The Mean Individual Biomass (MIB) of Ground Beetles (Carabidae): A Review of Its Application to Ecosystem Succession, Biodiversity, and Climate Change Research
Simple Summary
Abstract
1. Introduction
2. Methodology of Literature Collection
3. Development of the Mean Individual Biomass (MIB) Concept
4. Application of the MIB Index in Different Habitat Types
4.1. Forest Succession and Forest Management
4.2. Agricultural Landscapes and Field Margins
4.3. Post-Industrial and Disturbed Habitats
4.4. Glacier Forelands and Extreme Environments
4.5. Synthesis of MIB Applications Across Habitats
5. Methodological Issues and Limitations
5.1. Length–Mass Relationships
5.2. Seasonal and Phenological Variability
5.3. Sampling Methods
5.4. Minimum Sample Size
5.5. Sensitivity to Community Structure
5.6. Spatial Scale and Habitat Heterogeneity
5.7. Summary
6. MIB in the Context of Global Change and Anthropogenic Pressure
6.1. Climate Change and Species Traits
6.2. Soil Processes and Carbon Sequestration
6.3. Anthropogenic Pressure and Landscape Transformation
6.4. Synthesis
7. Integration of MIB with Other Indicators
7.1. Classical Diversity Indices, Traditional Taxonomic Diversity Metrics
7.2. Functional Traits and Ecological Strategies
7.3. Molecular Methods
7.4. The Index of Natural Value (INV)
7.5. The Importance of an Integrated Approach
8. Future Perspectives and Research Agenda
- (1)
- verification of the applicability of MIB in tropical and non-European landscapes, where comparative data are still lacking;
- (2)
- analysis of relationships between MIB values and soil processes, particularly carbon sequestration, through experimental and long-term studies;
- (3)
- development and standardization of methodologies, including body length–mass equations and the use of new databases and bioinformatic tools;
- (4)
- integration of MIB with molecular analyses and functional indicators to establish more comprehensive frameworks for biodiversity and habitat quality monitoring.
9. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIB | Mean Individual Biomass |
| SOC | Soil Organic Carbon |
| FAI | Forest Affinity Index |
| FS | Flower Strip |
| DNA | Deoxyribonucleic Acid |
| eDNA | Environmental DNA |
References
- Lorenz, W. Systematic List of Extant Ground Beetles of the World (Coleoptera “Geadephaga”: Trachypachidae and Carabidae, incl. Paussinae, Cicindelinae, Rhysodinae), 2nd ed.; W. Lorenz: Tutzing, Germany, 2005; 530p. [Google Scholar]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe—From taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Koivula, M.J. Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef]
- Niemelä, J.; Langor, D.; Spence, J.R. Effects of clear-cut harvesting on boreal ground-beetle assemblages (Coleoptera: Carabidae) in western Canada. Conserv. Biol. 1993, 7, 551–561. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles in sustainable agriculture: A review on pest control efficacy, cultivation impacts and enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Holland, J.M.; Luff, M.L. The effects of agricultural practices on Carabidae in temperate agroecosystems. Integr. Pest Manag. Rev. 2000, 5, 109–129. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L.; Tóthmérész, B. Does urbanization decrease diversity in ground beetle (Carabidae) assemblages? Glob. Ecol. Biogeogr. 2010, 19, 16–26. [Google Scholar] [CrossRef]
