Effects of Low Benzoic Acid Concentrations on Growth and Substrate Utilization in Black Soldier Fly Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. BSF Larvae
2.2. Feed Substrates
2.3. Rearing Experiments
2.4. CO2 Production Rates
2.5. Analytical Procedures
2.6. Growth Model
2.7. Performance Indicators
2.8. Conversion Factors and Statistical Analysis
3. Results and Discussion
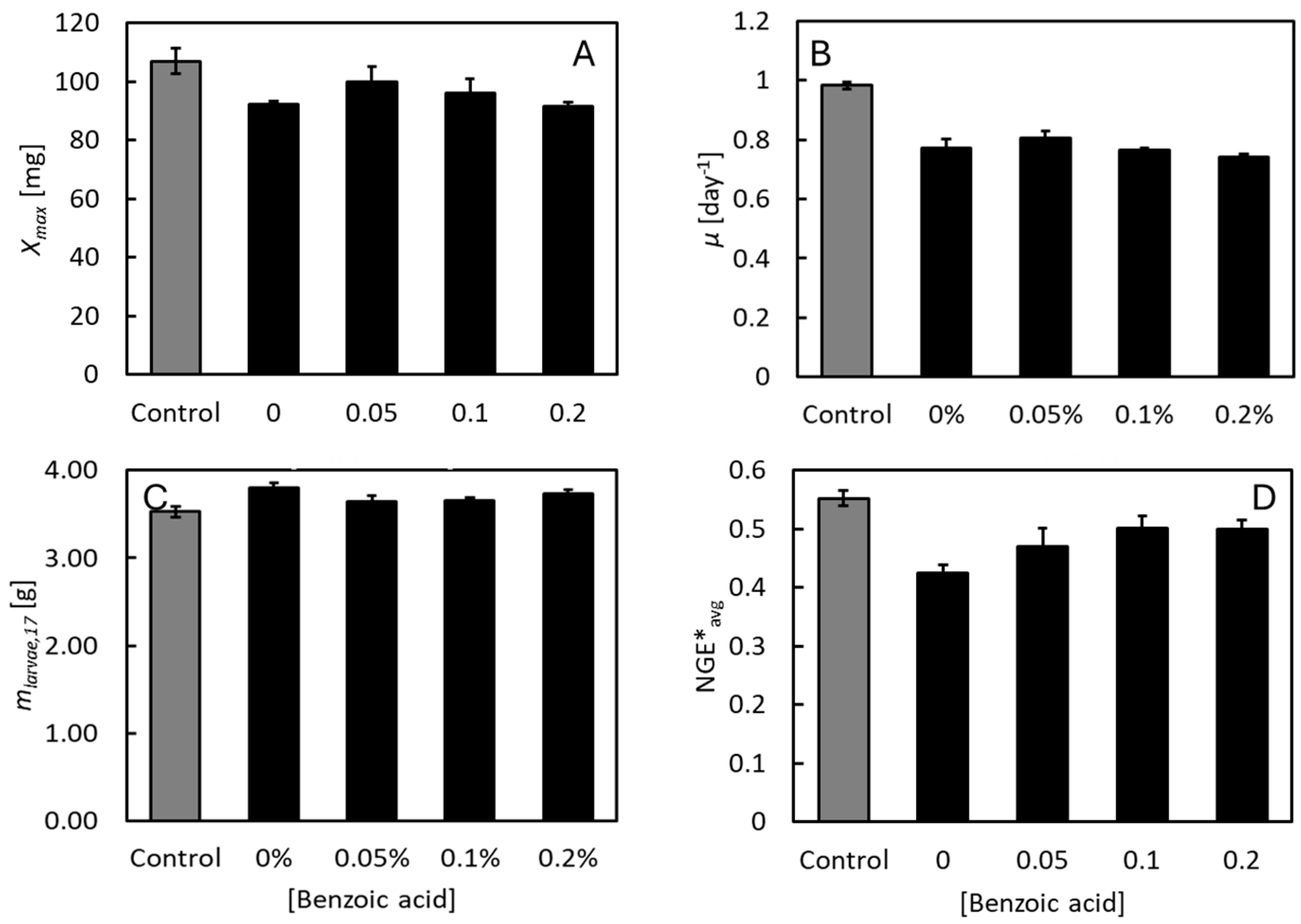
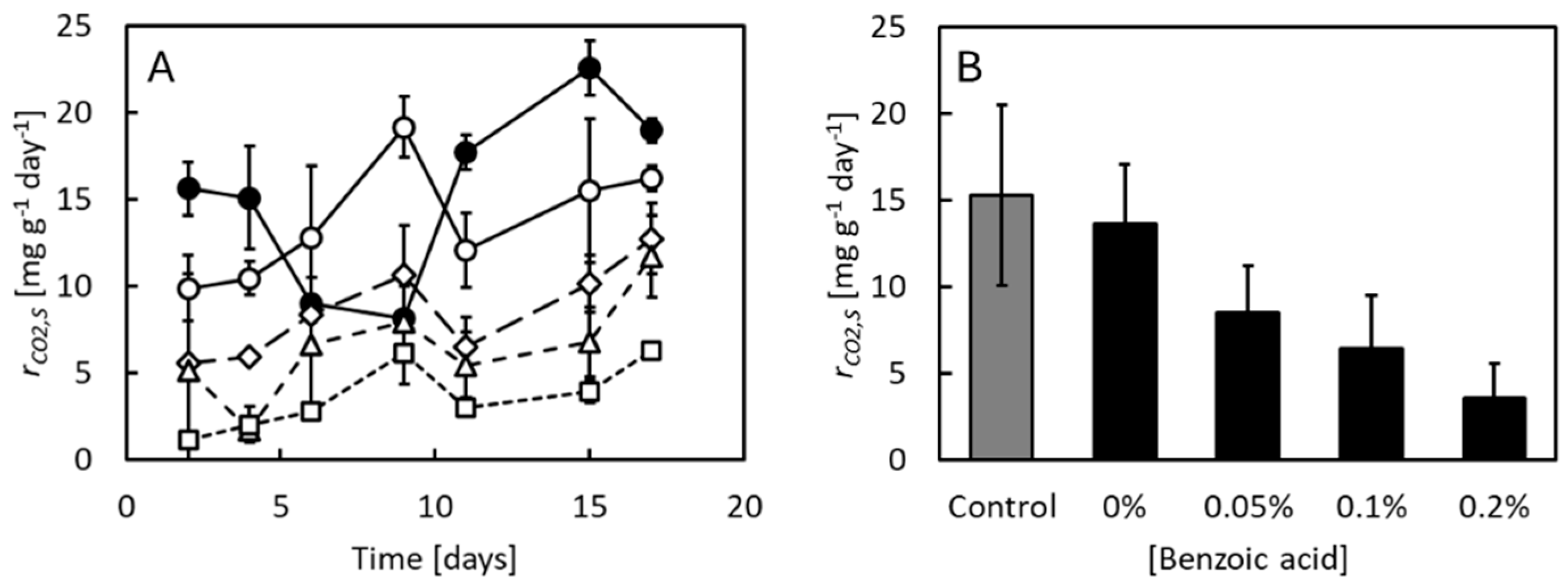
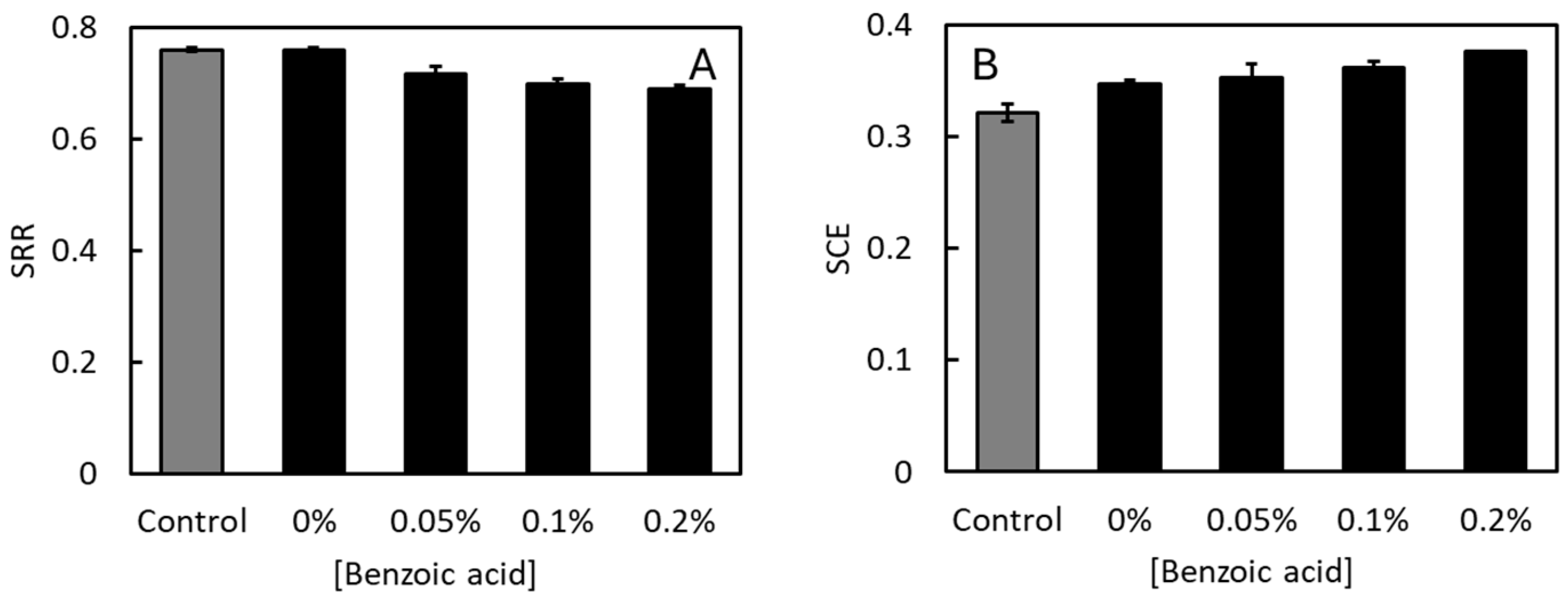
3.1. Effects of Benzoic Acid on Growth and Feed Efficiency at pH 4
