.; Wang, C.Y.; Xiao, C. Occurrence of parthenogenesis in potato tuber moth. J. Insect Sci. 2018, 18, 14. [Google Scholar] [CrossRef]
- Dethier, V.G. Mechanism of host-plant recognition. Entomol. Exp. Appl. 1982, 31, 49–56. [Google Scholar] [CrossRef]
- Miller, J.R.; Strickler, K.L. Chapter6—Finding and accepting host plants. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Springer: New York, NY, USA, 1984; pp. 127–157. [Google Scholar]
- Conti, E.; Frati, F.; Salerno, G. Oviposition behavior of Lygus rugulipennnis and its preferences for plant wounds. J. Insect Behav. 2012, 25, 339–351. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F. Olfactory recognition and behavioural avoidance of angiosperm non host volatiles by conifer-inhabiting bark beetles. Agric. For. Entomol. 2004, 6, 1–20. [Google Scholar] [CrossRef]
- De Bruyne, M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Sun, X.L.; Xin, Z.J.; Luo, Z.X.; Gao, Y.; Bian, L.; Chen, Z.M. Identification and field evaluation of non-host volatiles disturbing host location by the tea geometrid, Ectropis oblique. J. Chem. Ecol. 2013, 39, 1284–1296. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef]
- Ma, Y.F.; Xiao, C. Push-pull effect of three plant secondary metabolites in oviposition manipulation against Phthorimaea operculella (Zeller). J. Insect Sci. 2013, 13, 128. [Google Scholar] [CrossRef][Green Version]
- Li, Z.W.; Zeng, X.N.; Luo, S.; Wang, M.Q.; Luo, J.B. Deterrent effect of essential oils on oviposition of Conopomorpha sinensis Bradley. Nat. Enemies Insects 2007, 29, 97–102. (In Chinese) [Google Scholar]
- Hidayat, Y.; Heatherand, N.; Hassan, E. Repellency and oviposition deterrence effects of plant essential and vegetable oils against female Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust. J. Entomol. 2013, 52, 379–386. [Google Scholar] [CrossRef]
- Sharaby, A.; Abdel-Rahman, A.H.; Moawad, S. Biological effects of some natural and chemical compounds on the potato tuber moth, Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae). Saudi J. Biol. Sci. 2009, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Han, R.; Dong, W.X.; Xiao, C. Fumigant toxicity and oviposition deterrent activity of volatile constituents from Asari Radix et Rhizoma against Phthorimaea operculella (Lepidoptera: Gelechiidae). J. Insect Sci. 2020, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Luo, Z.X.; Gao, Y.; Bian, L.; Sun, X.L.; Chen, Z.M. Volatiles from non-host aromatic plants repel tea green leafhopper Empoascavitis. Neth. Entomol. Soc. Entomol. Appl. 2014, 153, 156–169. [Google Scholar] [CrossRef]
- Sharaby, A.; Rahman, H.A.; Abdel-Aziz, A.A.; Moawad, S.S. Natural plant oils and terpenes as protector for the potato tubers against Phthorimaea operculella infestation by different application methods. Ecol. Balk. 2014, 6, 45–59. [Google Scholar]
- Hannour, K.; Boughdad, A.; Maataoui, A.; Bouchelta, A. Chemical composition and toxicity of Moroccan Rosmarinus officinalis (Lamiaceae) essential oils against the potato tuber moth, Phthorimaea operculella (Zeller, 1873) Zeller (Lepidoptera, Gelechiidae). J. Mater. Environ. 2017, 8, 758–769. [Google Scholar]
- Martins, A.P.; Salgueiro, L.R.; Cunha, A.P.D.; Vila, R.; Canigueral, S.; Tomi, F.; Casanova, J. Essential oil composition of Eryngiym foetidum from S. Tome e Principe. J. Essent. Oil Res. 2003, 15, 93–95. [Google Scholar]