- Schwerk, A.; Szyszko, J. Model of succession in degraded areas based on carabid beetles (Coleoptera, Carabidae). ZooKeys 2011, 100, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Skłodowski, J. Anthropogenic transformation of ground beetle assemblages (Coleoptera: Carabidae) in Białowieża Forest, Poland: From primeval to managed stands. Entomol. Fenn. 2006, 17, 296–314. [Google Scholar] [CrossRef]
- Skłodowski, J. Consequences of the transformation of a primeval forest into a managed forest for carabid beetles (Coleoptera: Carabidae)—A case study from Białowieża (Poland). Eur. J. Entomol. 2014, 111, 639–648. [Google Scholar] [CrossRef]
- Niemelä, J.; Koivula, M.; Kotze, D.J. The effects of forestry on carabid beetles (Coleoptera: Carabidae) in boreal forests. J. Insect Conserv. 2007, 11, 5–18. [Google Scholar] [CrossRef]
- Taboada, A.; Johan Kotze, D.; Salgado, J.M. Carabid beetle occurrence at the edges of oak and beech forests in NW Spain. Eur. J. Entomol. 2004, 101, 555–563. [Google Scholar] [CrossRef]
- Haack, R.A.; Acciavatti, R.E.; Davidson, R.L.; Petrice, T.R. Initial responses of Carabidae (Coleoptera) to intensive biomass harvesting and soil compaction in aspen stands of the Huron-Manistee National Forest, Oscoda County, Michigan. Great Lakes Entomol. 2024, 57, 7. [Google Scholar] [CrossRef]
- Makwela, M.M.; Slotow, R.; Munyai, T.C. Ecological response of carabid beetles (Coleoptera: Carabidae) to contrasting agroecosystem management. Community Ecol. 2025, 26, 1–13. [Google Scholar] [CrossRef]
- Middendorf, F.; Eitzinger, B.; Entling, M.; Schirmel, J. Carabid beetles as indicators of stream zonation. Ecol. Indic. 2025, 170, 113036. [Google Scholar] [CrossRef]
- Jelaska, L.S.; Durbesic, P. Comparison of the body size and wing form of carabid species (Coleoptera: Carabidae) between isolated and continuous forest habitats. Ann. Soc. Entomol. Fr. 2009, 45, 327–338. [Google Scholar] [CrossRef]
- Cvetkovska-Gjorgjievska, A.; Hristovski, S.; Prelić, D.; Šerić Jelaska, L.; Slavevska-Stamenković, V.; Ristovska, M. Body size and mean individual biomass variation of ground-beetles community (Coleoptera: Carabidae) as a response to increasing altitude and associated vegetation types in mountainous ecosystem. Biologia 2017, 72, 1059–1066. [Google Scholar] [CrossRef]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar] [CrossRef]
- Honek, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Szyszko, J. Methods of macrofauna investigations. In The Process of Forest Soil Macrofauna Formation after Afforestation of Farmland; Szyszko, J., Ed.; Warsaw Agricultural University Press: Warsaw, Poland, 1983; pp. 10–16. [Google Scholar]
- Szyszko, J.; Vermeulen, H.J.W.; Klimaszewski, K.; Abs, M.; Schwerk, A. Mean Individual Biomass (MIB) of Carabidae as an indicator of the state of the environment. In Natural History and Applied Ecology of Carabid Beetles; Brandmayr, P., Lövei, G., Zetto Brandmayr, T., Casale, A., Vigna Taglianti, A., Eds.; Pensoft Publishers: Sofia, Russia; Bulgaria, Moscow, 2000; pp. 288–294. [Google Scholar]
- Brooks, D.R.; Bater, J.E.; Clark, S.J.; Monteith, D.T.; Andrews, C.; Corbett, S.J.; Beaumont, D.A.; Chapman, J.W. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J. Appl. Ecol. 2012, 49, 1009–1019. [Google Scholar] [CrossRef]
- Pizzolotto, R.; Gobbi, M.; Brandmayr, P. Changes in ground beetle assemblages above and below the treeline of the Dolomites after almost 30 years (1980/2009). Ecol. Evol. 2014, 4, 1284–1294. [Google Scholar] [CrossRef]
- Angst, G.; Potapov, A.; Joly, F.X.; Angst, Š.; Frouz, J.; Ganault, P.; Eisenhauer, N. Conceptualizing soil fauna effects on labile and stabilized soil organic matter. Nat. Commun. 2024, 15, 5005. [Google Scholar] [CrossRef]
