Transforming Tuta absoluta Management: A Synergistic Approach Integrating Sustainability, Biological Control, and Biotechnological Innovations
Simple Summary
Abstract
1. Introduction
2. Advancing Integrated Management Strategies for Tuta absoluta
2.1. Enhanced Strategies: Surveillance, Mass Trapping, and Mating Disruption
2.2. Tomato Resistance and Breeding Strategies
3. Host Plant Resistance for the Control of T. absoluta
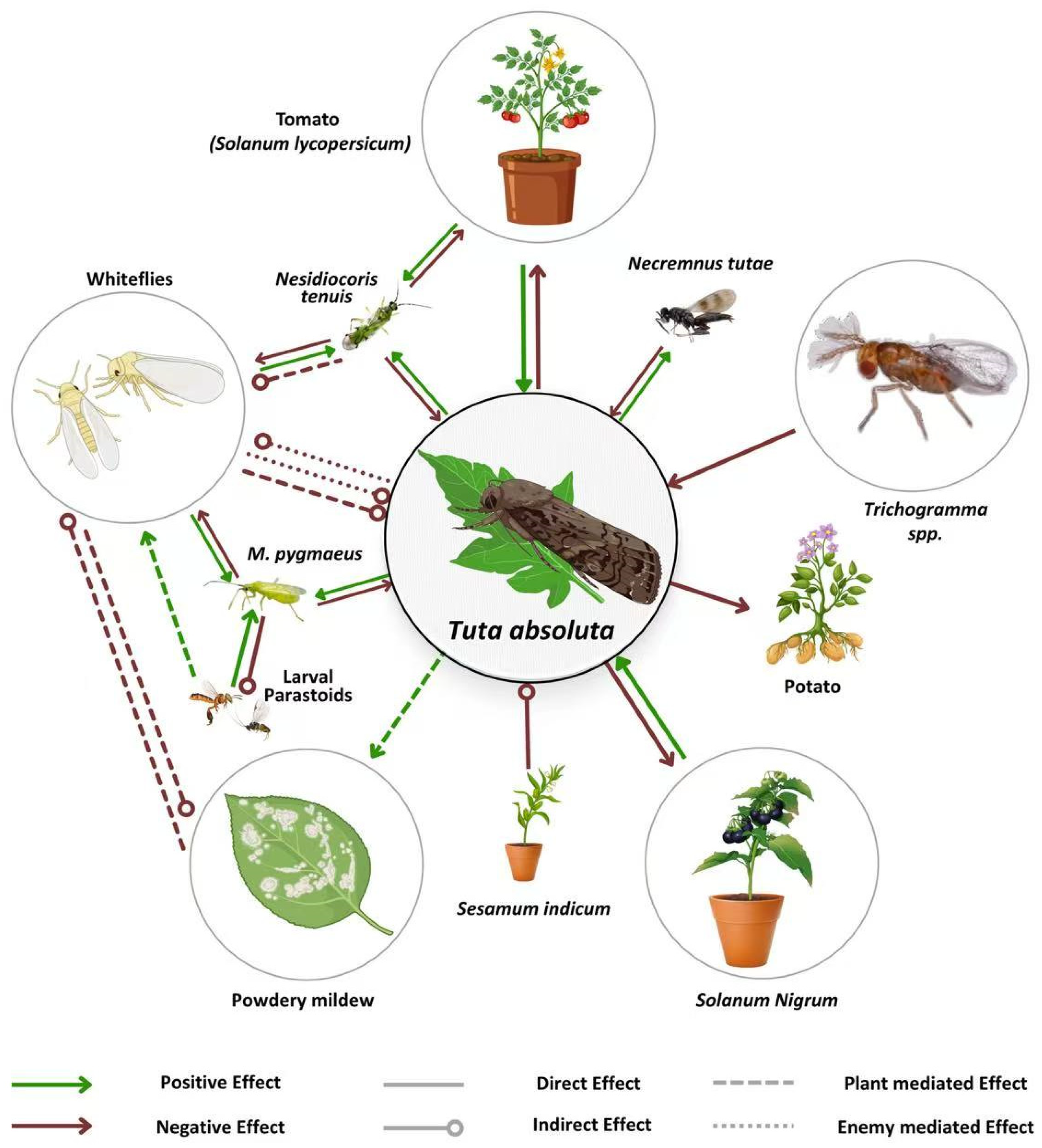
4. Cutting-Edge Strategies in the Biological Control of T. absoluta
5. Microbial Control and Biopesticides
6. Arthropod-Based Biological Control of Tuta absoluta
Enhancing T. absoluta Management with Sex Pheromones and Hormonal Control Strategies
7. Unlocking the Power of Botanical Pesticides for T. absoluta
| Botanical | Insect Stage | References |
|---|---|---|
| Azadirachta indica A. Juss | Egg and Larva | Kona et al. (2014) [140] |
| Allium sativum L. | Second instar larva | Ghanim and Abdel Ghani (2014) [141] |
| Eucalyptus globulus Labill | Larva | Sanda et al. (2018) [142] |
| Jatropa curcas L. | Larva | Moreno et al. (2012) [118] |
| Piper amalago var. medium | Larva and pupa | (Brito et al., 2015) [143] |
| Simmondsia chinensis | Second instar larva | (Abdel-Baky and Al-Soqeer 2017) [138] |
| Melia azedarach L. | Second instar larva | (Ghanim and Abdel Ghani 2014) [141] |
| Acmella oleracea (L.) R.K. Jansen | All | (Moreno et al., 2012) [118] |
| Allium cepa L. | Second instar larva | (Ghanim and Abdel Ghani 2014) [141] |
8. Evaluating the Effectiveness of Chemical Pesticides in Controlling T. absoluta Infestations
9. Exploring Cutting-Edge Biotechnological Innovations for the Comprehensive Control of Tuta absoluta
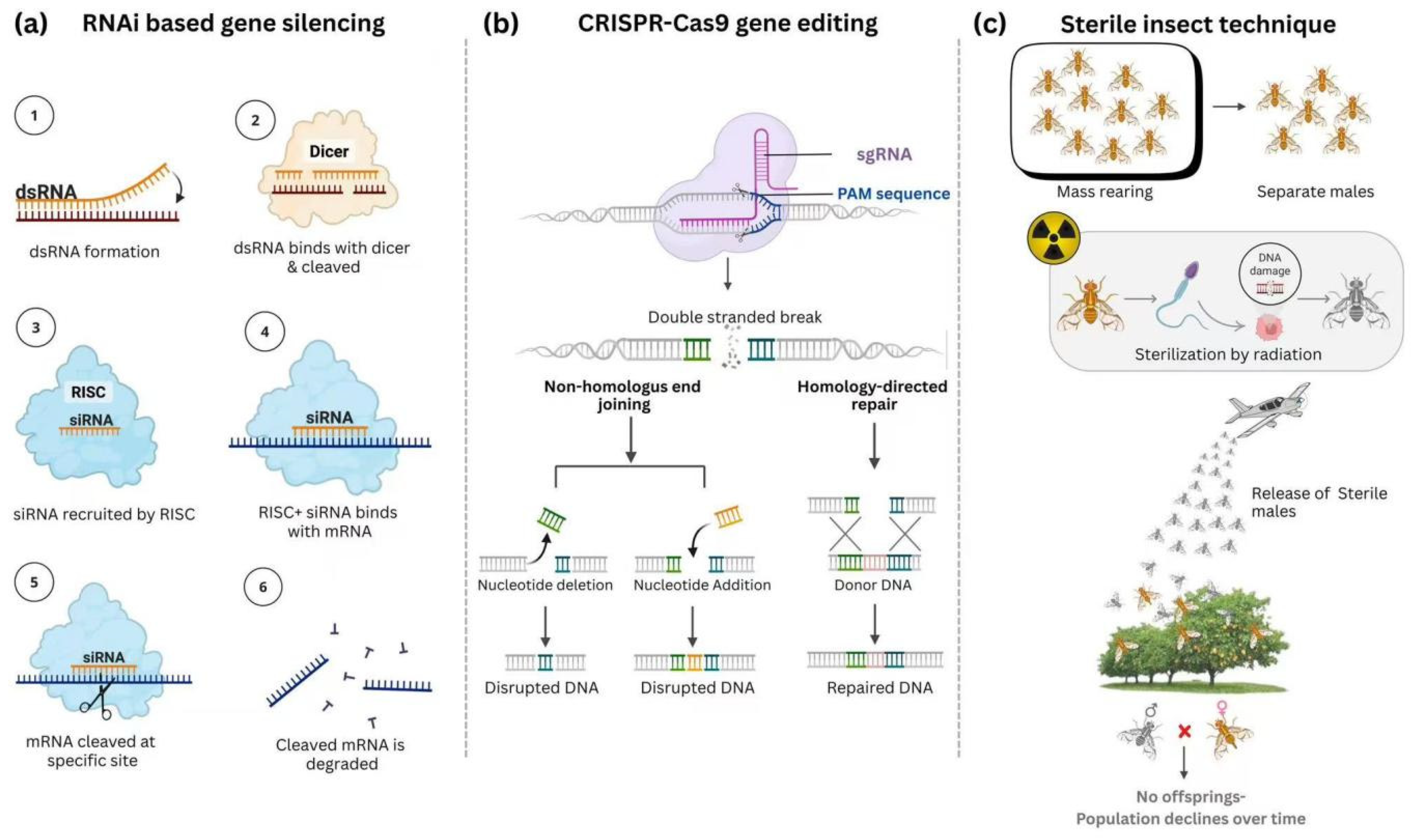
9.1. Advancing Tuta absoluta Control with Sterile Insect Technique
9.2. RNAi-Mediated Gene Silencing for Targeted Control of Tuta absoluta
9.3. CRISPR/Cas9-Based Genome Editing
9.4. Nano-Bioinsecticides for Tuta absoluta Control
10. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIT | Sterile Insect Technique |
| IPM | Integrated Pest Management |
| RNAi | RNA interference |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9. |
| EPNs | Entomopathogenic Nematodes |
| VOCs | Volatile Organic Compounds |
| NHEJ | Non-homologous End Joining |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| N. tenuis | Nesidiocoris tenuis |
| S. carpocapsae | Steinernema carpocapsae |
References
- Guillemaud, T.; Blin, A.; Le Goff, I.; Desneux, N.; Reyes, M.; Tabone, E.; Tsagkarakou, A.; Nino, L.; Lombaert, E. The Tomato Borer, Tuta absoluta, Invading the Mediterranean Basin, Originates from a Single Introduction from Central Chile. Sci. Rep. 2015, 5, 8371. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The Invasive South American Tomato Pinworm, Tuta absoluta, Continues to Spread in Afro-Eurasia and beyond: The New Threat to Tomato World Production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.; Lu, Z.; Wang, S.; Ma, D. Are We Ready for the Invasion of Tuta absoluta? Unanswered Key Questions for Elaborating an Integrated Pest Management Package in Xinjiang, China. Entomol. Gen. 2018, 38, 113–125. [Google Scholar] [CrossRef]
- Mansour, R.; Brévault, T.; Chailleux, A.; Cherif, A.; Grissa-Lebdi, K.; Haddi, K.; Mohamed, S.A.; Nofemela, R.S.; Oke, A.; Sylla, S. Occurrence, Biology, Natural Enemies and Management of Tuta absoluta in Africa. Entomol. Gen. 2018, 38, 83–112. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K. Tuta absoluta Continues to Disperse in Asia: Damage, Ongoing Management and Future Challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J. Integrated Pest Management of Tuta absoluta: Practical Implementations across Different World Regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Bawin, T.; Dujeu, D.; De Backer, L.; Francis, F.; Verheggen, F.J. Ability of Tuta absoluta (Lepidoptera: Gelechiidae) to Develop on Alternative Host Plant Species. Can. Entomol. 2016, 148, 434–442. [Google Scholar] [CrossRef]
- Caruso, A.G.; Tortorici, S.; Davino, S.; Bertacca, S.; Ragona, A.; Lo Verde, G.; Biondi, A.; Noris, E.; Rizzo, R.; Panno, S. The Invasive Tomato Pest Tuta absoluta Can Transmit the Emergent Tomato Brown Rugose Fruit Virus. Entomol. Gen. 2024, 44, 289–296. [Google Scholar] [CrossRef]
- Cherif, A.; Attia-Barhoumi, S.; Mansour, R.; Zappalà, L.; Grissa-Lebdi, K. Elucidating Key Biological Parameters of Tuta absoluta on Different Host Plants and under Various Temperature and Relative Humidity Regimes. Entomol. Gen. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide Resistance in the Tomato Pinworm Tuta absoluta: Patterns, Spread, Mechanisms, Management and Outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, R.; Kuang, M. First Report of the South American Tomato Leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Wang, M.; Ismoilov, K.; Li, H.; Zhang, X.; Lu, Z.; Feng, L.; Han, P. Polygyny of Tuta absoluta May Affect Sex Pheromone-Based Control Techniques. Entomol. Gen. 2021, 41, 357–367. [Google Scholar] [CrossRef]
- Zhang, G.; Xian, X.; Zhang, Y.; Liu, W.; Liu, H.; Feng, X.; Ma, D.; Wang, Y.; Gao, Y.; Zhang, R. Outbreak of the South American Tomato Leafminer, Tuta absoluta, in the Chinese Mainland: Geographic and Potential Host Range Expansion. Pest Manag. Sci. 2021, 77, 5475–5488. [Google Scholar] [CrossRef]
- Ouattara, S.S.S.; Konate, M. The Tomato: A Nutritious and Profitable Vegetable to Promote in Burkina Faso. Alex. Sci. Exch. J. 2024, 45, 11–20. [Google Scholar] [CrossRef]
