Effects of High Larval Density on Wing Shape Deformations of Culex pipiens (Culicidae: Diptera) via Geometric Morphometrics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Design
2.2. Preparation of the Wings
2.3. Digitization and Validation of Data
2.4. Landmark-Based Geometric Morphometrics
2.5. Outline-Based Geometric Morphometrics
3. Results
3.1. Landmark-Based Geometric Morphometrics
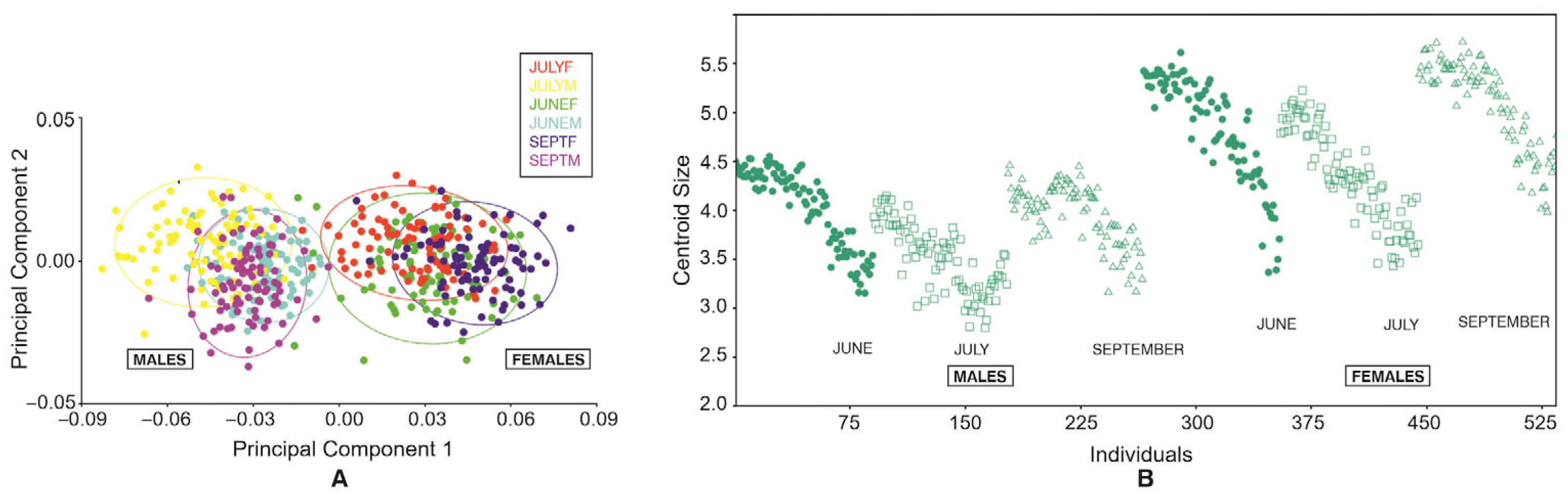
3.1.1. Two-Dimensional Principal Component Analysis
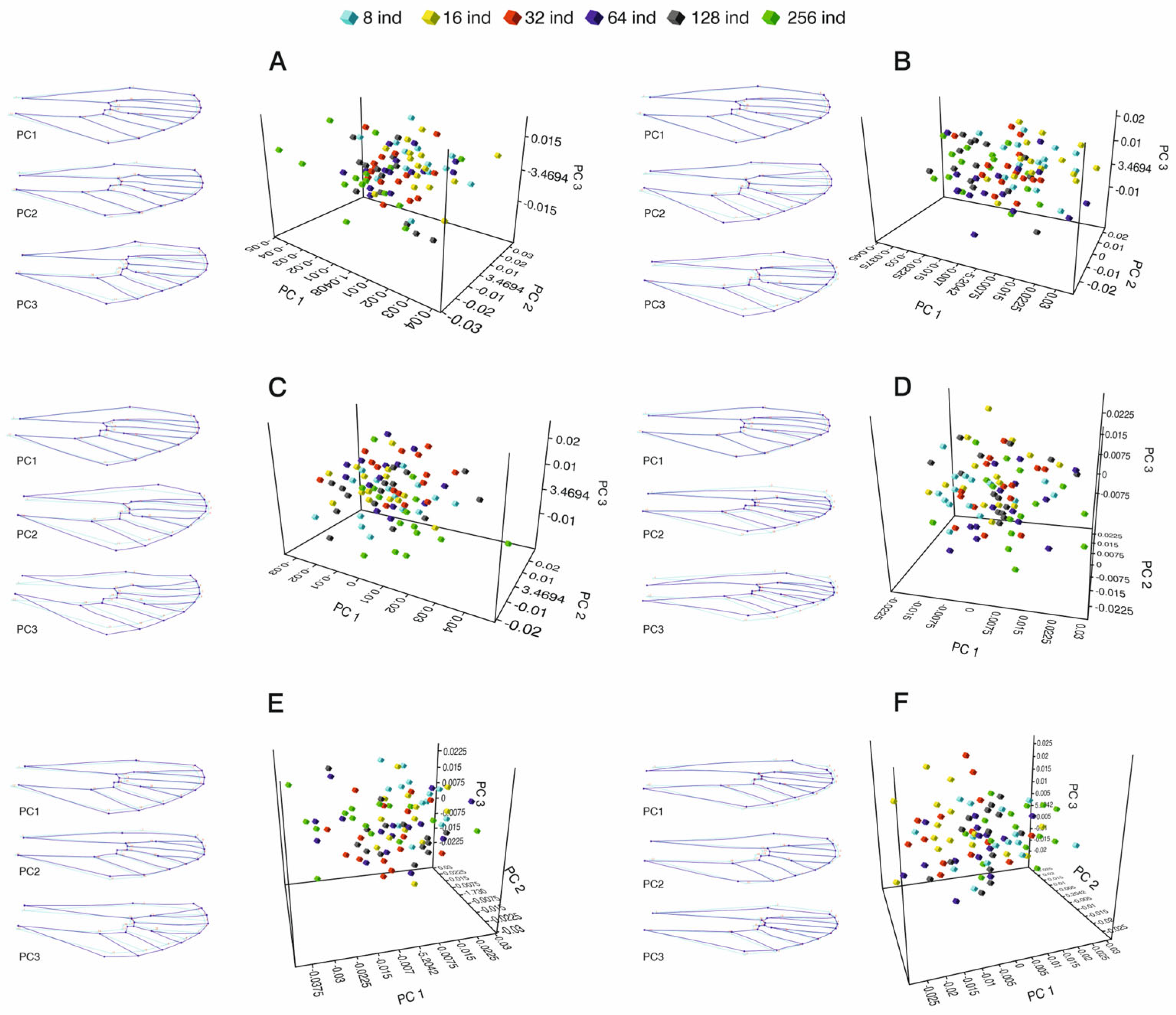
3.1.2. Three-Dimensional Principal Component Analysis
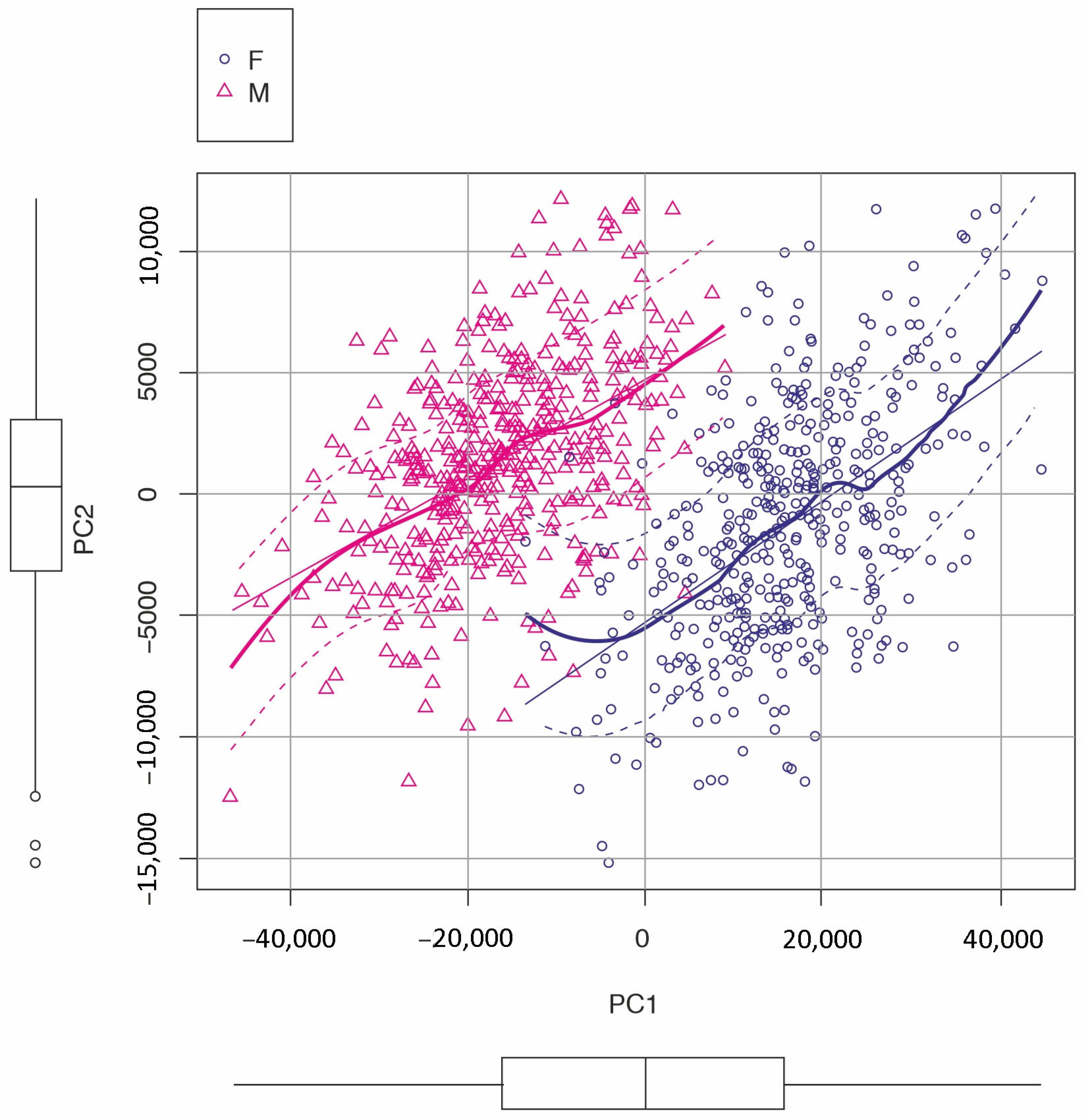
3.1.3. Canonical Variate Analysis (CVA)
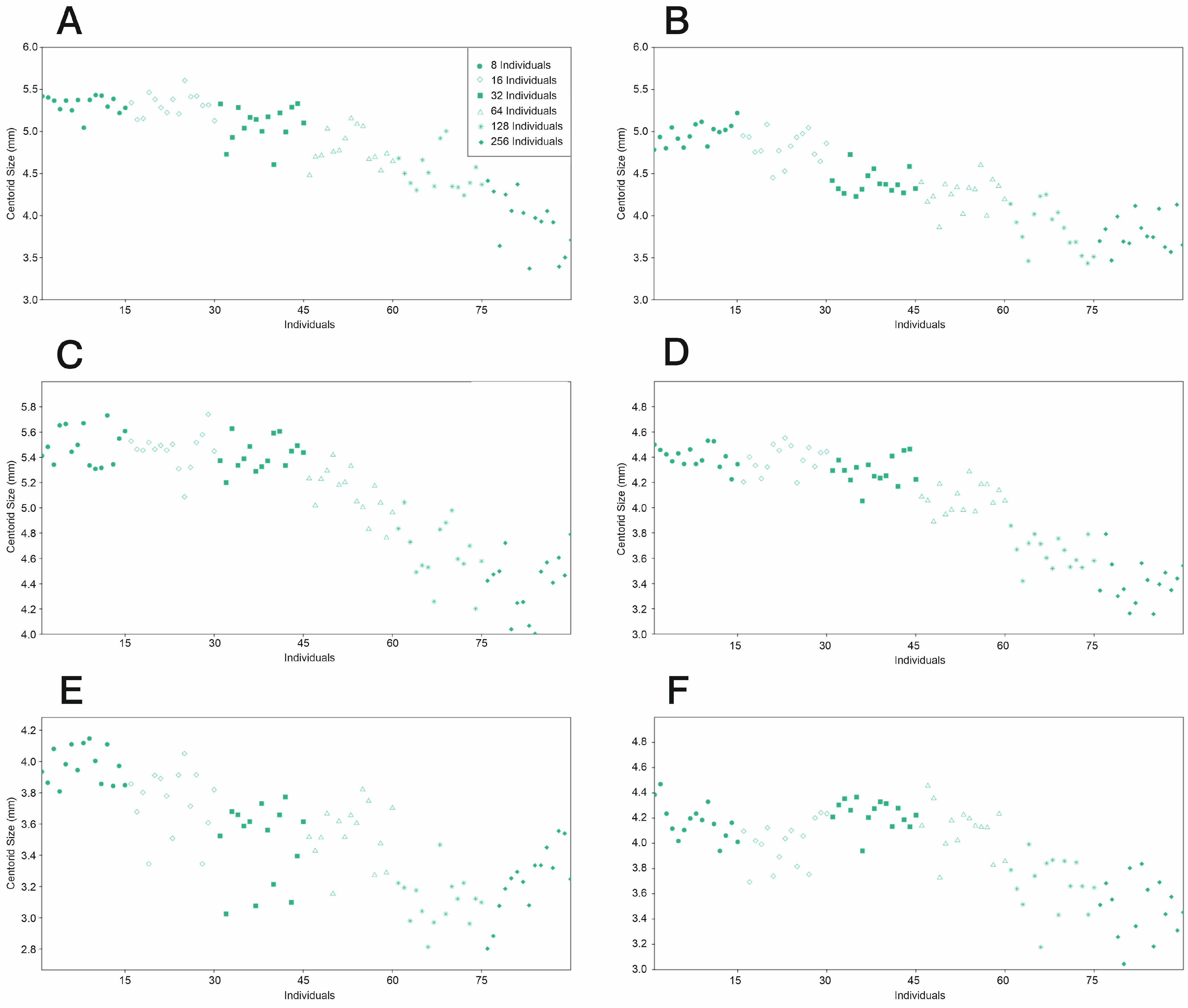
3.1.4. Centroid Sizes
3.2. Outline-Based Geometric Morphometric-Based Elliptic Fourier Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| mm | Millimeter |
| PCA | Principal Component Analysis |
References
- Wang, J.; Lo, S.; Wang, Q.; Sun, J.; Mu, H. Risk of large-scale evacuation based on the effectiveness of rescue strategies under different crowd densities. Risk Anal. 2013, 33, 1553–1563. [Google Scholar] [CrossRef]
- Fantinou, K.; Pafka, E. Mapping Urbanities: Morphologies, Flows, Possibilities; Taylor & Francis: Oxfordshire, UK, 2017; 218p. [Google Scholar]
- Fantinou, A.; Perdikis, D.; Stamogiannis, N. Effect of Larval Crowding on the Life History Traits of Sesamia nonagrioides (Lepidoptera: Noctuidae). Eur. J. Entomol. 2008, 105, 625–630. [Google Scholar] [CrossRef]
- Henry, Y.; Renault, D.; Colinet, H. Hormesis-Like Effect of Mild Larval Crowding on Thermo tolerance in Drosophila Flies. J. Exp. Biol. 2018, 221, jeb169342. [Google Scholar] [CrossRef] [PubMed]
- Alaidrous, W.; Villa, S.M.; de Roode, J.C.; Majewska, A.A. Crowding Does Not Affect Monarch Butterflies’ Resistance to a Protozoan Parasite. Ecol. Evol. 2022, 12, e8791. [Google Scholar] [CrossRef] [PubMed]