- Bonfanti, J.; Potapov, A.; Angst, G.; Ganault, P.; Briones, M.J.; Calderón-Sanou, I.; Chen, T.W.; Conti, E.; Degrune, F.; Eisenhauer, N.; et al. Linking effect traits of soil fauna to processes of organic matter turnover. Funct. Ecol. 2025, 39, 14720. [Google Scholar] [CrossRef]
- Den Boer, P.J. Spreading of risk and stabilization of animal numbers. Acta Biotheor. 1968, 18, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, P.J. On the significance of dispersal power for populations of carabid-beetles (Coleoptera, Carabidae). Oecologia 1970, 4, 1–28. [Google Scholar] [CrossRef]
- Den Boer, P.J. Density limits and survival of local populations in 64 carabid species with different powers of dispersal. J. Evol. Biol. 1990, 3, 19–48. [Google Scholar] [CrossRef]
- Den Boer, P.J. The survival value of dispersal in terrestrial arthropods. Biol. Conserv. 1990, 54, 175–192. [Google Scholar] [CrossRef]
- Den Boer, P.J.; den Boer-Daanje, W. On life history tactics in carabid beetles: Are there only spring- and autumn-breeders? In The Role of Ground Beetles in Ecological and Environmental Studies; Stork, N.E., Ed.; Intercept: Andover, UK, 1990; pp. 247–258. [Google Scholar]
- Booij, C.J.H.; den Nijs, L.; Heijerman, T.; Jorritsma, I.; Lock, C.; Noorlander, J. Size and weight of carabid beetles: Ecological applications. Proc. Sect. Exp. Appl. Entomol. Neth. Entomol. Soc. 1994, 5, 93–98. [Google Scholar]
- Weiss, F.; Linde, A. How to estimate carabid biomass?—An evaluation of size–weight models for ground beetles (Coleoptera: Carabidae) and perspectives for further improvement. J. Insect Conserv. 2022, 26, 481–495. [Google Scholar] [CrossRef]
- Weiss, F. Collection of size–weight equations for carabid beetles. Res. Gate 2023. [Google Scholar] [CrossRef]
- Schreiner, A.; Schwerk, A. Does the Mean Individual Biomass (MIB) of carabids as a bioindicator of forest succession follow a logistic function? Examples from Western German beech and Polish Scots pine forests. Baltic J. Coleopterol. 2012, 12, 57–64. [Google Scholar]
- Nietupski, M.; Kosewska, A.; Ludwiczak, E. Ground beetle assemblages inhabiting various age classes of Norway spruce stands in north-eastern Poland. PeerJ 2023, 11, e16502. [Google Scholar] [CrossRef]
- Schwerk, A. Influence of mowing measures on carabid beetle fauna (Coleoptera: Carabidae) in a post-agricultural area. Period. Biol. 2016, 118, 163–169. [Google Scholar] [CrossRef]
- Jelaska, L.Š.; Dumbović, V.; Kučinić, M. Carabid beetle diversity and mean individual biomass in beech forests of various ages. ZooKeys 2011, 100, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyk, M.; Błońska, E.; Wojas, T. Deadwood, soil and carabid beetle-based interaction networks—An initial case study from montane coniferous forests in Poland. Forests 2021, 12, 382. [Google Scholar] [CrossRef]
- Błaszkiewicz, M.; Schwerk, A. Carabid beetle (Coleoptera: Carabidae) diversity in agricultural and post-agricultural areas in relation to the surrounding habitats. Baltic J. Coleopterol. 2013, 13, 15–26. [Google Scholar]
- Döring, T.F.; Kromp, B. Which carabid beetles benefit from organic agriculture? A review of comparative studies in winter cereals from Germany and Switzerland. Agric. Ecosyst. Environ. 2003, 98, 153–161. [Google Scholar] [CrossRef]