- Xian, X.; Han, P.; Wang, S.; Zhang, G.; Liu, W.; Wan, F. The Potential Invasion Risk and Preventive Measures against the Tomato Leafminer Tuta absoluta in China. Entomol. Gen. 2017, 36, 319–333. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, L.; Song, C.; Xue, Z.; Zheng, R.; Yan, X.; Hao, C. Modelling Potential Distribution of Tuta absoluta in China under Climate Change Using CLIMEX and MaxEnt. J. Appl. Entomol. 2023, 147, 895–907. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Arnó, J.; Kriticos, D.J.; Desneux, N.; Zalucki, M.P.; Lu, Z. Protected Agriculture Matters: Year-Round Persistence of Tuta absoluta in China Where It Should Not. Entomol. Gen. 2023, 44, 279–287. [Google Scholar] [CrossRef]
- Xi, M.; Wang, Z.L.; Liu, X.X.; Li, Z.H.; Zhang, X.; Lu, Z.Z.; Han, P. Assessment of the Economic Loss to the Tomato Industry Caused by Tuta absoluta in China Based On@ RISK. J. Biosaf. 2022, 31, 300–308. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Ullah, F.; Güncan, A.; Gul, H.; Hafeez, M.; Zhou, S.; Wang, Y.; Zhang, Z.; Huang, J.; Ghramh, H.A.; Guo, W. Spinosad-Induced Intergenerational Sublethal Effects on Tuta absoluta: Biological Traits and Related Genes Expressions. Entomol. Gen. 2024, 44, 395–404. [Google Scholar] [CrossRef]
- Ullah, F.; Güncan, A.; Abbas, A.; Gul, H.; Guedes, R.N.C.; Zhang, Z.; Huang, J.; Khan, K.A.; Ghramh, H.A.; Chavarín-Gómez, L.E. Sublethal Effects of Neonicotinoids on Insect Pests. Entomol. Gen. 2024, 44, 1145–1160. [Google Scholar] [CrossRef]
- Han, P.; Lavoir, A.-V.; Le Bot, J.; Amiens-Desneux, E.; Desneux, N. Nitrogen and Water Availability to Tomato Plants Triggers Bottom-up Effects on the Leafminer Tuta absoluta. Sci. Rep. 2014, 4, 4455. [Google Scholar] [CrossRef]
- Dong, Y.; Han, P.; Niu, C.; Zappalà, L.; Amiens-Desneux, E.; Bearez, P.; Lavoir, A.; Biondi, A.; Desneux, N. Nitrogen and Water Inputs to Tomato Plant Do Not Trigger Bottom-up Effects on a Leafminer Parasitoid through Host and Non-host Exposures. Pest Manag. Sci. 2018, 74, 516–522. [Google Scholar] [CrossRef]
- Aygel, G.; Aslan, M.M. Population Density and Infestation Rate of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) on Different Tomato Varieties in Mersin Field Conditions. KSU J. Agric. Nat. 2023, 26, 27–37. [Google Scholar] [CrossRef]
- Borges, I.; Oliveira, L.; Durão, A.; Arruda, P.; Soares, A.O. Feeding Preference and Intraguild Interactions between the Parasitoid Trichogramma achaeae and the Predator Macrolophus pygmaeus, Two Biological Agents of Tuta absoluta. Pest Manag. Sci. 2023, 79, 4376–4382. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F. Pheromone-Based Management Strategies to Control the Tomato Leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A Review. Biotechnol. Agron. Société Environ. 2013, 17, 475–482. [Google Scholar]
- Benvenga, S.R.; Fernandes, O.A.; Gravena, S. Decision Making for Integrated Pest Management of the South American Tomato Pinworm Based on Sexual Pheromone Traps. Hortic. Bras. 2007, 25, 164–169. [Google Scholar] [CrossRef]
- Cocco, A.; Serra, G.; Lentini, A.; Deliperi, S.; Delrio, G. Spatial Distribution and Sequential Sampling Plans for Tuta absoluta (Lepidoptera: Gelechiidae) in Greenhouse Tomato Crops. Pest Manag. Sci. 2015, 71, 1311–1323. [Google Scholar] [CrossRef]
- Lobos, E.; Occhionero, M.; Werenitzky, D.; Fernandez, J.; Gonzalez, L.M.; Rodriguez, C.; Calvo, C.; Lopez, G.; Oehlschlager, A.C. Optimization of a Trap for Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) and Trials to Determine the Effectiveness of Mass Trapping. Neotrop. Entomol. 2013, 42, 448–457. [Google Scholar] [CrossRef]
- Cocco, A.; Deliperi, S.; Delrio, G. Control of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Greenhouse Tomato Crops Using the Mating Disruption Technique. J. Appl. Entomol. 2013, 137, 16–28. [Google Scholar] [CrossRef]
- Vacas, S.; Alfaro, C.; Primo, J.; Navarro-Llopis, V. Studies on the Development of a Mating Disruption System to Control the Tomato Leafminer, Tuta absoluta Povolny (Lepidoptera: Gelechiidae). Pest Manag. Sci. 2011, 67, 1473–1480. [Google Scholar] [CrossRef]
- Cagnotti, C.L.; Andorno, A.V.; Hernández, C.M.; Paladino, L.C.; Botto, E.N.; López, S.N. Inherited Sterility in Tuta absoluta (Lepidoptera: Gelechiidae): Pest Population Suppression and Potential for Combined Use with a Generalist Predator. Florida Entomol. 2016, 99, 87–94. [Google Scholar] [CrossRef]
- Gharekhani, G.H.; Salek-Ebrahimi, H. Life Table Parameters of Tuta absoluta (Lepidoptera: Gelechiidae) on Different Varieties of Tomato. J. Econ. Entomol. 2014, 107, 1765–1770. [Google Scholar] [CrossRef]
- Ecole; Picanço; Guedes; Brommonschenkel. Effect of Cropping Season and Possible Compounds Involved in the Resistance of Lycopersicon hirsutum f Typicum to Tuta absoluta (Meyrick)(Lep Gelechiidae). J. Appl. Entomol. 2001, 125, 193–200. [Google Scholar] [CrossRef]
- Sohrabi, F.; Nooryazdan, H.; Gharati, B.; Saeidi, Z. Evaluation of Ten Tomato Cultivars for Resistance against Tomato Leaf Miner, Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae) under Field Infestation Conditions. Entomol. Gen. 2016, 36, 163–175. [Google Scholar] [CrossRef]
- Leite, G.L.D.; Picanço, M.; Guedes, R.N.C.; Zanuncio, J.C. Role of Plant Age in the Resistance of Lycopersicon hirsutum f. glabratum to the Tomato Leafminer Tuta Absoluta (Lepidoptera: Gelechiidae). Sci. Hortic. 2001, 89, 103–113. [Google Scholar] [CrossRef]
- Pereira, G.V.N.; Maluf, W.R.; Gonçalves, L.D.; do Nascimento, I.R.; Gomes, L.A.A.; Licursi, V. Selection towards High Acylsugar Levels in Tomato Genotypes and Its Relationship with Resistance to Spider Mite (Tetranychus Evansi) and to the South American Pinworm (Tuta absoluta). Ciência e Agrotecnologia 2008, 32, 996–1004. [Google Scholar] [CrossRef]