- Opare, L.O.; Jensen, A.B.; Lecocq, A.; Holm, S.; Esperk, T. Exposure to entomopathogenic fungus and high larval density induce a strong immune response and life-history costs in black soldier fly, a commercially important insect. Entomol. Exp. Appl. 2024, 172, 710–719. [Google Scholar] [CrossRef]
- Therry, L.; Swaegers, J.; Van Dinh, K.; Bonte, D.; Stoks, R. Low larval densities in northern populations reinforce range expansion by a Mediterranean damselfly. Freshw. Biol. 2016, 61, 1430–1441. [Google Scholar] [CrossRef]
- Heath, K.; Paton, R.S.; Wilson, A.J.; Bonsall, M.B. Nutritional Availability and Larval Density Dependence in Aedes aegypti. Ecol. Entomol. 2020, 45, 929–944. [Google Scholar] [CrossRef]
- Gama, R.A.; Alves, K.C.; Martins, R.F.; Eiras, A.E.; Resende, M.C. Effect of Larvae Density on Adult Size of Aedes aegypti Reared under Laboratory Conditions. Rev. Soc. Bras. Med. Trop. 2005, 38, 64–66. [Google Scholar] [CrossRef]
- Jirakanjanakit, N.; Leemingsawat, S.; Thongrungkiat, S.; Apiwathnasorn, C.; Singhaniyom, S.; Bellec, C.; Dujardin, J.-P. Influence of Larval Density or Food Variation on the Geometry of the Wing of Aedes (Stegomyia) aegypti. Trop. Med. Int. Health 2007, 12, 1354–1360. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data; Cambridge University Press: Cambridge, UK, 1991; 435p. [Google Scholar]
- Oxnard, C.E. Evolution of the human shoulder: Some possible pathways. Am. J. Phys. Anthropol. 1969, 30, 319–331. [Google Scholar] [CrossRef]
- Adams, D.C.; Otárola-Castillo, E. geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Marcus, L.F. A revolution morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Mitteroecker, P.; Schaefer, K. Thirty years of Geometric Morphometrics: Achievements, Challenges and the Ongoing Quest for Biological Meaningfulness. Yearb. Biol. Anthropol. 