- Pfiffner, L.; Luka, H. Overwintering of arthropods in soils of arable fields and adjacent semi-natural habitats. Agric. Ecosyst. Environ. 2000, 78, 215–222. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Kędzior, R.; Szwalec, A.; Mundala, P. Mean Individual Biomass (MIB) of ground beetles (Coleoptera, Carabidae) as indicator of succession processes in postindustrial areas. Acta Sci. Pol. Form. Circumiectus 2018, 17, 23. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Hidalgo, J.M. Application of the mean individual biomass (MIB) of ground beetles (Coleoptera, Carabidae) to assess the recovery process of the Guadiamar Green Corridor (southern Iberian Peninsula). Biodivers. Conserv. 2007, 16, 4131–4146. [Google Scholar] [CrossRef]
- Tropek, R.; Kadlec, T.; Hejda, M.; Spitzer, L.; Kocarek, P.; Malenovsky, I.; Banar, P.; Tuf, I.H.; Hejda, M.; Konvicka, M. Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J. Appl. Ecol. 2010, 47, 139–147. [Google Scholar] [CrossRef]
- Skłodowski, J.; Garbalińska, P. Ground beetle assemblages (Coleoptera: Carabidae) in the third year of regeneration after a hurricane in the Puszcza Piska pine forests. Baltic J. Coleopterol. 2007, 7, 17–36. [Google Scholar]
- Gobbi, M. Application of the mean individual biomass of ground beetles (Coleoptera: Carabidae) to assess the assemblage successions along areas of recent glacier retreats. Eur. J. Entomol. 2014, 111, 537–541. [Google Scholar] [CrossRef]
- Hodkinson, I.D.; Webb, N.R.; Coulson, S.J.; Block, W. Invertebrate community structure along environmental gradients in glacier foreland ecosystems. Ecol. Entomol. 2004, 29, 566–573. [Google Scholar] [CrossRef]
- Kaufmann, R. Invertebrate succession on an Alpine glacier foreland. Ecology 2001, 82, 2261–2278. [Google Scholar] [CrossRef]
- Thiele, H.U. Carabid Beetles in Their Environments; Springer: Berlin, Germany, 1977. [Google Scholar]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Adis, J. Problems of interpreting arthropod sampling with pitfall traps. Zool. Anz. 1979, 202, 177–184. [Google Scholar]
- Spence, J.R.; Niemelä, J.K. Sampling carabid assemblages with pitfall traps: The madness and the method. Can. Entomol. 1994, 126, 881–894. [Google Scholar] [CrossRef]
- Litavský, J.; Prokop, P. Coverings on pitfall traps influence the abundance of ground-dwelling arthropods. Diversity 2024, 16, 19. [Google Scholar] [CrossRef]
- Nakládal, O.; Havránková, E.; Zumr, V. Trapping liquids may bias the results of beetle diversity assessment. PeerJ 2023, 11, e16531. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, A.; Murray, T.; Robertson, B.C.; Monks, J.M. Pitfall trapping outperforms other methods for surveying ground-dwelling large-bodied alpine invertebrates. J. Insect Conserv. 2023, 27, 679–692. [Google Scholar] [CrossRef]
- Schwerk, A.; Szyszko, J. Increase of Mean Individual Biomass (MIB) of Carabidae in relation to succession in forest habitats. Wiad. Entomol. 2007, 26, 195–206. [Google Scholar]
- Magura, T.; Tóthmérész, B.; Molnár, T. Changes in carabid beetle assemblages along an urbanisation gradient in the city of Debrecen, Hungary. Landsc. Ecol. 2004, 19, 747–759. [Google Scholar] [CrossRef]
- Weiss, F.; von Wehrden, H.; Linde, A. Long-term drought triggers severe declines in carabid beetles in a temperate forest. Ecography 2024, 47, e07020. [Google Scholar] [CrossRef]
- Weiss, F.; Winter, S.; Pflugmacher, D.; Kolling, T.; Linde, A. Evidence for regional-scale declines in carabid beetles in old lowland beech forests following a period of severe drought. Landsc. Ecol. 2024, 39, 123. [Google Scholar] [CrossRef]