- Maluf, W.R.; de Fátima Silva, V.; das Graças Cardoso, M.; Gomes, L.A.A.; Neto, Á.C.G.; Maciel, G.M.; Nízio, D.A.C. Resistance to the South American Tomato Pinworm Tuta absoluta in High Acylsugar and/or High Zingiberene Tomato Genotypes. Euphytica 2010, 176, 113–123. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; VanDoorn, A.; Tissier, A.; Kant, M.R.; Prins, M.; de Vos, M.; Haring, M.A.; Schuurink, R.C. Improved Herbivore Resistance in Cultivated Tomato with the Sesquiterpene Biosynthetic Pathway from a Wild Relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, S.M.; Ventura Faria, M.; Maluf, W.R.; Barneche de Oliveira, A.C.; de Freitas, J.A. Zingiberene-Mediated Resistance to the South American Tomato Pinworm Derived from Lycopersicon Hirsutum Var. Hirsutum. Euphytica 2003, 134, 347–351. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Picanço, M.C. The Tomato Borer Tuta absoluta in South America: Pest Status, Management and Insecticide Resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- de Resende, J.T.V.; Maluf, W.R.; Faria, M.V.; Pfann, A.Z.; Nascimento, I.R. do Acylsugars in Tomato Leaflets Confer Resistance to the South American Tomato Pinworm, Tuta absoluta Meyr. Sci. Agric. 2006, 63, 20–25. [Google Scholar] [CrossRef]
- Dias, D.M.; Resende, J.T.V.; Faria, M.V.; Camargo, L.K.P.; Chagas, R.R.; Lima, I.P. Selection of Processing Tomato Genotypes with High Acyl Sugar Content that Are Resistant to the Tomato Pinworm. Genet. Mol. Res. 2013, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mouttet, R.; Kaplan, I.; Bearez, P.; Amiens-Desneux, E.; Desneux, N. Spatiotemporal Patterns of Induced Resistance and Susceptibility Linking Diverse Plant Parasites. Oecologia 2013, 173, 1379–1386. [Google Scholar] [CrossRef]
- Leckie, B.M.; Halitschke, R.; De Jong, D.M.; Smeda, J.R.; Kessler, A.; Mutschler, M.A. Quantitative Trait Loci Regulating the Fatty Acid Profile of Acylsugars in Tomato. Mol. Breed. 2014, 34, 1201–1213. [Google Scholar] [CrossRef]
- Yan, L.; Zhai, Q.; Wei, J.; Li, S.; Wang, B.; Huang, T.; Du, M.; Sun, J.; Kang, L.; Li, C.-B. Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores. PLoS Genet. 2013, 9, e1003964. [Google Scholar] [CrossRef]
- Strapasson, P.; Pinto-Zevallos, D.M.; Paudel, S.; Rajotte, E.G.; Felton, G.W.; Zarbin, P.H.G. Enhancing Plant Resistance at the Seed Stage: Low Concentrations of Methyl Jasmonate Reduce the Performance of the Leaf Miner Tuta absoluta but Do Not Alter the Behavior of Its Predator Chrysoperla externa. J. Chem. Ecol. 2014, 40, 1090–1098. [Google Scholar] [CrossRef]
- De Backer, L.; Megido, R.C.; Fauconnier, M.-L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-Induced Plant Volatiles: Attractiveness towards the Generalist Predator Macrolophus pygmaeus. Arthropod. Plant. Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Mouttet, R.; Bearez, P.; Thomas, C.; Desneux, N. Phytophagous Arthropods and a Pathogen Sharing a Host Plant: Evidence for Indirect Plant-Mediated Interactions. PLoS ONE 2011, 6, e18840. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Fidantsef, A.L.; Duffey, S.S.; Bostock, R.M. Trade-Offs in Plant Defense against Pathogens and Herbivores: A Field Demonstration of Chemical Elicitors of Induced Resistance. J. Chem. Ecol. 1999, 25, 1597–1609. [Google Scholar] [CrossRef]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-Mediated Interactions between Pathogenic Microorganisms and Herbivorous Arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef]
- Bitew, M.K. Significant Role of Wild Genotypes of Tomato Trichomes for Tuta absoluta Resistance. J. Plant Genet. Breed. 2018, 2, 104. [Google Scholar]
- de Almeida, K.C.; de Resende, J.T.V.; Hata, F.T.; Oliveira, L.V.B.; Neto, J.G. Characterization of Solanum Sp. Lycopersicon Section for Density and Types of Leaf Trichomes and Resistance to Whitefly and Tomato Pinworm. Sci. Hortic. 2023, 310, 111746. [Google Scholar] [CrossRef]
- Sridhar, K.; Makroo, H.A.; Srivastava, B. Effect of Cold-and Hot-Break Heat Treatments on the Physicochemical Characteristics of Currant Tomato (Solanum Pimpinellifolium) Pulp and Paste. Foods 2022, 11, 1730. [Google Scholar] [CrossRef] [PubMed]
- Maluf, W.R.; Maciel, G.M.; Gomes, L.A.A.; das Cardoso, M.G.; Gonçalves, L.D.; da Silva, E.C.; Knapp, M. Broad-spectrum Arthropod Resistance in Hybrids between High-and Low-acylsugar Tomato Lines. Crop Sci. 2010, 50, 439–450. [Google Scholar] [CrossRef]
- Silva, D.M.D.; de Bueno, A.F.; Andrade, K.; Stecca, C.D.S.; Neves, P.M.O.J.; de Oliveira, M.C.N. Biology and Nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) Fed on Different Food Sources. Sci. Agric. 2017, 74, 18–31. [Google Scholar] [CrossRef]
- D’Esposito, D.; Manzo, D.; Ricciardi, A.; Garonna, A.P.; De Natale, A.; Frusciante, L.; Pennacchio, F.; Ercolano, M.R. Tomato Transcriptomic Response to Tuta absoluta Infestation. BMC Plant Biol. 2021, 21, 358. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control Failure Likelihood and Spatial Dependence of Insecticide Resistance in the Tomato Pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Roumani, M.; Ropars, A.; Robin, C.; Duval, R.E.; Frippiat, J.-P.; Boisbrun, M.; Larbat, R. Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts. Molecules 2023, 28, 1552. [Google Scholar] [CrossRef]
- Aynalem, B. Empirical Review of Tuta absoluta Meyrick Effect on the Tomato Production and Their Protection Attempts. Adv. Agric. 2022, 2022, 2595470. [Google Scholar] [CrossRef]