2022, 178 (Suppl. S74), 181–210. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Oliveira-Christe, R.; Multini, L.C.; Vidal, P.O.; Wilk-da-Silva, R.; Carvalho, G.C.; Marrelli, M.T. Morphometric wing characters as a tool for mosquito identification. PLoS ONE 2016, 11, e0161643. [Google Scholar] [CrossRef]
- Lorenz, C.; Almeida, F.; Almeida-Lopes, F.; Louise, C.; Pereira, S.N.; Petersen, V.; Vidal, P.O.; Virginio, F.; Suesdek, L. Geometric Morphometrics in Mosquitoes: What Has Been Measured? Infect. Genet. Evol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Doğan, M.; Günay, F.; Puggioli, A.; Balestrino, F.; Öncü, C.; Alten, B.; Bellini, R. Establishment of a Satellite Rearing Facility to Support the Release of Sterile Aedes albopictus Males. I. Optimization of Mass Rearing Parameters. Acta Trop. 2016, 159, 62–68. [Google Scholar] [CrossRef]
- Briegel, H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990, 36, 165–172. [Google Scholar] [CrossRef]
- Blackmore, M.S.; Lord, C.C. The relationship between size and fecundity in Aedes albopictus and Aedes aegypti. J. Vector Ecol. 2000, 25, 212–217. [Google Scholar]
- Alcalay, Y.; Puzhevsky, D.; Tsurim, I.; Scharf, I.; Ovadia, S.O. Interactive and Sex-Specific Life-History Responses of Culex pipiens Mosquito Larvae to Multiple Environmental Factors. J. Zool. 2018, 306, 268–278. [Google Scholar] [CrossRef]
- Sakaci, Z.; Talay, S.; Erguler, K.; Korkmaz, A.; Sirin, D.; Er, A.; Alten, B.; Kar, S. Interindividual variation among Culex pipiens larvae in terms of thermal response. Med. Vet. Entomol. 2024, 38, 205–215. [Google Scholar] [CrossRef]
- Briegel, H. Physiological bases of mosquito ecology. J. Vector Ecol. 2002, 27, 1–8. [Google Scholar]