- Harris, J.E.; Rodenhouse, N.L.; Holmes, R.T. Decline in beetle abundance and diversity in an intact temperate forest linked to climate warming. Biol. Conserv. 2019, 240, 108219. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K.; Kondras, M.; Dymitryszyn, I.; Matracka, A.; Cimoch, M.; Żyfka-Zagrodzińska, E. Influence of soil macrofauna on soil organic carbon content. Environ. Prot. Nat. Resour. 2018, 29, 20–25. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K.; Dymitryszyn, I.; Jankiewicz, U.; Kondras, M.; Żyfka-Zagrodzińska, E.; Schwerk, A. Assemblage characteristics of butterflies and carabid beetles as a function of soil characteristics and plant diversity in differently managed fields, forests and ecotones: A case study in Tuczno Forest District, Poland. Land 2022, 11, 25. [Google Scholar] [CrossRef]
- Szyszko, J.; Schwerk, A.; Malczyk, J. Animals as an indicator of carbon sequestration and valuable landscapes. ZooKeys 2011, 100, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N. Soil organic carbon storage as a key function of soils: A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Don, A.; Seidel, F.; Leifeld, J.; Kätterer, T.; Martin, M.; Pellerin, S.; Emde, D.; Seitz, D.; Chenu, C. Carbon sequestration in soils and climate change mitigation—Definitions and pitfalls. Glob. Change Biol. 2024, 30, e16983. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, J. Carabid beetles (Coleoptera: Carabidae) and habitat fragmentation: A review. Eur. J. Entomol. 2001, 98, 127–132. [Google Scholar] [CrossRef]
- Eversham, B.C.; Roy, D.B.; Telfer, M.G. Urban, industrial and other man-made sites as analogues of natural habitats for Carabidae. Ann. Zool. Fenn. 1996, 33, 149–156. [Google Scholar]
- Kotze, D.J.; O’Hara, R.B. Species decline—But why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 2003, 135, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; Choi, M.B. Effects of urbanization on carabid beetles in an urban riparian area. Int. J. Ecol. Environ. Sci. 2022, 52, 281–294. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L. Consequences of urban living: Urbanization and ground beetles. Curr. Landsc. Ecol. Rep. 2021, 6, 9–21. [Google Scholar] [CrossRef]
- Zimmermann, E.E.; Chittaro, Y.; Wider, S.; Zemp, D.C. Composition and diversity of ground beetles within wooded pastures and alternative land-use systems in Swiss Jura mountains. Agrofor. Syst. 2024, 98, 2411–2430. [Google Scholar] [CrossRef]
- Riley, K.; Browne, R. Changes in ground beetle diversity and community in age structured forests (Coleoptera: Carabidae). ZooKeys 2011, 147, 601–621. [Google Scholar] [CrossRef]
- Allegro, G.; Sciaky, R. Assessing the potential role of ground beetles (Coleoptera: Carabidae) as bioindicators in poplar stands with a newly proposed ecological index (FAI). For. Ecol. Manag. 2003, 175, 275–284. [Google Scholar] [CrossRef]
- Porhajašová, J.; Babošová, M. Impact of arable farming management on the biodiversity of Carabidae (Coleoptera). Saudi J. Biol. Sci. 2022, 29, 103371. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A.; Sienkiewicz, P.; Matysiak, K.; Sienkiewicz, P. Botanical evaluation of the two-year-old flower strip with analysis of the local Carabidae population: Case study. Sustainability 2025, 17, 3223. [Google Scholar] [CrossRef]
- Bouraga, I.; Guezoul, O.; Abdelwahab, C. Diversity of Carabidae (Coleoptera) in Ouargla palm groves (Algerian Sahara). Zool. Ecol. 2025, 35, 1–12. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K.; Ukalska, J.; Ukalski, K.; Kondras, M. Impact of anthropogenic landscape transformation on soil fertility and diversity of Carabid beetles and butterflies in Poland. Glob. Ecol. Conserv. 2025, 53, e03693. [Google Scholar] [CrossRef]