- Zannou, A.J.; Romeis, J.; Collatz, J. Response of the Tomato Leaf Miner Phthorimaea Absoluta to Wild and Domesticated Tomato Genotypes. Pest Manag. Sci. 2025, 81, 1345–1359. [Google Scholar] [CrossRef]
- Oliveira, C.M. de Resistência de Linhagens de Tomateiro à Traça Tuta Absoluta, Relacionada a Aleloquímicos e à Densidade de Tricomas. Ciênc. Agrotec. 2013, 36, 45–52. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Shen, Y.; Gao, H.; Zhang, G.; Liu, W.; Jiang, H.; Zhang, Y. Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China. Insects 2024, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.H.P.; Lins, J.C., Jr.; Silva, D.B.; van Lenteren, J.C. Is Predation of Tuta absoluta by Three Neotropical Mirid Predators Affected by Tomato Lines with Different Densities in Glandular Trichomes? Arthropod. Plant. Interact. 2019, 13, 41–48. [Google Scholar] [CrossRef]
- Bottega, D.B.; de Souza, B.H.S.; Rodrigues, N.E.L.; Eduardo, W.I.; Barbosa, J.C.; Júnior, A.L.B. Resistant and Susceptible Tomato Genotypes Have Direct and Indirect Effects on Podisus Nigrispinus Preying on Tuta Absoluta Larvae. Biol. Control 2017, 106, 27–34. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J. Biological Invasion of European Tomato Crops by Tuta absoluta: Ecology, Geographic Expansion and Prospects for Biological Control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Zappala, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arno, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y. Natural Enemies of the South American Moth, Tuta absoluta, in Europe, North Africa and Middle East, and Their Potential Use in Pest Control Strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant Release of Nesidiocoris Tenuis and Supplementary Tactics for Control of Tuta absoluta and Bemisa tabaci in Greenhouse Tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Balzan, M.V.; Wäckers, F.L. Flowers to Selectively Enhance the Fitness of a Host-Feeding Parasitoid: Adult Feeding by Tuta absoluta and Its Parasitoid Necremnus Artynes. Biol. Control 2013, 67, 21–31. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Di Mauro, A.; Tropea Garzia, G.; Russo, A.; Desneux, N.; Siscaro, G. Can Alternative Host Plant and Prey Affect Phytophagy and Biological Control by the Zoophytophagous Mirid Nesidiocoris Tenuis? BioControl 2016, 61, 79–90. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Candian, V.; Psomadelis, I.; Bodino, N.; Tavella, L. The Potential of Host Plants for Biological Control of Tuta absoluta by the Predator Dicyphus Errans. Bull. Entomol. Res. 2017, 107, 340–348. [Google Scholar] [CrossRef]
- Mollá, O.; Biondi, A.; Alonso-Valiente, M.; Urbaneja, A. A Comparative Life History Study of Two Mirid Bugs Preying on Tuta absoluta and Ephestia Kuehniella Eggs on Tomato Crops: Implications for Biological Control. BioControl 2014, 59, 175–183. [Google Scholar] [CrossRef]
- Jaworski, C.C.; Chailleux, A.; Bearez, P.; Desneux, N. Apparent Competition between Major Pests Reduces Pest Population Densities on Tomato Crop, but Not Yield Loss. J. Pest Sci. 2015, 88, 793–803. [Google Scholar] [CrossRef]
- Mollá, O.; González-Cabrera, J.; Urbaneja, A. The Combined Use of Bacillus thuringiensis and Nesidiocoris tenuis against the Tomato Borer Tuta absoluta. BioControl 2011, 56, 883–891. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Ferracini, C.; Gallinotti, D.; Alma, A.; Tavella, L. Evaluation of the Effectiveness of Dicyphus Errans (Wolff) as Predator of Tuta absoluta (Meyrick). Biol. Control 2013, 67, 246–252. [Google Scholar] [CrossRef]
- Abbas, S.; Pérez-Hedo, M.; Colazza, S.; Urbaneja, A. The Predatory Mirid Dicyphus maroccanus as a New Potential Biological Control Agent in Tomato Crops. BioControl 2014, 59, 565–574. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A. Impact of the Zoophytophagous Plant Bug Nesidiocoris tenuis (Heteroptera: Miridae) on Tomato Yield. J. Econ. Entomol. 2008, 101, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Abbes, K.; Chermiti, B. Propensity of Three Tunisian Populations of the Tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae) for Deuterotokous parthenogenetic Reproduction. Afr. Entomol. 2014, 22, 538–544. [Google Scholar] [CrossRef]
- Abbes, K.; Biondi, A.; Kurtulus, A.; Ricupero, M.; Russo, A.; Siscaro, G.; Chermiti, B.; Zappala, L. Combined Non-Target Effects of Insecticide and High Temperature on the Parasitoid Bracon nigricans. PLoS ONE 2015, 10, e0138411. [Google Scholar] [CrossRef]
- Bompard, A.; Jaworski, C.C.; Bearez, P.; Desneux, N. Sharing a Predator: Can an Invasive Alien Pest Affect the Predation on a Local Pest? Popul. Ecol. 2013, 55, 433–440. [Google Scholar] [CrossRef]
- Picanço, M.C.; Bacci, L.; Queiroz, R.B.; Silva, G.A.; Miranda, M.M.M.; Leite, G.L.D.; Suinaga, F.A. Social Wasp Predators of Tuta absoluta. Sociobiology 2011, 58, 621–633. [Google Scholar]
- González-Cabrera, J.; Mollá, O.; Montón, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) in Controlling the Tomato Borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). BioControl 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Contreras, J.; Mendoza, J.E.; Martínez-Aguirre, M.R.; García-Vidal, L.; Izquierdo, J.; Bielza, P. Efficacy of Enthomopathogenic Fungus Metarhizium anisopliae against Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2014, 107, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Borgi, I.; Dupuy, J.-W.; Blibech, I.; Lapaillerie, D.; Lomenech, A.-M.; Rebai, A.; Ksantini, M.; Bonneu, M.; Gargouri, A. Hyper-Proteolytic Mutant of Beauveria bassiana, a New Biological Control Agent against the Tomato Borer. Agron. Sustain. Dev. 2016, 36, 60. [Google Scholar] [CrossRef]