- Noor-E Jannat, K.; Roitberg, B.D. Effects of larval density and feeding rates on larval life history traits in Anopheles gambiae s.s. (Diptera: Culicidae). J. Vector Ecol. 2013, 38, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, Y.; Tsurim, I.; Ovadia, O. Multi-scale oviposition site selection in two mosquito species. Ecol. Entomol. 2018, 44, 347–356. [Google Scholar] [CrossRef]
- Yan, J.; Kibech, R.; Stone, C.M. Differential Effects of Larval and Adult Nutrition on Survival, Fecundity, and Size of the Yellow Fever Mosquito Aedes aegypti. Front. Zool. 2021, 18, 10. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar] [CrossRef]
- Lorenz, C.; Suesdek, L. The Use of Wing Shape for Characterising Macroevolution in Mosquitoes. Infect. Genet. Evol. 2020, 77, 104052. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Mårtensson, T. Measurement Error in Geometric Morphometrics: Empirical Strategies to Assess and Reduce Its Impact on Measures of Shape. Acta Zool. Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric Morphometrics: Ten Years of Progress Following the ‘Revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Fruciano, C. Measurement Error in Geometric Morphometrics. Dev. Genes Evol. 2016, 226, 139–158. [Google Scholar] [CrossRef]
- Fruciano, C.; Çelik, M.A.; Butler, K.; Dooley, T.; Weisbecker, V.; Phillips, M.J. Sharing Is Caring? Measurement Error and the Issues Arising from Combining 3D Morphometric Datasets. Ecol. Evol. 2017, 7, 7034–7046. [Google Scholar] [CrossRef]
- Beriotto, A.C.; Garzon, M.J.; Schweigmann. Is There a Minimum Number of Landmarks That Optimizes the Geometric Morphometric Analysis of Mosquito (Diptera: Culicidae) Wings? J. Med. Entomol. 2021, 58, 576–587. [Google Scholar] [CrossRef]
- De Lima, V.R.; de Morais, M.C.C.; Kirchgatter, K. Integrating Artificial Intelligence and Wing Geometric Morphometry to Automate Mosquito Classification. Acta Trop. 2024, 249, 107089. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. TpsDig; Department of Ecology and Evolution, State University of New York at Stony Brook: Stony Brook, NY, USA, 2004. [Google Scholar]
