Abiotic Factors Affecting Vector-Borne Plant Pathogen Complexes: Elevated CO2 and the Barley Yellow Dwarf Pathosystem
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Insect Material
2.2. Experimental Design and Environmental Conditions
2.3. Transpiration Measurements
2.4. Plant Biomass and Water-Soluble Carbohydrates Analysis
2.5. Virus Detection and Quantification
2.6. Statistical Analysis
3. Results
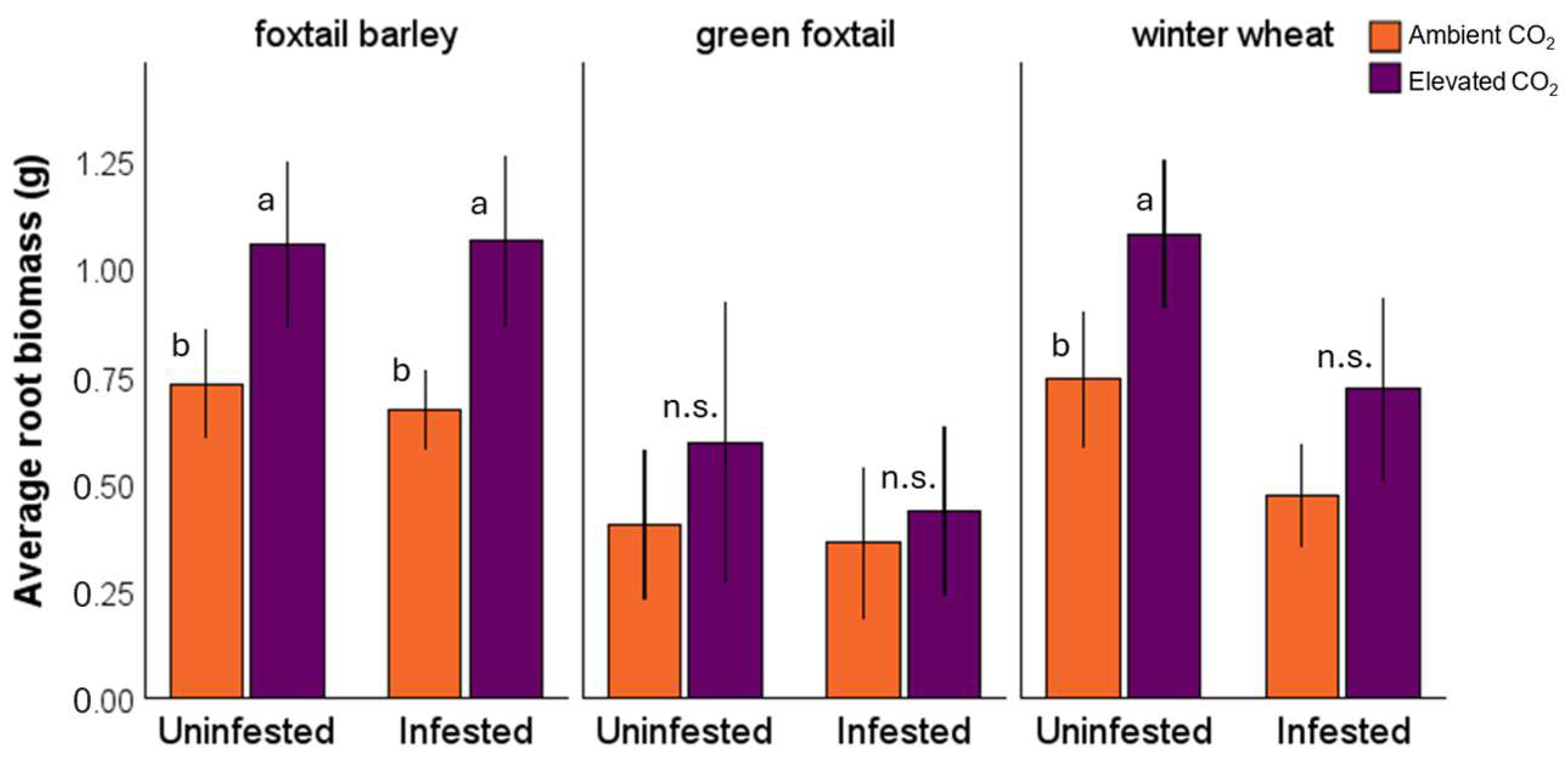
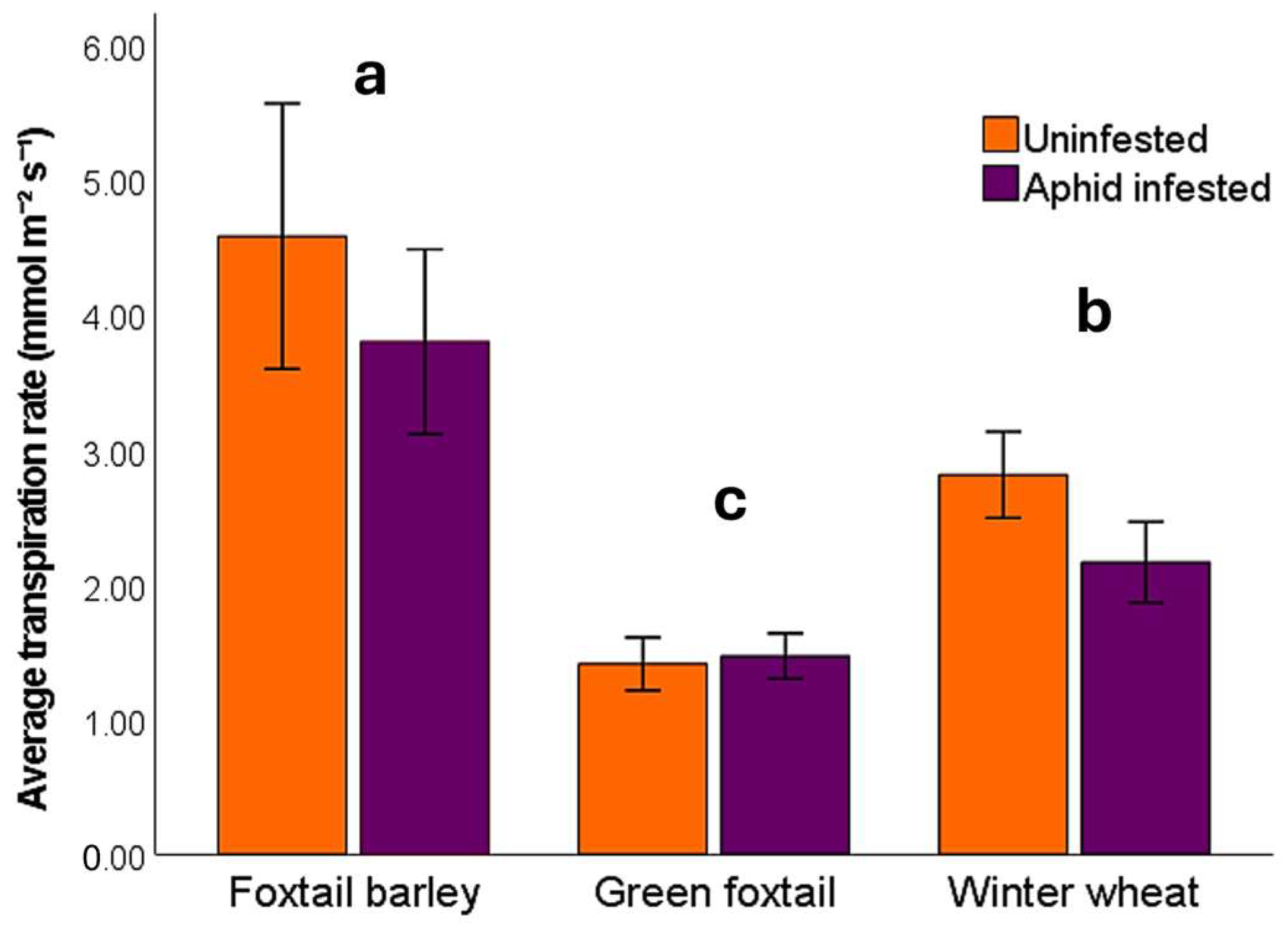
3.1. Plant Response