- Batalla-Carrera, L.; Morton, A.; García-del-Pino, F. Efficacy of Entomopathogenic Nematodes against the Tomato Leafminer Tuta absoluta in Laboratory and Greenhouse Conditions. BioControl 2010, 55, 523–530. [Google Scholar] [CrossRef]
- Chailleux, A.; Bearez, P.; Pizzol, J.; Amiens-Desneux, E.; Ramirez-Romero, R.; Desneux, N. Potential for Combined Use of Parasitoids and Generalist Predators for Biological Control of the Key Invasive Tomato Pest Tuta absoluta. J. Pest Sci. 2013, 86, 533–541. [Google Scholar] [CrossRef]
- Chailleux, A.; Wajnberg, E.; Zhou, Y.; Amiens-Desneux, E.; Desneux, N. New Parasitoid-Predator Associations: Female Parasitoids Do Not Avoid Competition with Generalist Predators When Sharing Invasive Prey. Naturwissenschaften 2014, 101, 1075–1083. [Google Scholar] [CrossRef]
- Chailleux, A.; Droui, A.; Bearez, P.; Desneux, N. Survival of a Specialist Natural Enemy Experiencing Resource Competition with an Omnivorous Predator When Sharing the Invasive Prey Tuta absoluta. Ecol. Evol. 2017, 7, 8329–8337. [Google Scholar] [CrossRef]
- Naselli, M.; Biondi, A.; Tropea Garzia, G.; Desneux, N.; Russo, A.; Siscaro, G.; Zappalà, L. Insights into Food Webs Associated with the South American Tomato Pinworm. Pest Manag. Sci. 2017, 73, 1352–1357. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Subramanian, S. Progress in the Commercialization of Entomopathogenic Fungi for Fall Armyworm Control. Insect Sci. 2020, 27, 123–131. [Google Scholar]
- Mawcha, K.T.; Kinyanjui, G.; Berhe, D.H.; Hategekimana, A.; Joelle, K.; Ndolo, D. An Overview of Sustainable Management Strategies for Tuta absoluta. Int. J. Pest Manag. 2025, 1–24. [Google Scholar] [CrossRef]
- Tijjani, A.; Bashir, K.A.; Mohammed, I.; Muhammad, A.; Gambo, A.; Musa, H. Biopesticides for Pests Control: A Review. J. Biopestic. Agric. 2016, 3, 6–13. [Google Scholar]
- Pandey, M.; Bhattarai, N.; Pandey, P.; Chaudhary, P.; Katuwal, D.R.; Khanal, D. A Review on Biology and Possible Management Strategies of Tomato Leaf Miner, Tuta absoluta (Meyrick), Lepidoptera: Gelechiidae in Nepal. Heliyon 2023, 9, e16474. [Google Scholar] [CrossRef]
- Aynalem, B.; Muleta, D.; Venegas, J.; Assefa, F. Isolation, Molecular Characterization and Pathogenicity of Native Bacillus thuringiensis, from Ethiopia, against the Tomato Leafminer, Tuta absoluta: Detection of a New High Lethal Phylogenetic Group. Microbiol. Res. 2021, 250, 126802. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Gerding, M.; France, A. Efectividad de Aislamientos de Hongos Entomopatógenos Sobre Larvas de Polilla Del Tomate Tuta absoluta Meyrick (Lepidoptera: Gelechiidae). Agric. Técnica 2006, 66, 159–165. [Google Scholar] [CrossRef]
- Abdel-Raheem, M.A.; Ismail, I.A.; Abdel-Rahman, R.S.; Abdel-Rhman, I.E.; Naglaa, F.R. Efficacy of Three Entomopathogenic Fungi on Tomato Leaf Miner, Tuta absoluta in Tomato Crop in Egypt. Swift J. Agric. Res. 2015, 1, 15–21. [Google Scholar]
- Tadele, S.; Emana, G. Entomopathogenic Effect of Beauveria bassiana (Bals.) and Metarrhizium anisopliae (Metschn.) on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Larvae under Laboratory and Glasshouse Conditions in Ethiopia. J. Plant Pathol. Microbiol. 2017, 8, 411–414. [Google Scholar]
- Shiberu, T.; Getu, E. Evaluation of Bio-Pesticides on Integrated Management of Tomato Leafminer, Tuta absoluta (Meyrick) (Gelechiidae: Lepidoptera) on Tomato Crops in Western Shewa of Central Ethiopia. Entomol. Ornithol. Herpetol 2018, 7, 10–14. [Google Scholar] [CrossRef]
- Hammad, A.M.A.; Bashir, H.A.A.A.; Abdelbagi, A.O.; Ishag, A.E.S.A.; Ali, M.M.Y.; Bashir, M.O.; Hur, J.-H.; Laing, M.D. Efficacy of Indigenous Entomopathogenic Fungi for the Control of the Tomato Leafminer Tuta absoluta (Meyrick) in Sudan. Int. J. Trop. Insect Sci. 2022, 42, 1449–1459. [Google Scholar] [CrossRef]
- Erol, A.B.; Erdoğan, O.; Karaca, İ. Effects of Some Bioinsecticides on the Tomato Leaf Miner Tuta absoluta (Meyrick 1917) (Lepidoptera: Gelechiidae). Egypt J. Biol. Pest Control 2021, 31, 4. [Google Scholar] [CrossRef]
- Karaca, G.; Erol, A.B.; Çığgın, B.A.; Acarbulut, H.; Karaca, İ. Efficacy of Some Entomopathogenic Fungi against Tomato Leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt J. Biol. Pest Control 2022, 32, 84. [Google Scholar] [CrossRef]
- Chouikhi, S.; Assadi, B.H.; Lebdi, K.; Belkadhi, M.S. Efficacy of the Entomopathogenic Fungi Beauveria bassiana and Lecanicillium muscarium in the Control of the Tomato Leaf Miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 2022, 32, 139. [Google Scholar] [CrossRef]
- Bali, G.K.; Singh, S.K.; Maurya, D.K.; Wani, F.J.; Pandit, R.S. Morphological and Molecular Identification of the Entomopathogenic Fungus Purpureocillium lilacinum and Its Virulence against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Larvae and Pupae. Egypt. J. Biol. Pest Control 2022, 32, 86. [Google Scholar] [CrossRef]
- Zekeya, N.; Mtambo, M.; Ramasamy, S.; Chacha, M.; Ndakidemi, P.A.; Mbega, E.R. First Record of an Entomopathogenic Fungus of Tomato Leafminer, Tuta absoluta (Meyrick) in Tanzania. Biocontrol Sci. Technol. 2019, 29, 626–637. [Google Scholar] [CrossRef]
- Mohamed Mahmoud, F.; Bendebbah, R.; Benssaci, B.; Toudji, F.; Tafifet, L.; Krimi, Z. Entomopathogenic Efficacy of the Endophytic Fungi: Clonostachys sp. and Beauveria bassiana on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Larvae under Laboratory and Greenhouse Conditions. Egypt. J. Biol. Pest Control 2021, 31, 43. [Google Scholar] [CrossRef]