- Klingenberg, C.P. Analyzing Fluctuating Asymmetry with Geometric Morphometrics: Concepts, Methods, and Applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Zulzahrin, Z.; Wong, M.L.; Naziri, M.R.A.; Lau, Y.; Vythilingam, I.; Lee, W. Digital microscope-assisted photography improves the accuracy of mosquito wing measurement. Heliyon 2024, 10, e25207. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Christe, R.; Carvalho, G.C.; Wilke, A.B.B.; Marrelli, M.T. Assessment of wing geometric morphometrics of urban Culex quinquefasciatus (Diptera: Culicidae) populations. Acta Trop. 2023, 245, 106971. [Google Scholar] [CrossRef]
- Rohlf, F.J. Tps Series; Department of Ecology and Evolution, State University of New York at Stony Brook: Stony Brook, NY, USA, 2006. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Simões, R.F.; Wilke, A.B.B.; Chagas, C.R.F.; Menezes, R.M.T.d.; Suesdek, L.; Multini, L.C.; Silva, F.S.; Grech, M.G.; Marrelli, M.T.; Kirchgatter, K. Wing geometric morphometrics as a tool for the identification of Culex subgenus mosquitoes of Culex (Diptera: Culicidae). Insects 2020, 11, 567. [Google Scholar] [CrossRef]
- Martinet, J.P.; Ferte, H.; Sientzoff, P.; Krupa, E.; Mathieu, B.; Depaquit, J. Wing morphometrics of Aedes mosquitoes from north-eastern France. Insects 2021, 12, 341. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Iwata, H.; Ukai, Y. SHAPE: A Computer Program Package for Quantitative Evaluation of Biological Shapes Based on Elliptic Fourier Descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier Features of a Closed Contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: www.R-project.org (accessed on 1 February 2024).