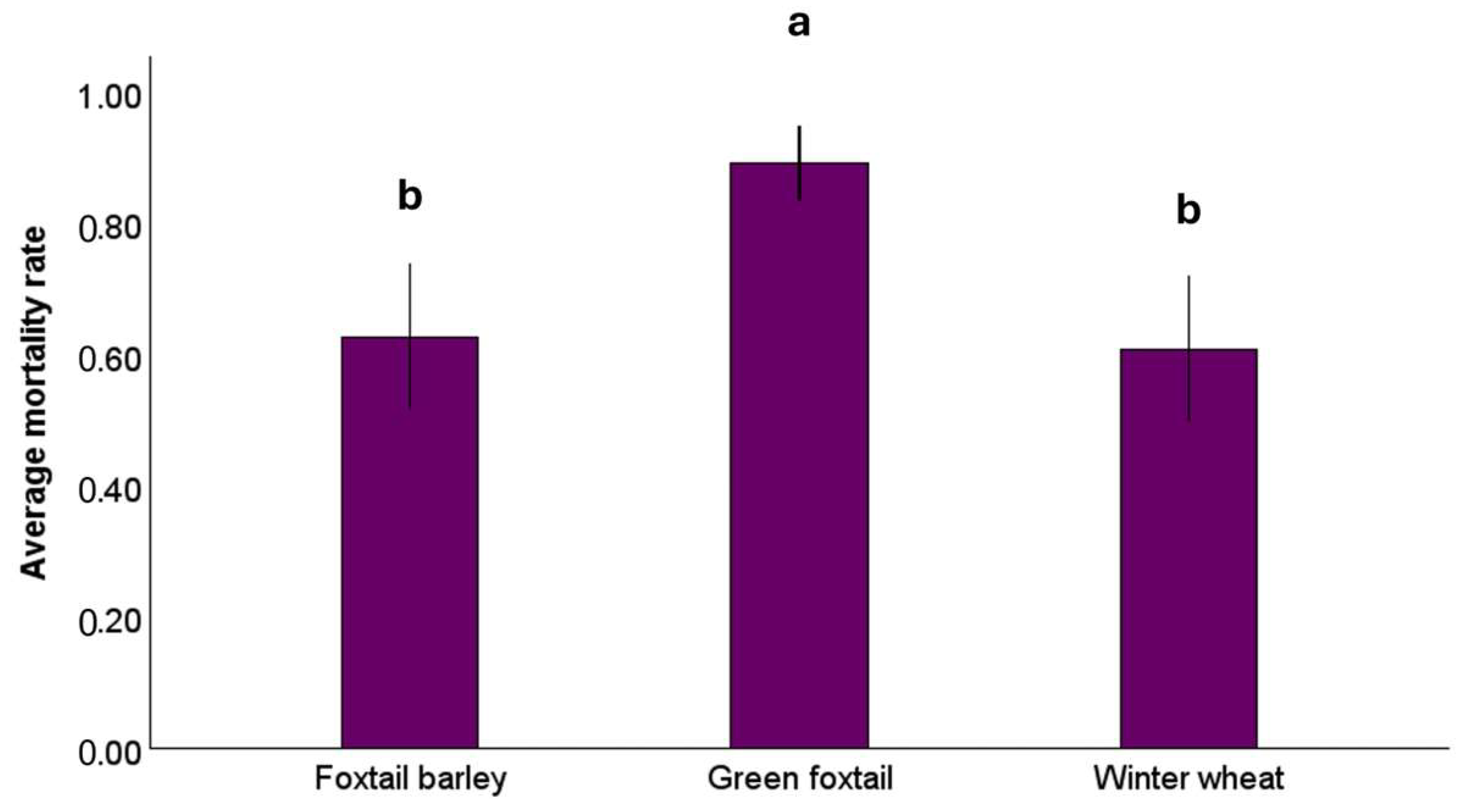
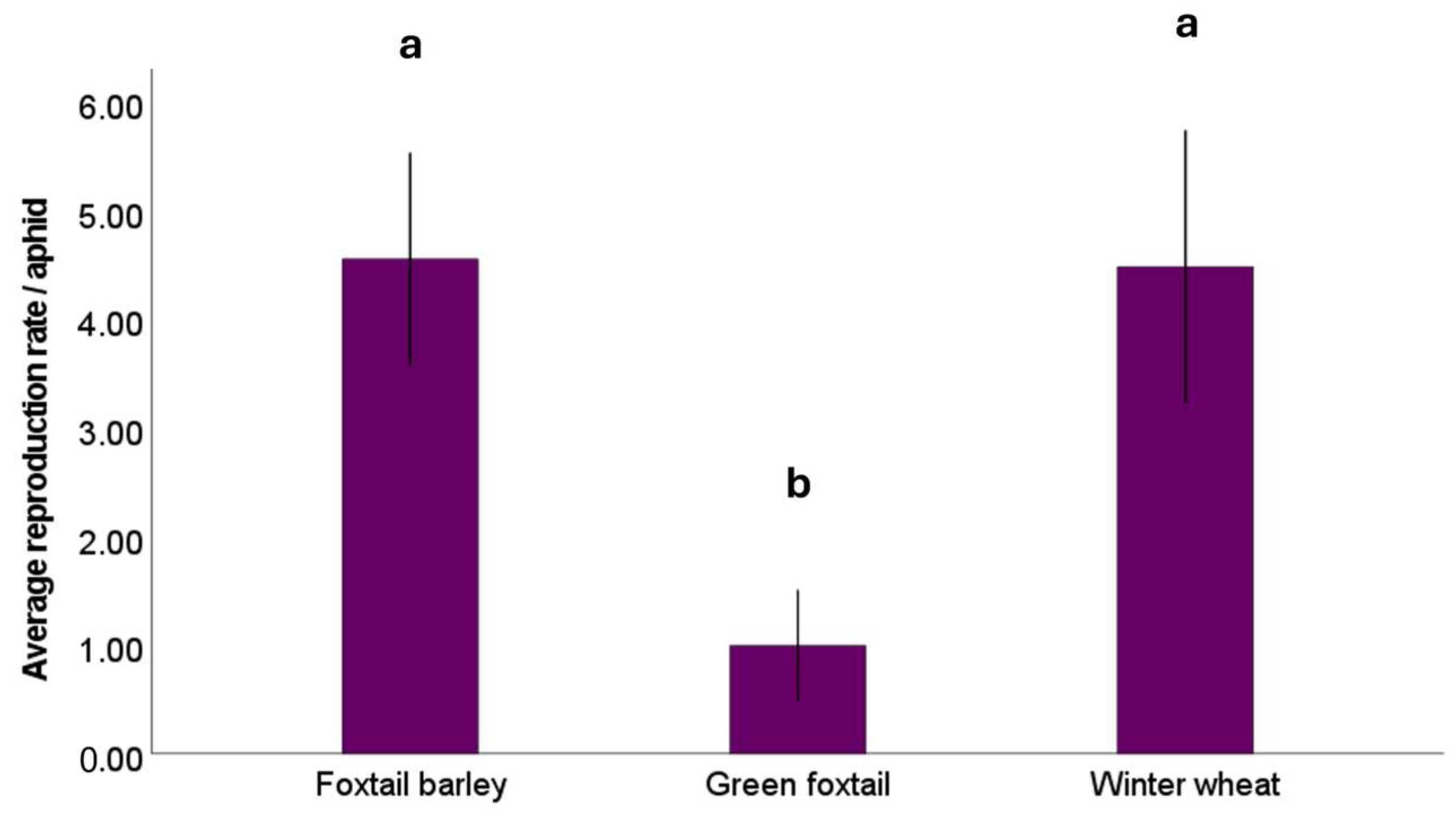
3.2. Aphid Response
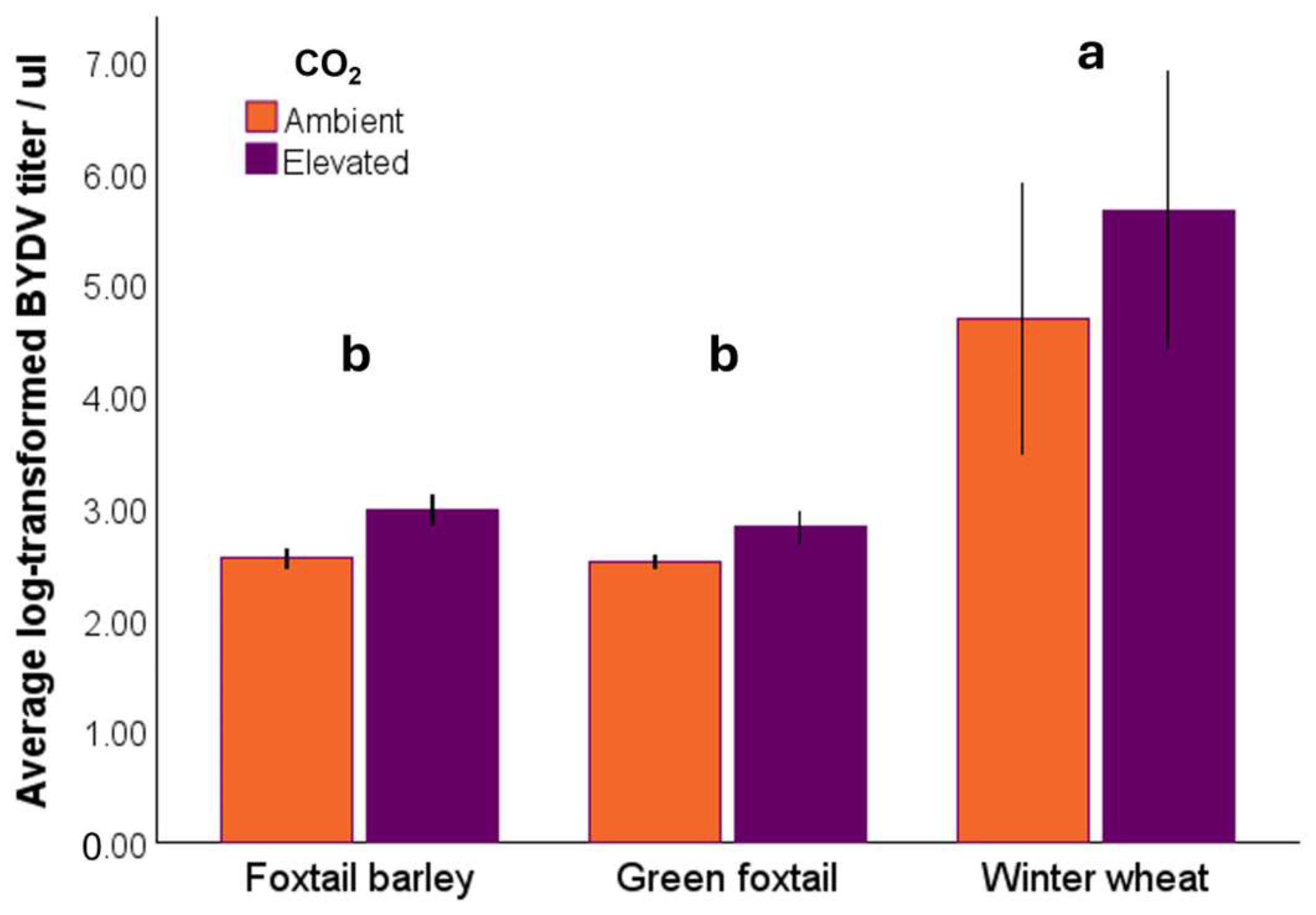
3.3. Virus Response
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: 2020 Release. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=2169986&lvl=3&lin=f&keep=1&srchmode=1&unlock (accessed on 15 November 2025).