- Gonthier, J.; Arnó, J.; Romeis, J.; Collatz, J. Few Indirect Effects of Baculovirus on Parasitoids Demonstrate High Compatibility of Biocontrol Methods against Tuta absoluta. Pest Manag. Sci. 2023, 79, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Kamali, S.; Karimi, J.; Koppenhöfer, A.M. New Insight into the Management of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae) with Entomopathogenic Nematodes. J. Econ. Entomol. 2018, 111, 112–119. [Google Scholar] [CrossRef]
- Husin, T.O.B.; Port, G.R. Efficacy of Entomopathogenic Nematodes against Tuta absoluta. Biol. Control 2021, 160, 104699. [Google Scholar] [CrossRef]
- Coleman, O. Efficacy of Entomopathogenic Nematodes for Control of Tuta absoluta in South Africa 2020. J. Plant Dis. Prot. 2025, 132, 156. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of Plant Immunity by Microbial Pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Sharma, N.; Bhandari, A.S.; Shukla, P.K. Entomopathogenic Biopesticides: Opportunities and Challenges. In Bio-Management of Postharvest Diseases and Mycotoxigenic Fungi; CRC Press: Boca Raton, FL, USA, 2020; pp. 121–144. [Google Scholar]
- Shalaby, H.H.; Faragalla, F.H.; El-Saadany, H.M.; Ibrahim, A.A. Efficacy of Three Entomopathogenic Agents for Control the Tomato Borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Nat. Sci. 2013, 11, 63–72. [Google Scholar]
- Halder, J.; Kushwaha, D.; Rai, A.B.; Singh, B. Biology and Biorational Management of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): A Global Challenge to Tomato Production. In Proceedings of the Zoological Society; Springer: Berlin/Heidelberg, Germany, 2019; Volume 72, pp. 107–110. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Ma, X.; Qu, C.; Yao, J.; Xia, J.; Luo, C.; Guedes, R.N.C.; Wang, R. Resistance Monitoring of Diamide Insecticides and Characterization of Field-Evolved Chlorantraniliprole Resistance among Chinese Populations of the Tomato Pinworm Phthorimaea Tuta absoluta (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2024, 205, 106140. [Google Scholar] [CrossRef]
- Qureshi, S.A. Pest Management in Tropical Vegetable Systems in Cucurbit Crops 2018. Ph.D. Thesis, La Trobe University, Victoria, ST, Australia, 2018. [Google Scholar]
- Moreno, S.C.; Carvalho, G.A.; Picanço, M.C.; Morais, E.G.F.; Pereira, R.M. Bioactivity of Compounds from Acmella Oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and Selectivity to Two Non-target Species. Pest Manag. Sci. 2012, 68, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Assis, C.P.O.; Ribeiro, L.M.S.; Siqueira, H.A.A. Field-Evolved Resistance and Cross-Resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) Populations to Diamide Insecticides. J. Econ. Entomol. 2016, 109, 2190–2195. [Google Scholar] [CrossRef]
- Calvo, F.J.; Knapp, M.; van Houten, Y.M.; Hoogerbrugge, H.; Belda, J.E. Amblyseius Swirskii: What Made This Predatory Mite Such a Successful Biocontrol Agent? Exp. Appl. Acarol. 2015, 65, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, X.; Shang, L.; Wang, Y.; Haq, I.U.; Wang, J.; Hou, Y. Adaptability Analysis of Tuta absoluta to Different Hosts and Related Salivary Genes Identification. J. Agric. Food Chem. 2025, 73, 2814–2829. [Google Scholar] [CrossRef]
- Schäfer, L.; Herz, A. Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta. Insects 2020, 11, 357. [Google Scholar] [CrossRef]
- Idriss, G.E.A.; Mohamed, S.A.; Khamis, F.; Du Plessis, H.; Ekesi, S. Biology and Performance of Two Indigenous Larval Parasitoids on Tuta absoluta (Lepidoptera: Gelechiidae) in Sudan. Biocontrol Sci. Technol. 2018, 28, 614–628. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Machekano, H.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives. Insects 2020, 11, 764. [Google Scholar] [CrossRef]
- Ferracini, C.; Bueno, V.H.P.; Dindo, M.L.; Ingegno, B.L.; Luna, M.G.; Salas Gervassio, N.G.; Sánchez, N.E.; Siscaro, G.; Van Lenteren, J.C.; Zappalà, L. Natural Enemies of Tuta absoluta in the Mediterranean Basin, Europe and South America. Biocontrol Sci. Technol. 2019, 29, 578–609. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Hill, M.P.; Azrag, A.G.A.; Zalucki, M.P.; Mohamed, S.A. Disentangling Thermal Effects Using Life Cycle Simulation Modelling on the Biology and Demographic Parameters of Dolichogenidea gelechiidivoris, a Parasitoid of Tuta absoluta. J. Therm. Biol. 2022, 107, 103260. [Google Scholar] [CrossRef]
- Lopes, P.C.; French, S.S.; Woodhams, D.C.; Binning, S.A.; Ezenwa, V.; Altizer, S.; Hall, R.J. Infection Avoidance Behaviors across Vertebrate Taxa: Patterns, Processes, and Future Directions. In Animal Behavior and Parasitism; OUP Oxford: Oxford, UK, 2022; Volume 237. [Google Scholar]
- Wang, L.; Keyhani, N.O.; Xia, Y.; Xie, J. The Potential and Limitations of Entomopathogenic Fungi as Biocontrol Agents for Insect Pest Management. Entomol. Gen. 2024, 44, 797–811. [Google Scholar] [CrossRef]
- Amizadeh, M.; Hejazi, M.J.; Niknam, G.; Arzanlou, M. Compatibility and Interaction between Bacillus thuringiensis and Certain Insecticides: Perspective in Management of Tuta absoluta (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 2015, 25, 671–684. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Nayera, Y.S. Evaluations of Three Bacillus thuringiensis against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Egypt. Int. J. Sci. Res. 2014, 3, 2319–7064. [Google Scholar]
- Inanli, C.; Yoldaș, Z.; Birgücü, A.K. Effects of Entomopathogenic Fungi, Beauveria bassiana (Bals.) and Metarhizium anisopliae (Metsch.) on Larvae and Egg Stages of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Plant Pathol. Microbiol. 2012, 8, 239–242. [Google Scholar]