- Fox, A.N. A Study of Late Woodland Projectile Point Typology in New York Using Elliptical Fourier Outline Analysis. J. Archeol. Sci. 2015, 4, 501–509. [Google Scholar] [CrossRef]
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alvial, I.E.; Hernandez-P, R.; Suazo, M.J.; Gonzalez, C.R.; Veliz, D.; Benitez, H.A. Unraveling Biotypes of the Northern House Mosquito, Culex pipiens s.l. (Diptera: Culicidae): Molecular Differentiation and Morphometric Analysis. J. Insect Sci. 2024, 24, ieae006. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex pipiens (Diptera: Culicidae): A Bridge Vector of West Nile Virus to Humans. J. Med. Entomol. 2008, 45, 125–128. [Google Scholar] [CrossRef]
- Ergunay, K.; Günay, F.; Kasap, O.E.; Öter, K.; Gargari, S.; Karaoğlu, T.; Tezcan, S.; Çabalar, M.; Yıldırım, Y.; Emekdaş, G.; et al. Serological, Molecular and Entomological Surveillance Demonstrates Widespread Circulation of West Nile Virus in Turkey. PLoS Negl. Trop. Dis. 2014, 8, 3028. [Google Scholar] [CrossRef]
- Günay, F.; Alten, B.; Şimsek, F.M.; Aldemir, A.; Linton, Y.M. Barcoding Turkish Culex Mosquitoes to Facilitate Arbovirus Vector Incrimination Studies Reveals Hidden Diversity and New Potential Vectors. Acta Trop. 2015, 143, 112–120. [Google Scholar] [CrossRef]
- Akiner, M.M.; Ozturk, M.; Beris, F.S.; Karacaoglu, C.; Simsek, F.M.; Akgeyik, A.U. Distribution and molecular differentiation of Culex pipiens complex species in the Middle and Eastern Black Sea Regions of Turkey. Turk. J. Zool. 2022, 46, 207–219. [Google Scholar] [CrossRef]
- Gómez, G.F.; Márquez, E.J.; Gutiérrez, L.A.; Conn, J.E.; Correa, M.M. Geometric Morphometric Analysis of Colombian Anopheles albimanus (Diptera: Culicidae) Reveals Significant Effect of Environmental Factors on Wing Traits and Presence of a Metapopulation. Acta Trop. 2014, 135, 75–85. [Google Scholar] [CrossRef]
- Sumruayphol, S.; Apiwathnasorn, C.; Ruangsittichai, J.; Sriwichai, P.; Attrapadung, S.; Samung, Y.; Dujardin, J.P. DNA barcoding and wing morphometrics to distinguish three Aedes vectors in Thailand. Acta Trop. 2016, 159, 1–10. [Google Scholar] [CrossRef]
- Aytekin, S.; Aytekin, A.M.; Alten, B. Effect of Different Larval Rearing Temperatures on the Productivity (Ro) and Morphology of the Malaria Vector Anopheles superpictus Grassi (Diptera: Culicidae) Using Geometric Morphometrics. J. Vector Ecol. 2009, 34, 32–42. [Google Scholar] [CrossRef]
- Dutra, C.L.H.; de Silva, V.L.; Fernandes, M.R.; Logullo, C.; Maciel-de-Freitas, R.; Moreira, L.A. The Influence of Larval Competition on Brazilian Wolbachia-Infected Aedes aegypti Mosquitoes. Parasit. Vectors. 2016, 9, 282. [Google Scholar] [CrossRef]
- Gleiser, R.M.; Urrutia, J.; Gorla, D.E. Effects of Crowding on Populations of Aedes albifasciatus Larvae under Laboratory Conditions. Entomol. Exp. Appl. 2000, 95, 135–140. [Google Scholar] [CrossRef]
- Adams, A.W.; Craig, J.V. Effect of Crowding and Cage Shape on Productivity and Profitability of Caged Layers: A Survey. Poult. Sci. 1985, 64, 238–242. [Google Scholar] [CrossRef]
- Lord, C.C. Density Dependence in Larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1998, 35, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, Larval Diet and Density Effects on Development Rate and Survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef]
- Macia, A. Effects of larval crowding on development time, survival and weight at metamorphosis in Aedes aegypti (Diptera: Culicidae). Rev. Soc. Entomol. Argent. 2009, 68, 107–114. [Google Scholar]