- Philpott, S.M.; Albuquerque, S.; Bichier, P.; Cohen, H.; Egerer, M.H.; Kirk, C.; Will, K.W. Local and landscape drivers of carabid activity, species richness, and traits in urban gardens in coastal California. Insects 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Maveety, S.A.; Browne, R.A. Patterns of morphology in carabid beetles (Coleoptera: Carabidae) along a Neotropical altitudinal gradient. Int. J. Trop. Insect Sci. 2014, 34, 157–171. [Google Scholar] [CrossRef]
- Hahs, A.K.; Fournier, B.; Aronson, M.F.J.; Nilon, C.H.; Herrera-Montes, A.; Salisbury, A.B.; Threlfall, C.G.; Rega-Brodsky, C.C.; Lepczyk, C.A.; La Sorte, F.A.; et al. Urbanisation generates multiple trait syndromes for terrestrial animal taxa worldwide. Nat. Commun. 2023, 14, 4751. [Google Scholar] [CrossRef]
- Charalabidis, A.; Dechaume-Moncharmont, F.-X.; Carbonne, B.; Bohan, D.A.; Petit, S. Diversity of foraging strategies and responses to predator interference in seed-eating carabid beetles. Basic Appl. Ecol. 2019, 36, 13–24. [Google Scholar] [CrossRef]
- Gobbi, M.; Fontaneto, D. Biodiversity of ground beetles (Coleoptera: Carabidae) in different habitats of the Italian Po lowland. Agric. Ecosyst. Environ. 2008, 127, 273–276. [Google Scholar] [CrossRef]
- Ji, Y.; Ashton, L.; Pedley, S.M.; Edwards, D.P.; Tang, Y.; Nakamura, A.; Kitching, R.; Dolman, P.M.; Woodcock, P.; Edwards, F.A.; et al. Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol. Lett. 2013, 16, 1245–1257. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Cantera, I.; Giachello, S.; Münkemüller, T.; Caccianiga, M.; Gobbi, M.; Losapio, G.; Marta, S.; Valle, B.; Zawierucha, K.; Thuiller, W.; et al. Describing functional diversity of communities from environmental DNA. Trends Ecol. Evol. 2025, 40, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Pizzolotto, R. Soil arthropods for faunal indices in assessing changes in natural value resulting from human disturbances. In Biodiversity, Temperate Ecosystems and Global Change; Boyle, T., Boyle, C.E.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 291–313. [Google Scholar]
- Brandmayr, P.; Brandmayr, T.Z.; Pizzolotto, R. I Coleotteri Carabidi per la valutazione ambientale e la conservazione delle biodiversità. In Manuale Operativo, Agenzia per la Protezione dell’Ambiente e per i Servizi Tecnici; IGER: Rome, Italy, 2005; Volume 34. [Google Scholar]
- Peretti, E.; Armanini, M.; Chirichella, R.; Mustoni, A.; Gobbi, M. Assessing the Conservation Priority of Alpine Carabid Beetle Communities by Mapping the Index of Natural Value (INV) in Natura 2000 Habitats in the Brenta Dolomites (Italian Alps). Insects 2025, 16, 602. [Google Scholar] [CrossRef] [PubMed]
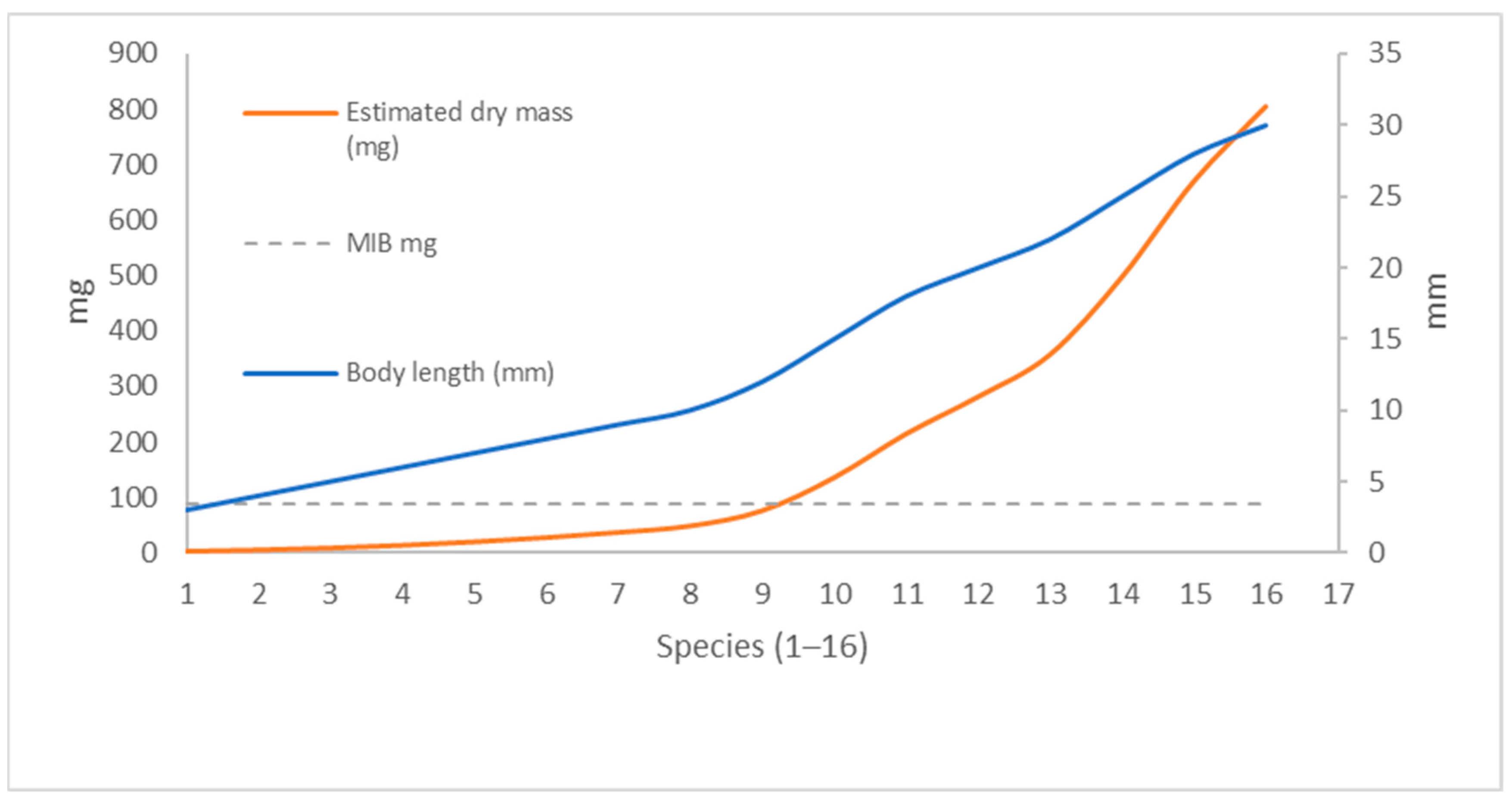
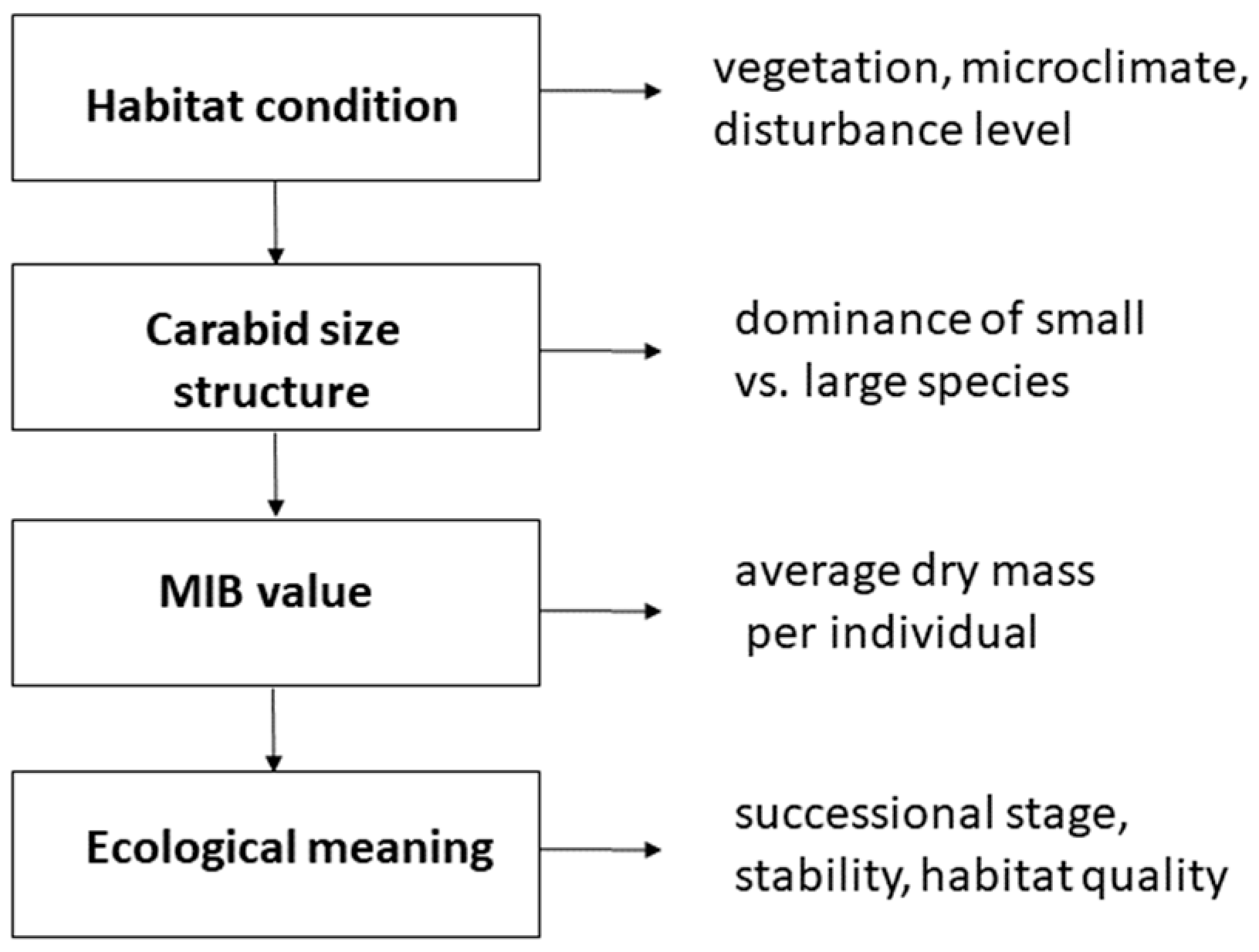
| Habitat Type | Region/Scale | Trend in MIB Values | Ecological Interpretation/Notes |
|---|---|---|---|
| Forests—secondary succession | Central and Southern Europe | ↑ with stand age | Reflects transition from small pioneer species to large, late-successional taxa; no data available from tropical regions |
| Agricultural landscapes/field margins | Central Europe | Fields: ↓; refuges: ↑ | Sensitive to land-use intensity and presence of semi-natural refuges; relatively few studies available |
| Post-industrial habitats | Poland, Spain | ↑ during regeneration | Accurately reflects habitat recovery and reclamation processes; number of studies still limited |