- Giustolin, T.A.; Vendramim, J.D.; Alves, S.B.; Vieira, S.A. Patogenicidade de Beauveria bassiana (Bals.) Vuill. Sobre Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Criada Em Dois Genótipos de Tomateiro. Neotrop. Entomol. 2001, 30, 417–421. [Google Scholar] [CrossRef]
- Sridhar, V.; Wu, S.; Shi, B.; Marathe, A.; Sah, L.P.; Giri, A.P.; Colavito, L.A.; Nitin, K.S.; Asokan, R.; Muniappan, R.M. Modeling Commodity Flow in the Context of Invasive Species Spread: Study of Tuta absoluta in Nepal. Crop Prot. 2017, 135, 104736. [Google Scholar]
- Sabbour, M.M. Biocontrol of the Tomato Pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Egypt. Middle East J. Agric. Res. 2014, 3, 499–503. [Google Scholar]
- Domínguez, A.; López, S.; Bernabé, A.; Guerrero, Á.; Quero, C. Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta absoluta. Insects 2019, 10, 256. [Google Scholar] [CrossRef]
- Tomé, H.V.V.; Cordeiro, E.M.G.; Rosado, J.F.; Guedes, R.N.C. Egg Exposure to Pyriproxyfen in the Tomato Leaf Miner T Uta Absoluta: Ovicidal Activity or Behavioural-modulated Hatching Mortality? Ann. Appl. Biol. 2012, 160, 35–42. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Soliman, M.H.A.; Abd Elaal, A.A. Efficiency of Sex Pheromone Traps and Some Bio and Chemical Insecticides against Tomato Borer Larvae, Tuta absoluta (Meyrick) and Estimate the Damages of Leaves and Fruit Tomato Plant. Ann. Agric. Sci. 2015, 60, 153–156. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Al-Soqeer, A.A. Controlling the 2nd Instar Larvae of Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) by Simmondsin Extracted from Jojoba Seeds in KSA. J. Entomol. 2017, 14, 73–80. [Google Scholar]
- Berxolli, A.; Shahini, S. Azadirachtin, a Useful Alternative for Controlling Tuta Absoluta (Myerick). Eur. J. Phys. Agric. Sci. 2017, 5, 40. [Google Scholar]
- Kona, N.E.M.; Taha, A.K.; Mahmoud, M.E.E. Effects of Botanical Extracts of Neem (Azadirachta indica) and Jatropha (Jatropha curcus) on Eggs and Larvae of Tomato Leaf Miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Persian Gulf Crop Prot. 2014, 3, 41–46. [Google Scholar]
- Ghanim, N.M.; Abdel Ghani, S.B. Controlling Tuta absoluta (Lepidoptera: Gelechiidae) and Aphis gossypii (Hemiptera: Aphididae) by Aqueous Plant Extracts. Life Sci. J. 2014, 11, 299–307. [Google Scholar]
- Sanda, N.; Sunusi, M.; Hamisu, H.; Wudil, B.; Sule, H.; Abdullahi, A. Biological Invasion of Tomato Leaf Miner, Tuta absoluta (Meyrick) in Nigeria: Problems and Management Strategies Optimization: A Review. Asian J. Agric. Hortic. Res. 2018, 1, 1–14. [Google Scholar] [CrossRef]
- Brito, E.F.D.; Baldin, E.L.L.; de Silva, R.C.M.; Ribeiro, L.D.P.; Vendramim, J.D. Bioatividade de Extratos de Piper Sobre Tuta absoluta (Lepidoptera: Gelechiidae) Em Tomateiro. Pesqui. Agropecuária Bras. 2015, 50, 196–202. [Google Scholar] [CrossRef]
- Arati Joshi, A.J.; Thapa, R.B.; Dharmendra Kalauni, D.K. Integrated Management of South American Tomato Leaf Miner [Tuta absoluta (Meyrick)]: A Review. J. Plant Prot. Soc. 2018, 5, 70–86. [Google Scholar] [CrossRef]
- Bastola, A.; Pandey, S.R.; Khadka, A.; Regmi, R. Efficacy of Commercial Insecticides against Tomato Leaf Miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Palpa, Nepal. Turkish J. Agric. Sci. Technol. 2020, 8, 2388–2396. [Google Scholar] [CrossRef]
- Simkhada, R.; Thapa, R.B.; Bajracharya, A.S.R.; Regmi, R. Efficacy of Novel Insecticides Against South American Tomato Leaf Miner (Tuta absoluta Meyrick) Under Plastic House Conditions in Kathmandu, Nepal. J. Agric. For. Univ. 2018, 2, 133–140. [Google Scholar]
- Braham, M.; Hajji, L. Management of Tuta Absoluta (Lepidoptera, Gelechiidae) with Insecticides on Tomatoes. In Insecticides Pest Engineering; Intech Open Acces Publisher: Rijeka, Croatia, 2012; pp. 333–354. [Google Scholar]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; del Rosario Martinez-Aguirre, M.; Bielza, P.; Morou, E. Ryanodine Receptor Point Mutations Confer Diamide Insecticide Resistance in Tomato Leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.G.; Barbieri, F.S.; Rocha, R.B.; Santos, A.P.L.; Silva, R.R.; Ribeiro, E.S.; Guerrero, F.; Foil, L.; Oliveira, M.C.S. Pyrethroid and Organophosphate Pesticide Resistance in Field Populations of Horn Fly in Brazil. Med. Vet. Entomol. 2019, 33, 121–130. [Google Scholar] [CrossRef]
- Sparks, T.C.; Dripps, J.E.; Watson, G.B.; Paroonagian, D. Resistance and Cross-Resistance to the Spinosyns—A Review and Analysis. Pestic. Biochem. Physiol. 2012, 102, 1–10. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Using Geographic Information Systems and Decision Support Systems for the Prediction, Prevention, and Control of Vector-Borne Diseases. Annu. Rev. Entomol. 2011, 56, 41–61. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Indirect Effect of Pesticides on Insects and Other Arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sun, S.; Tan, H.; Sun, X.; Shang, D.; Yao, C.; Qin, C.; Ji, S.; Li, X.; Zhang, J. Compatibility of Chlorantraniliprole with the Generalist Predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) Based Toxicity, Life-Cycle Development and Population Parameters in Laboratory Microcosms. Chemosphere 2019, 225, 182–190. [Google Scholar] [CrossRef]
- Abegão, K.G.B.; Bracale, B.N.; Delfim, I.G.; Santos, E.S.D.; Laposy, C.B.; Nai, G.A.; Giuffrida, R.; Nogueira, R.M.B. Effects of Heterologous Platelet-Rich Plasma Gel on Standardized Dermal Wound Healing in Rabbits. Acta Cir. Bras. 2015, 30, 209–215. [Google Scholar] [CrossRef]