- Jaramillo, B.; Duarte, E.; Martelo, I. Volatile chemical composition of the essential oil from Colombian Eryngium foetidum L. and determination of its antioxidant activity. Rev. Cuba. Plantas Med. 2011, 16, 140–150. [Google Scholar]
- Paul, S.T.; Emmanuel, E.E.; Samuel, J.N.; Mohammad, I.C. Eryngium foetidum L. essential oils: Chemical composition and antioxidant capacity. Medicines 2017, 4, 24. [Google Scholar] [CrossRef]
- Punareewattana, K.; Borlace, G.N.; Seubsasana, S.; Thongkham, E.; Aiemsaard, J. In vitro antimicrobial examination and efficacy of Eryngium foetidum L. extract for skin ointment in animal infectious dermatitis treatment. Sci. Asia 2022, 49, 248–255. [Google Scholar] [CrossRef]
- Forbes, W.M.; Gallimore, W.A.; Mansingh, A.; Reese, P.B.; Robinson, R.D. Eryngial (trans-2-dodecenal), a bioactive compound from Eryngium foetidum: Its identification, chemical isolation, characterization and comparison with ivermectin in vitro. Parasitology 2014, 141, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, X.X.; Zhang, X.G.; Jia, S.K.; Tang, L.P.; Xiao, C.; Dong, W.X. Behavioral responses of potato tubermoth (Phthorimaea operculella) to volatiles from wilted Yunnan poplar (Populus yunnanensis). Entomol. Exp. Appl. 2024, 172, 280–288. [Google Scholar] [CrossRef]
- Udayagiri, S.; Mason, C.E. Epicuticular wax chemicals in Zea mays influence oviposition in Ostrinia nubilalis. J. Chem. Ecol. 1997, 23, 1675–1687. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef]
- Ma, Y.F.; Zhang, X.M.; Xu, Y.; Xiao, C. Effect of Lindera glauca and its volatiles on the oviposition preferences of the potato tuber moth, Phthorimaea operculella. Chin. J. Appl. Entomol. 2016, 53, 843–850. (In Chinese) [Google Scholar]
- Mahdavi, V.; Rafiee-Dastjerdi, H.; Asadi, A.; Razmjou, J.; Achachlouei, B.F.; Kamita, S.G. Effective management of the Phthorimaea operculella (Zeller) using PVA nanofibers loaded with Cinnamomum zeylanicum essential oil. Am. J. Potato Res. 2017, 94, 647–657. [Google Scholar] [CrossRef]
- Moawad, S.S.; Ebadah, I.M.A. Impact of some natural plant oils on some biological aspects of the potato tuber moth, Phthorimaea opercullela, (Zeller) (Lepidoptera: Gelechiidae). Res. J. Agric. Biol. Sci. 2007, 3, 119–123. [Google Scholar]
- Sharaby, A.M.F.; Gesraha, A.M.; Fallatah, S.A.B. Botanical extracts against the potato tuber moth, Phthorimaea operculella (Zeller1873) (Lepidoptera: Gelechiidae), during storage conditions. Egypt. J. Biol. Pest Control 2020, 30, 93. [Google Scholar] [CrossRef]
- Elsayed, G. Bioassay of Lantana camara and Solanum nigrum extracts on potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Afr. Entomol. 2020, 1, 55–61. [Google Scholar] [CrossRef]
- Acharya, G.C.; Ponnam, N.; Kumari, M.; Roy, T.K.; Shivashankara, K.S.; Sahoo, M.R. Phytochemical profiling of spiny coriander (Eryngium foetidum L.)—A potential perennial spicing-culinary herb of eastern India. Acta Chromatogr. 2021, 34, 197–202. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Nandi, N.C.; Yusuf, M. Chemical constituents of essential oil of the leaves of Erygium foetidum from Bangladesh. Bangladesh J. Sci. Ind. Res. 2007, 42, 347–352. [Google Scholar] [CrossRef]
- Quynh, C.T.T.; Kubota, K. Aroma constituents and enzyme activities of Japanese long coriander leaves (Culantro, Eryngium foetidum L.). Food Sci. Technol. Res. 2012, 18, 287–294. [Google Scholar] [CrossRef]