| Glacier forelands and extreme environments | European Alps | ↑ with stabilization | Indicator of primary succession and climate-related changes; most data originate from Alpine and other European glacial forelands |
| Urbanization/fragmentation | Europe, global | Generally: ↓ | Selection for small, mobile generalist species and assemblage homogenization; requires studies outside Europe |
| Element/Issue | Why it Matters | What to Report/Minimum Standard |
|---|---|---|
| Length–mass equations | The choice of regression model affects biomass estimation and comparability among studies. | Provide the exact equation(s) used, length range, and source; if mixed models are applied, specify assignment rules; indicate the software or tool used (e.g., R script). |
| Barber traps—design and preservative liquid | Pitfall traps measure activity density rather than absolute abundance; trap design and preservative type may selectively influence species composition. | Report trap diameter and depth, cover type, preservative used, exposure duration, and inspection frequency; note any corrections applied. |
| Sampling effort and season | Species phenology causes seasonal variation in MIB values. | Full-season or multi-year sampling recommended; report sampling schedule and total effort (trap-days). |
| Minimum sample size | Small samples produce unstable and difficult-to-interpret MIB values. | Report number of individuals (N) and species; ≥50 individuals per comparison unit recommended, or aggregate data across time/space. |
| Spatial/temporal unit | Averaging may obscure habitat mosaic patterns and local differences. | Provide exact plot size, trap spacing and number, number of replicates, and their spatial arrangement. |
| MIB calculation method | Reproducibility requires clear description of the computation process. | Specify whether biomass was measured directly or estimated via length–mass regression; indicate aggregation method (weighted or unweighted mean) and units used. |
| Habitat context | Interpreting MIB without environmental data may be misleading. | Include vegetation structure, dead wood volume, and soil parameters (e.g., SOC, pH), when available. |
| Interpretation caution | Dominance of large-bodied species (e.g., Carabus spp.) may artificially increase MIB. | Report dominant species and their relative abundance; consider complementary indicators (e.g., functional traits or diversity indices). |
| Indicator/Approach | Advantages/Strengths | Limitations | Data Requirements and Costs | Common Interpretative Pitfalls |
|---|---|---|---|---|
| MIB (Mean Individual Biomass) | Simple to calculate; synthetically reflects successional changes; intuitive to interpret. | Sensitive to small sample sizes; lack of standardized length–mass equations; method-dependent (pitfall traps). | Body length data and L–M equations; sufficient sample size (N > 50). | Overinterpretation as a universal measure of “ecosystem maturity”; comparing results without specifying the applied L–M equation. |
| Diversity indices (Shannon, Simpson, Jaccard) | Accurately capture species richness and evenness; widely used and comparable across studies. | Do not account for functional traits; limited interpretation of ecological processes. | Full species-level identification across assemblages. | Reducing ecological complexity to a single number; neglecting functional dominance patterns. |