- Ullah, F.; Ullah, Z.; Gul, H.; Li, X.; Pan, Y.; Zhang, H.; Zhang, Z.; Huang, J.; Emmanouil, R.; Guedes, R.N.C. Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole. Int. J. Mol. Sci. 2025, 26, 5180. [Google Scholar] [CrossRef]
- Alemu, M. Trend of Biotechnology Applications in Pest Management: A Review. Int. J. Appl. Sci. Biotechnol. 2020, 8, 108–131. [Google Scholar] [CrossRef]
- Pan, X.; Guo, X.; Zhai, T.; Zhang, D.; Rao, W.; Cao, F.; Guan, X. Nanobiopesticides in Sustainable Agriculture: Developments, Challenges, and Perspectives. Environ. Sci. Nano 2023, 10, 41–61. [Google Scholar] [CrossRef]
- Hadapad, A.B.; Hire, R.S. Molecular Characterisation of Tomato Leaf Miner Tuta absoluta Populations Obtained from Different Geographical Locations of India. J. Biol. Control 2019, 33, 147–154. [Google Scholar] [CrossRef]
- Marec, F.; Vreysen, M.J.B. Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, E.C.; Hendrichs, J. History of the Sterile Insect Technique. In Sterile Insect Technique; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–44. [Google Scholar]
- Zhou, S.; Li, X.; Zhang, J.; Liu, C.; Huang, J.; Zhang, Z.; Ren, X.; Chen, L.; Han, P.; Wang, B. Screening the Optimal Dose of Gamma Radiation for Tuta absoluta Sterility: Paving the Way for Sterile Insect Technique Programs. Entomol. Gen. 2024, 44, 415–422. [Google Scholar] [CrossRef]
- Cagnotti, C.; Conte, C.; Kramar, J.; Lanzavecchia, S.; López, S. Molecular Detection of Reproductive Symbionts and Parthenogenesis Experiments in Tuta absoluta from Argentina: Facing Potential for Sustainable and Specific Pest Control Strategies. Entomol. Exp. Appl. 2023, 171, 681–690. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-Based Pest Control: Production, Application and the Fate of DsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.A.; Barbosa, G.O.; Possignolo, I.P.; Peres, L.E.P.; Lam, E.; Lima, J.E.; Figueira, A.; Marques-Souza, H. RNA Interference as a Gene Silencing Tool to Control Tuta absoluta in Tomato (Solanum Lycopersicum). PeerJ 2016, 4, e2673. [Google Scholar] [CrossRef]
- de Camargo, R.A.; Herai, R.H.; Santos, L.N.; Bento, F.M.M.; Lima, J.E.; Marques-Souza, H.; Figueira, A. De Novo Transcriptome Assembly and Analysis to Identify Potential Gene Targets for RNAi-Mediated Control of the Tomato Leafminer (Tuta absoluta). BMC Genomics 2015, 16, 635. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Bandani, A.R. Caspase Gene Silencing Affects the Growth and Development of Tuta Absoluta. Biocatal. Agric. Biotechnol. 2021, 34, 102044. [Google Scholar] [CrossRef]
- Yang, W.-J.; Yan, X.; Han, P.; Wang, M.; Zhang, C.; Song, J.-H.; Zhang, G.-F.; Zhang, Y.-B.; Wan, F.-H. Ovarian Development and Role of Vitellogenin Gene in Reproduction of the Tomato Leaf Miner Tuta absoluta. Entomol. Gen. 2024, 44, 423–432. [Google Scholar] [CrossRef]
- Ji, S.-X.; Bi, S.-Y.; Wang, X.-D.; Wu, Q.; Tang, Y.-H.; Zhang, G.-F.; Wan, F.-H.; Lü, Z.-C.; Liu, W.-X. First Report on CRISPR/Cas9-Based Genome Editing in the Destructive Invasive Pest Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Front. Genet. 2022, 13, 865622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, B.; Zhang, G.; Qi, X.; Cao, S.; Akami, M.; Huang, Y.; Niu, C. Mutation of Bdpaired Induces Embryo Lethality in the Oriental Fruit Fly, Bactrocera Dorsalis. Pest Manag. Sci. 2020, 76, 944–951. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.A.N.; Aumann, R.A.; Haecker, I.; Schetelig, M.F. CRISPR-Based Genetic Control Strategies for Insect Pests. J. Integr. Agric. 2023, 22, 651–668. [Google Scholar]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M. Enhancement of Tomato Resistance to Tuta absoluta Using a New Efficient Mesoporous Silica Nanoparticle-Mediated Plant Transient Gene Expression Approach. Sci. Hortic. 2019, 243, 367–375. [Google Scholar] [CrossRef]

| Microbial Agents | Insect Stage | References |
|---|---|---|
| Beauveria bassiana | Just hatched to the fourth instar | Giustolin et al. (2001) [132] |
| Metarhizium anisopliae | Larva and pupa | Sridhar et al. 2017 [133] |
| Bacillus thuringiensis var. kurstaki | Newly hatched, second, and third instar larvae | Shalaby et al. (2013) [113] |
| Beauveria bassiana Bals. Criv. Metarhizium anisopliae (Metchnikoff) Sorokın | Third instar larvae | Sabbour (2014) [134] |
| Bacillus thuringiensis Berliner | Second instar larvae | Halder et al. (2019) [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, A.; Ullah, F.; Akhtar, M.R.; Humza, M.; Ghafar, M.A.; Hyder, M.; Haq, I.U.; Hou, Y. Transforming Tuta absoluta Management: A Synergistic Approach Integrating Sustainability, Biological Control, and Biotechnological Innovations. Insects 2025, 16, 1173. https://doi.org/10.3390/insects16111173
Basit A, Ullah F, Akhtar MR, Humza M, Ghafar MA, Hyder M, Haq IU, Hou Y. Transforming Tuta absoluta Management: A Synergistic Approach Integrating Sustainability, Biological Control, and Biotechnological Innovations. Insects. 2025; 16(11):1173. https://doi.org/10.3390/insects16111173
Chicago/Turabian StyleBasit, Abdul, Farman Ullah, Muhammad Rehan Akhtar, Muhammad Humza, Muhammad Adeel Ghafar, Moazam Hyder, Inzamam Ul Haq, and Youming Hou. 2025. "Transforming Tuta absoluta Management: A Synergistic Approach Integrating Sustainability, Biological Control, and Biotechnological Innovations" Insects 16, no. 11: 1173. https://doi.org/10.3390/insects16111173
APA StyleBasit, A., Ullah, F., Akhtar, M. R., Humza, M., Ghafar, M. A., Hyder, M., Haq, I. U., & Hou, Y. (2025). Transforming Tuta absoluta Management: A Synergistic Approach Integrating Sustainability, Biological Control, and Biotechnological Innovations. Insects, 16(11), 1173. https://doi.org/10.3390/insects16111173