- Rashidi, M.; Cruzado, R.K.; Hutchinson, P.J.S.; Bosque-Pérez, N.A.; Marshall, J.M.; Rashed, A. Grassy weeds and corn as potential sources of barley yellow dwarf virus spread into winter wheat. Plant Dis. 2021, 105, 444–449. [Google Scholar] [CrossRef]
- Irwin, M.E.; Thresh, J.M. Epidemiology of barley yellow dwarf: A study in ecological complexity. Annu. Rev. Phytopathol. 1990, 28, 393–424. [Google Scholar] [CrossRef]
- Power, A.G.; Gray, S.M. Aphid transmission of barley yellow dwarf viruses: Interactions between viruses, vectors, and host plants. In Barley Yellow Dwarf—40 Years of Progress; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 259–289. [Google Scholar]
- Alquicer, G.; Ibrahim, E.; Maruthi, M.N.; Kundu, J.K. Identifying putative resistance genes for barley yellow dwarf virus-PAV in wheat and barley. Viruses 2023, 15, 716. [Google Scholar] [CrossRef] [PubMed]
- Mastari, J.; Lapierre, H.; Dessens, J.T. Asymmetrical distribution of barley yellow dwarf virus PAV variants between host plant species. Phytopathology 1998, 88, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Gildow, F.E. Evidence for receptor-mediated endocytosis regulating Luteovirus acquisition by aphids. Phytopathology 1993, 83, 270–277. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.W.; Langham, M.A.C.; Hesler, L.S. Root and shoot responses to bird cherry-oat aphids and barley yellow dwarf virus in spring wheat. Crop Sci. 2003, 43, 1380–1386. [Google Scholar] [CrossRef]
- Bockus, W.W.; De Wolf, E.D.; Todd, T.C. Management strategies for barley yellow dwarf on winter wheat in Kansas. Plant Health Prog. 2016, 17, 122–127. [Google Scholar] [CrossRef]
- Marshall, J.M.; Rashed, A. Barley Yellow Dwarf Virus in Idaho Cereal Crops; University of Idaho Current Information Series, 1210; University of Idaho Extension: Moscow, ID, USA, 2014; Available online: https://objects.lib.uidaho.edu/uiext/uiext33170.pdf (accessed on 15 November 2025).
- Enders, L.S.; Hefley, T.J.; Girvin, J.J.; Whitworth, R.J.; Smith, C.M. Spatiotemporal distribution and environmental drivers of barley yellow dwarf virus and vector abundance in Kansas. Phytopathology 2018, 108, 1196–1205. [Google Scholar] [CrossRef]
- Dedryver, C.A.; Harrington, R. Epidemiology and forecasting of small grain viruses of the Luteoviridae family. In Viruses and Virus Diseases of Poaceae (Gramineae); Lapierre, H., Signoret, P., Eds.; INRA: Versailles, France, 2004; pp. 155–170. [Google Scholar]
- Parizad, S.; Rashed, A. Exploring the role of tall fescue-endophyte association in shaping Rhopalosiphum Padi host choice and reproduction and barley yellow dwarf virus transmission. Entomol. Gen. 2025, 45, 473–480. [Google Scholar] [CrossRef]
- D’Arcy, C.J. Symptomatology and host range of barley yellow dwarf. In Barley Yellow Dwarf—40 Years of Progress; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 9–28. [Google Scholar]
- Hadi, B.A.R.; Flanders, K.L.; Bowen, K.L.; Murphy, J.F.; Blount, A.R. Survey of barley yellow dwarf virus and cereal yellow dwarf virus on three perennial pasture grasses in Florida. J. Entomol. Sci. 2012, 47, 35–43. [Google Scholar] [CrossRef]
- Bergès, S.E.; Vile, D.; Vazquez-Rovere, C.; Blanc, S.; Yvon, M.; Bédiée, A.; Rolland, G.; Dauzat, M.; van Munster, M. Interactions between drought and plant genotype change epidemiological traits of cauliflower mosaic virus. Front. Plant Sci. 2018, 9, 703. [Google Scholar] [CrossRef]