- Yang, F.; Hu, G.; Shi, J.J.; Zhai, B.P. Effects of larval density and food stress on life-history traits of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Appl. Entomol. 2015, 139, 370–380. [Google Scholar] [CrossRef]
- Christe, R.O.; Wilke, A.B.B.; Vidal, P.O.; Marrelli, M.T. Wing Sexual Dimorphism in Aedes fluviatilis (Diptera: Culicidae). Infect. Genet. Evol. 2016, 45, 434–436. [Google Scholar] [CrossRef]
- Shin, S.M.; Akram, W.; Lee, J.J. Effect of body size on energy reserves in Culex pipiens pallens females (Diptera: Culicidae). Entomol. Res. 2012, 42, 163–167. [Google Scholar] [CrossRef]
- Blom, R.; Spitzen, J.; de Haan, T.; Koenraadt, C.J.M. Phenotypical Aspects of Culex pipiens Biotype pipiens during Diapause: Lipid Utilization, Body Size, Insemination, and Parity. J. Insect Physiol. 2024, 159, 104714. [Google Scholar] [CrossRef]
- Reisen, W.K.; Milby, M.M.; Bock, M.E. The Effects of Immature Stress on Selected Events in the Life History of Culex tarsalis. Mosq. News 1984, 44, 385–395. [Google Scholar]
- Bédhomme, S.; Agnew, P.; Sidobre, C.; Michalakis, Y. Pollution by Conspecifics as a Component of Intraspecific Competition among Aedes aegypti Larvae. Ecol. Entomol. 2005, 30, 1–7. [Google Scholar] [CrossRef]
- Tsurim, I.; Silberbush, A.; Ovadia, O.; Blaustein, L.; Margalith, Y. Inter- and Intra-Specific Density-Dependent Effects on Life History and Development Strategies of Larval Mosquitoes. PLoS ONE 2013, 8, e57875. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, E.O.; Takken, W.; Koella, J.C. Effect of Rearing Temperature and Larval Density on Larval Survival, Age at Pupation and Adult Size of Anopheles gambiae. Entomol. Exp. Appl. 1992, 63, 265–271. [Google Scholar] [CrossRef]
- Barrera, R.; Amador, M.; Clark, G.G. Ecological Factors Influencing Aedes aegypti (Diptera: Culicidae) Productivity in Artificial Containers in Salinas, Puerto Rico. J. Med. Entomol. 2006, 43, 484–492. [Google Scholar] [CrossRef]
- Ezeakacha, N.F.; Yee, D.A. The Role of Temperature in Affecting Carry-Over Effects and Larval Competition in the Globally Invasive Mosquito Aedes albopictus. Parasites Vectors 2019, 12, 123. [Google Scholar] [CrossRef]
- Cui, J.; Li, S.; Zhao, P.; Zou, F. Flight Capacity of Adult Culex pipiens pallens (Diptera: Culicidae) in Relation to Gender and Day-Age. J. Med. Entomol. 2013, 50, 1055–1058. [Google Scholar] [CrossRef]
- Warren, B.; Gibson, G.; Russell, I.J. Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr. Biol. 2009, 19, 485–491. [Google Scholar] [CrossRef]
- Devicari, M.; Lopes, A.R.; Suesdek, L. Wing Sexual Dimorphism in Aedes scapularis (Diptera: Culicidae). Biota Neotrop. 2011, 11, 165–169. [Google Scholar] [CrossRef]
- Gomes, B.; Sousa, C.A.; Novo, M.T.; Freitas, F.B.; Alves, R.; Côrte-Real, A.R.; Salgueiro, P.; Donnelly, M.J.; Almeida, A.P.; Pinto, J. Asymmetric Introgression between Sympatric molestus and pipiens Forms of Culex pipiens (Diptera: Culicidae) in the Comporta Region, Portugal. BMC Evol. Biol. 2009, 9, 262. [Google Scholar] [CrossRef]
- Kassim, N.F.A.; Webb, C.E.; Russell, R.C. The Importance of Males: Larval Diet and Adult Sugar Feeding Influences Reproduction in Culex molestus. J. Am. Mosq. Control Assoc. 2012, 28, 312–316. [Google Scholar] [CrossRef]
- Liu, Y.; Du, G. Effects of Blood-Feeding on Mosquitoes Hovering Kinematics and Aerodynamics. Phys. Fluids 2024, 36, 031909. [Google Scholar] [CrossRef]