- Banout, J.; Havlik, J.; Kulik, M.; Kloucek, P.; Lojka, B.; Valterova, I. Effect of solar drying on the composition of essential oil of Sacha Culantro (Eryngium Foetidum L.) grown in the Peruvian amazon. J. Food Process Eng. 2010, 33, 83–103. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wen, G.Z.; Xu, F.L. Repellent effect of 10 plant essential oil compounds and their mixtures against Drosophila melanogaster. Plant Prot. 2022, 48, 242–246. (In Chinese) [Google Scholar]
- Li, X.; Zhang, X.G.; Xiao, C.; Gao, Y.L.; Dong, W.X. Behavioral responses of potato tuber moth (Phthorimaea operculella) to tobacco plant volatiles. J. Integr. Agric. 2020, 19, 325–332. [Google Scholar] [CrossRef]
- Anfora, G.; Vitagliano, S.; Larsson, M.C.; Witzgall, P.; Tasin, M.; Germinara, G.S.; De Cristofaro, A. Disruption of Phthorimaea operculella (Lepidoptera: Gelechiidae) oviposition by the application of host plant volatiles. Pest Manag. Sci. 2014, 70, 628–635. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and application of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef]
- Ma, Y.F.; Xu, Y.; Xiao, C. Oviposition attraction effect of ten host-plant volatiles to potato tuber moth, Phthorimaea operculella. Chin. J. Biol. Control 2012, 28, 448–452. (In Chinese) [Google Scholar]


| Compound | CAS | Purity (%) | Source |
|---|---|---|---|
| Dichloromethane | 75-09-2 | Analytically pure (99.9%) | Tianjin Reagent Co., Ltd., Tianjin, China |
| Anhydrous sodium sulfate | 7757-82-6 | Analytically pure (99.9%) | Tianjin Reagent Co., Ltd., Tianjin, China |
| trans-2-Dodecenal | 20407-84-5 | ≥90.0 | TCI, Tokyo, Japan |
| trans-2-Tridecenal | 7069-41-2 | ≥96.0 | Sigma-Aldrich, Shanghai, China |
| Number | Compound | RT (Min) | Relative Area (%) | GC-EAD Active or Not |
|---|---|---|---|---|
| 1 | Undecanal | 22.87 | 0.21 | |
| 2 | 2,4,5-Trimethylbenzaldehyde | 24.33 | 0.15 | |
| 3 | 2-Undecenal | 24.44 | 0.65 | |
| 4 | trans-4-Nonenal | 25.36 | 0.47 | |
| 5 | Dodecanal | 25.60 | 3.88 | |
| 6 | trans-Caryophyllene | 25.93 | 0.20 | |
| 7 | 2-Dodecenal | 26.72 | 1.96 | |
| 8 | trans-2-Dodecenal | 27.22 | 68.39 | ※ |
| 9 | 3-Dodecenal | 29.62 | 1.31 | |
| 10 | Tetradecanal | 30.63 | 0.50 | |
| 11 | Unknown | 31.77 | 3.87 | |
| 12 | trans-2-Tridecenal | 31.97 | 9.46 | ※ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Yang, X.; Wu, M.; Guo, Y.; Dong, W.; Tang, R.; Xiao, C. Oviposition Deterrents from Extracts of Eryngium foetidum Against Potato Tuber Moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Insects 2025, 16, 158. https://doi.org/10.3390/insects16020158
Ma Y, Yang X, Wu M, Guo Y, Dong W, Tang R, Xiao C. Oviposition Deterrents from Extracts of Eryngium foetidum Against Potato Tuber Moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Insects. 2025; 16(2):158. https://doi.org/10.3390/insects16020158
Chicago/Turabian StyleMa, Yanfen, Xinzhou Yang, Mei Wu, Yunjiao Guo, Wenxia Dong, Rui Tang, and Chun Xiao. 2025. "Oviposition Deterrents from Extracts of Eryngium foetidum Against Potato Tuber Moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae)" Insects 16, no. 2: 158. https://doi.org/10.3390/insects16020158
APA StyleMa, Y., Yang, X., Wu, M., Guo, Y., Dong, W., Tang, R., & Xiao, C. (2025). Oviposition Deterrents from Extracts of Eryngium foetidum Against Potato Tuber Moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Insects, 16(2), 158. https://doi.org/10.3390/insects16020158