- Susi, H.; Laine, A.-L. Agricultural land use disrupts biodiversity mediation of virus infections in wild plant populations. New Phytol. 2021, 230, 2447–2458. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Barzman, M.; Booij, K.; Boonekamp, P.; Desneux, N.; Huber, L.; Kudsk, P.; Langrell, S.R.H.; Ratnadass, A.; Ricci, P.; et al. Robust cropping systems to tackle pests under climate change. A Review. Agron. Sustain. Dev. 2015, 35, 443–459. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Viñuela, E.; Fereres, A.; Medina, P.; Trębicki, P. Combined effects of elevated CO2 and temperature on multitrophic interactions involving a parasitoid of plant virus vectors. BioControl 2021, 66, 307–319. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Hughes, L.; Bazzaz, F.A. Effects of elevated CO2 on five plant-aphid interactions. Entomol. Exp. Appl. 2001, 99, 87–96. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of Free-Air CO2 Enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Sun, Y.C.; Chen, F.J.; Ge, F. Elevated CO2 changes interspecific competition among three species of wheat aphids: Sitobion avenae, Rhopalosiphum padi, and Schizaphis graminum. Environ. Entomol. 2009, 38, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Klaiber, J.; Najar-Rodriguez, A.J.; Piskorski, R.; Dorn, S. Plant acclimation to elevated CO2 affects important plant functional traits, and concomitantly reduces plant colonization rates by an herbivorous insect. Planta 2013, 237, 29–42. [Google Scholar] [CrossRef]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Oehme, V.; Högy, P.; Zebitz, C.P.W.; Fangmeier, A. Effects of elevated atmospheric CO2 concentrations on phloem sap composition of spring crops and aphid performance. J. Plant Interact. 2013, 8, 74–84. [Google Scholar] [CrossRef]
- Ryan, G.D.; Emiljanowicz, L.; Härri, S.A.; Newman, J.A. Aphid and host-plant genotype × genotype interactions under elevated CO2. Ecol. Entomol. 2014, 39, 309–315. [Google Scholar] [CrossRef]
- Ryan, G.D.; Newman, J.A.; Xue, H.; Parsons, A.J.; Newman, J.A. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant Cell Environ. 2014, 37, 204–212. [Google Scholar] [CrossRef]
- Malmström, C.M.; Field, C.B. Virus-induced differences in the response of oat plants to elevated carbon dioxide. Plant Cell Environ. 1997, 20, 178–188. [Google Scholar] [CrossRef]
- Dáder, B.; Fereres, A.; Moreno, A.; Trębicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 2016, 6, 19120. [Google Scholar] [CrossRef]
- Mattson, W. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Evol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Jones, T.H.; Knight, K.J. Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and Its parasitoid Aphidius matricariae. Oecologia 1998, 116, 128–135. [Google Scholar] [CrossRef]
- Coviella, C.E.; Trumble, J.T. Review: Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conserv. Biol. 1999, 13, 700–712. [Google Scholar] [CrossRef]
- Newman, J.A.; Gibson, D.J.; Parsons, A.J.; Thornley, J.H.M. How predictable are aphid population responses to elevated CO2? J. Anim. Ecol. 2003, 72, 556–566. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Feng, L.; Gao, F.; Ge, F. Effects of elevated CO2 and plant genotype on interactions among cotton, aphids and parasitoids. Insect Sci. 2011, 18, 451–461. [Google Scholar] [CrossRef]
- Johnson, S.N.; Ryalls, J.M.W.; Karley, A.J. Global climate change and crop resistance to aphids: Contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 2014, 165, 62–72. [Google Scholar] [CrossRef]
- Trębicki, P.; Nancarrow, N.; Cole, E.; Bosque-Pérez, N.A.; Constable, F.E.; Freeman, A.J.; Rodoni, B.; Yen, A.L.; Luck, J.E.; Fitzgerald, G.J. Virus disease in wheat predicted to increase with a changing climate. Glob. Change Biol. 2015, 21, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Balaji, B.; Bucholtz, D.B.; Anderson, J.M. Barley yellow dwarf virus and cereal yellow dwarf virus quantification by Real-Time Polymerase Chain Reaction in resistant and susceptible plants. Phytopathology 2003, 93, 1386–1392. [Google Scholar] [CrossRef]
- Choudhury, S.; Hu, H.; Meinke, H.; Shabala, S.; Westmore, G.; Larkin, P.; Zhou, M. Barley yellow dwarf viruses: Infection mechanisms and breeding strategies. Euphytica 2017, 213, 168. [Google Scholar] [CrossRef]