- Jung, H.; Oh, S.; Choi, H. Role of the Deviation Motion on the Aerodynamic Performance of a Mosquito Wing in Hover. Comput. Fluids 2024, 270, 106146. [Google Scholar] [CrossRef]
- Barr, J.S.; Estevez-Lao, T.Y.; Khalif, M.; Saksena, S.; Yarlagadda, S.; Farah, O.; Shivere, Y.; Hillyer, J.F. Temperature and Age, Individually and Interactively, Shape the Size, Weight, and Body Composition of Adult Female Mosquitoes. J. Insect Physiol. 2023, 148, 104525. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Archie, J.W. A comparison of Fourier methods for the description of wing shape in mosquitoes (Diptera: Culicidae). Syst. Zool. 1984, 33, 302–317. [Google Scholar] [CrossRef]
- Baylac, M.; Friess, M. Fourier Descriptors, Procrustes Superimposition, and Data Dimensionality: An Example of Cranial Shape Analysis in Human Populations. In Modern Morphometrics in Physical Anthropology; Slice, D.E., Ed.; Plenum: New York, NY, USA, 2005; pp. 145–165. [Google Scholar]
- Brophyl, J.K.; de Ruiter, D.J.; Athreya, S.; DeWitt, T.J. Quantitative Morphological Analysis of Bovid Teeth and Implications for Paleoenvironmental Reconstruction of Plovers Lake, Gauteng Province, South Africa. J. Archaeol. Sci. 2014, 41, 376–388. [Google Scholar] [CrossRef]
- Dujardin, J.; Kaba, D.; Solano, P.; Dupraz, M.; McCoy, K.D.; Jaramillo-O, N. Outline-Based Morphometrics, an Overlooked Method in Arthropod Studies? Infect. Genet. Evol. 2014, 28, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.A. The Effect of Larval Density on Adult Longevity of a Mosquito, Aedes sierrensis: Epidemiological Consequences. J. Anim. Ecol. 1985, 54, 955–964. [Google Scholar] [CrossRef]
- Juliano, S.A. Species Interactions among Larval Mosquitoes: Context Dependence across Habitat Gradients. Annu. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef]
- Madeira, S.; Bernardino, R.; Osório, H.C.; Boinas, F. Mosquito (Diptera: Culicidae) Fauna of a Zoological Park in an Urban Setting: Analysis of Culex pipiens s.l. and Their Biotypes. Insects 2024, 15, 45. [Google Scholar] [CrossRef]



| Period | Tmin (°C ± SD) | Tmax (°C ± SD) | Tmean (°C ± SD) |
|---|---|---|---|
| June | 18.1 ± 2.1 (14.6–23.4) | 29.7 ± 2.1 (26.4–33.6) | 23.8 ± 1.6 (21.2–27.6) |
| July | 20.3 ± 1.5 (17.3–23.6) | 31.8 ± 1.9 (27.9–35.1) | 25.8 ± 1.2 (23.6–27.8) |
| September | 14.7 ± 3.8 (7.9–21.8) | 26.1 ± 2.5 (21.2–31.3) | 20.2 ± 3.1 (14.9–26.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aytekin, S.; Sakaci, Z.; Talay, S.; Alten, B. Effects of High Larval Density on Wing Shape Deformations of Culex pipiens (Culicidae: Diptera) via Geometric Morphometrics. Insects 2025, 16, 1185. https://doi.org/10.3390/insects16121185
Aytekin S, Sakaci Z, Talay S, Alten B. Effects of High Larval Density on Wing Shape Deformations of Culex pipiens (Culicidae: Diptera) via Geometric Morphometrics. Insects. 2025; 16(12):1185. https://doi.org/10.3390/insects16121185
Chicago/Turabian StyleAytekin, Seçil, Zafer Sakaci, Sengul Talay, and Bulent Alten. 2025. "Effects of High Larval Density on Wing Shape Deformations of Culex pipiens (Culicidae: Diptera) via Geometric Morphometrics" Insects 16, no. 12: 1185. https://doi.org/10.3390/insects16121185
APA StyleAytekin, S., Sakaci, Z., Talay, S., & Alten, B. (2025). Effects of High Larval Density on Wing Shape Deformations of Culex pipiens (Culicidae: Diptera) via Geometric Morphometrics. Insects, 16(12), 1185. https://doi.org/10.3390/insects16121185