| Functional traits (body size, wings, trophic type) | Reflect underlying ecological mechanisms; high interpretative value for ecosystem functioning. | Time-consuming trait assessment; challenges in standardizing trait categories. | Morphological and ecological trait data for multiple species. | Ignoring intraspecific variability; overgeneralization of trait categories. |
| Trophic strategies | Explain the role of Carabidae in nutrient cycling and biological control. | Require feeding experiments; difficult to upscale across habitats. | Dietary data, feeding trials, or isotopic analyses. | Treating species as trophically uniform without accounting for variability. |
| Molecular methods (DNA metabarcoding, eDNA) | Rapid and precise species identification; allows environmental sample analysis. | Lack functional information; dependent on the quality of reference databases. | Laboratory analyses, sequencing, and bioinformatics expertise. | Confusing DNA presence with actual occurrence or abundance of individuals in the assemblage. |
| INV (Index of Natural Value) | Integrates taxonomic richness with functional traits (e.g., endemism, brachyptery, trophic specialization); enables assessment of habitat naturalness and conservation value. | Requires expert-based trait evaluation; functional weighting partly subjective; still limited applications outside specific regions (mainly Italy). | Comprehensive species lists with ecological trait data; expert ecological scoring. | Assuming cross-regional comparability without calibration; interpreting INV as a direct biodiversity measure instead of naturalness. |
| Indicator/Approach | Integration Potential with MIB |
|---|---|
| Diversity indices | Combining MIB with diversity metrics allows linking species richness with the dynamics of functional traits. |
| Functional traits | Integration enables the association of mean body size with other traits such as dispersal ability and wing morphology. |
| Trophic strategies | MIB can be combined with trophic group analyses to assess the functional stability of communities. |
| Molecular methods | Provide rapid identification of species composition, while integration with MIB adds a functional interpretation. |
| Index of Natural Value (INV) | The combined use of MIB and INV may provide a complementary bioindication framework, linking biomass-based and trait-based perspectives on habitat integrity. |
| Soil and climatic parameters | Integration with environmental data (SOC, moisture, temperature) allows MIB to be used as an indicator of ecosystem services. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszko-Podgórska, K. The Mean Individual Biomass (MIB) of Ground Beetles (Carabidae): A Review of Its Application to Ecosystem Succession, Biodiversity, and Climate Change Research. Insects 2025, 16, 1191. https://doi.org/10.3390/insects16121191
Szyszko-Podgórska K. The Mean Individual Biomass (MIB) of Ground Beetles (Carabidae): A Review of Its Application to Ecosystem Succession, Biodiversity, and Climate Change Research. Insects. 2025; 16(12):1191. https://doi.org/10.3390/insects16121191
Chicago/Turabian StyleSzyszko-Podgórska, Katarzyna. 2025. "The Mean Individual Biomass (MIB) of Ground Beetles (Carabidae): A Review of Its Application to Ecosystem Succession, Biodiversity, and Climate Change Research" Insects 16, no. 12: 1191. https://doi.org/10.3390/insects16121191
APA StyleSzyszko-Podgórska, K. (2025). The Mean Individual Biomass (MIB) of Ground Beetles (Carabidae): A Review of Its Application to Ecosystem Succession, Biodiversity, and Climate Change Research. Insects, 16(12), 1191. https://doi.org/10.3390/insects16121191