- Liang, X.; Rashidi, M.; Rogers, C.W.; Marshall, J.M.; Price, W.J.; Rashed, A. Winter Wheat (Triticum aestivum) response to barley yellow dwarf virus at various nitrogen application rates in the presence and absence of its aphid vector, Rhopalosiphum padi. Entomol. Exp. Appl. 2019, 167, 98–107. [Google Scholar] [CrossRef]
- Togawa-Urakoshi, Y.; Ueno, O. Photosynthetic nitrogen- and water-use efficiencies in C3 and C4 subtype grasses grown under two nitrogen supply levels. Plant Prod. Sci. 2021, 25, 183–194. [Google Scholar] [CrossRef]
- Jensen, S.G. Metabolism and carbohydrate composition in barley yellow dwarf virus-infected wheat. Phytopathology 1972, 62, 587–592. [Google Scholar] [CrossRef]
- Fereres, A.; Araya, J.E.; Housley, T.L.; Foster, J.E. Carbohydrate composition of wheat infected with barley yellow dwarf virus. J. Plant Dis. Prot. 1990, 97, 600–608. [Google Scholar]
- Trębicki, P.; Vandegeer, R.K.; Bosque-Pérez, N.A.; Powell, K.S.; Dader, B.; Freeman, A.J.; Yen, A.L.; Fitzgerald, G.J.; Luck, J.E. Virus infection mediates the effects of elevated CO2 on plants and vectors. Sci. Rep. 2016, 6, 22785. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef]
- Bede, J.C.; Blande, J.D. Effects of Elevated CO2 and O3 on Aboveground Brassicaceous Plant-Insect Interactions. Annu. Rev. Entomol. 2025, 70, 205–232. [Google Scholar] [CrossRef]
- Trębicki, P.; Nancarrow, N.; Bosque-Pérez, N.A.; Rodoni, B.; Aftab, M.; Freeman, A.; Yen, A.; Fitzgerald, G.J. Virus incidence in wheat increases under elevated CO2: A 4-year study of yellow dwarf viruses from a free air carbon dioxide facility. Virus Res. 2017, 241, 137–144. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Nancarrow, N.; Constable, F.E.; Finlay, K.J.; Freeman, A.J.; Rodoni, B.C.; Trebicki, P.; Vassiliadis, S.; Yen, A.L.; Luck, J.E. The effect of elevated temperature on barley yellow dwarf virus-PAV in wheat. Virus Res. 2014, 186, 97–103. [Google Scholar] [CrossRef]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]
- Telesnicki, M.C.; Martínez-Ghersa, M.A.; Arneodo, J.D.; Ghersa, C.M. direct effect of ozone pollution on aphids: Revisiting the evidence at individual and population scales. Entomol. Exp. Appl. 2015, 155, 71–79. [Google Scholar] [CrossRef]
- Kansman, J.T.; Basu, S.; Casteel, C.L.; Crowder, D.W.; Lee, B.W.; Nihranz, C.T.; Finke, D.L. plant water stress reduces aphid performance: Exploring mechanisms driven by water stress intensity. Front. Ecol. Evol. 2022, 10, 846908. [Google Scholar] [CrossRef]
- Xie, H.; Shi, F.; Li, J.; Yu, M.; Yang, X.; Li, Y.; Fan, J. The reciprocal effect of elevated CO2 and drought on wheat-aphid interaction system. Front. Plant Sci. 2022, 13, 853220. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A. Climate change and cereal aphids: The relative effects of increasing CO2 and temperature on aphid population dynamics. Glob. Change Biol. 2003, 10, 5–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parizad, S.; Yang, J.; Oeller, L.; Nikoukar, A.; Liang, X.; Rashed, A. Abiotic Factors Affecting Vector-Borne Plant Pathogen Complexes: Elevated CO2 and the Barley Yellow Dwarf Pathosystem. Insects 2025, 16, 1186. https://doi.org/10.3390/insects16121186
Parizad S, Yang J, Oeller L, Nikoukar A, Liang X, Rashed A. Abiotic Factors Affecting Vector-Borne Plant Pathogen Complexes: Elevated CO2 and the Barley Yellow Dwarf Pathosystem. Insects. 2025; 16(12):1186. https://doi.org/10.3390/insects16121186
Chicago/Turabian StyleParizad, Shirin, Jingya Yang, Liesl Oeller, Atoosa Nikoukar, Xi Liang, and Arash Rashed. 2025. "Abiotic Factors Affecting Vector-Borne Plant Pathogen Complexes: Elevated CO2 and the Barley Yellow Dwarf Pathosystem" Insects 16, no. 12: 1186. https://doi.org/10.3390/insects16121186
APA StyleParizad, S., Yang, J., Oeller, L., Nikoukar, A., Liang, X., & Rashed, A. (2025). Abiotic Factors Affecting Vector-Borne Plant Pathogen Complexes: Elevated CO2 and the Barley Yellow Dwarf Pathosystem. Insects, 16(12), 1186. https://doi.org/10.3390/insects16121186