3.2. Effects of Benzoic Acid on Growth and Feed Efficiency at Neutral pH
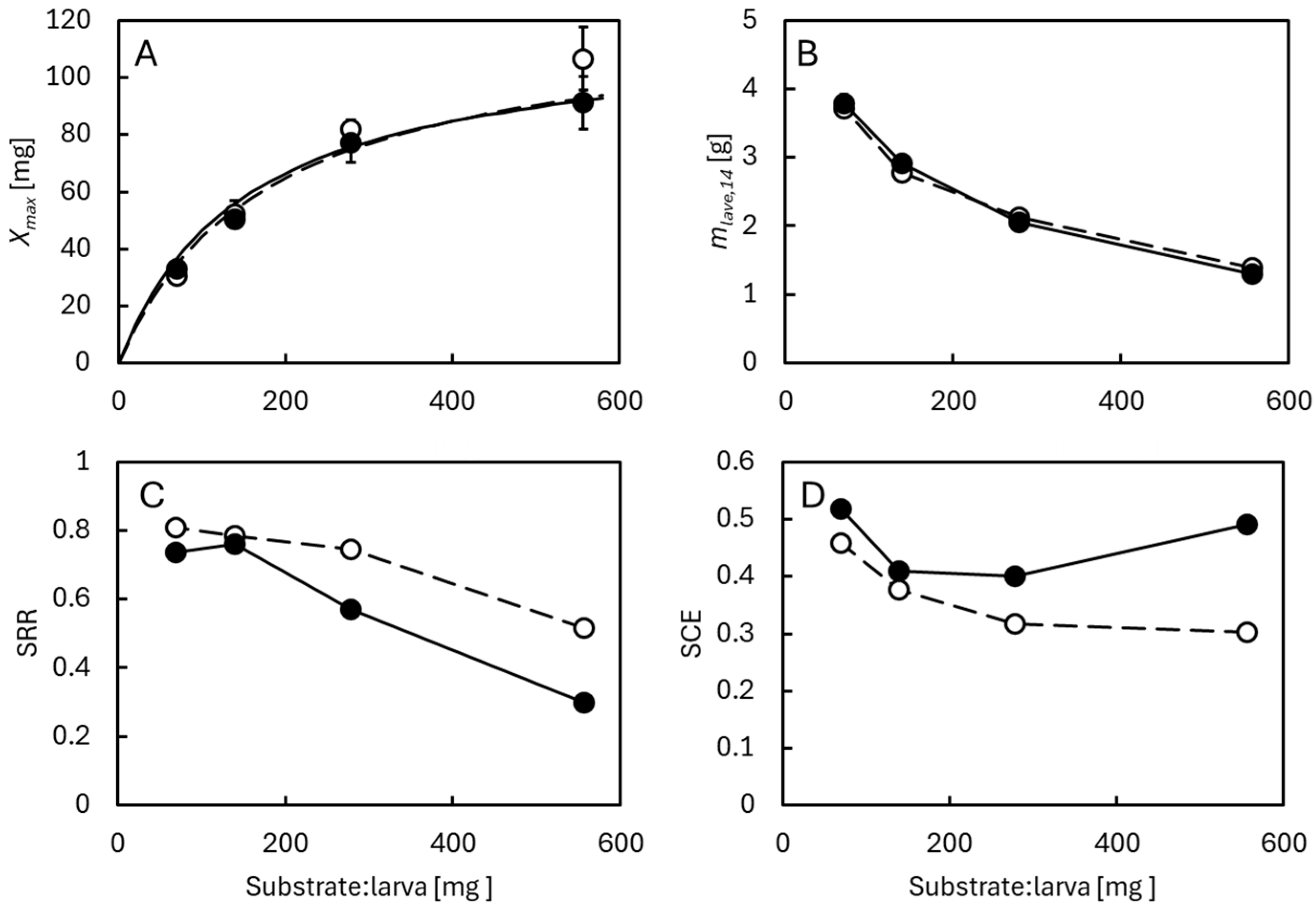
3.3. Effects of Larval Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caparros Megido, R.; Francis, F.; Haubruge, E.; Le Gall, P.; Tomberlin, J.K.; Miranda, C.D.; Jordan, H.R.; Picard, C.J.; Pino, M.J.M.; Ramos-Elordy, J.; et al. A Worldwide Overview of the Status and Prospects of Edible Insect Production. Entomol. Gen. 2024, 44, 3–27. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Influence of Larval Density and Dietary Nutrient Concentration on Performance, Body Protein, and Fat Contents of Black Soldier Fly Larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Guillaume, J.B.; Mezdour, S.; Marion-Poll, F.; Terrol, C.; Schmidely, P. Asymptotic Estimated Digestibility, a New Indicator of Black Soldier Fly (Hermetia Illucens) Conversion Efficiency in Relation to Larval Density. J. Insects Food Feed 2023, 9, 893–906. [Google Scholar] [CrossRef]
- Muurmann, A.T.; Eriksen, N.T.; Jacobsen, J.A.; Limborg, M.T.; Tomberlin, J.K.; Gilbert, M.T.P.; Bahrndorff, S. Growth and Metabolic Performance of House Fly and Black Soldier Fly Larvae Differ across Densities and Waste-Based Growth Substrates. Waste Manag. 2025, 193, 529–538. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste Treatment with Black Soldier Fly Larvae: Increasing Performance through the Formulation of Biowastes Based on Protein and Carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Laganaro, M.; Bahrndorff, S.; Eriksen, N.T. Growth and Metabolic Performance of Black Soldier Fly Larvae Grown on Low and High-Quality Substrates. Waste Manag. 2021, 121, 198–205. [Google Scholar] [CrossRef]
- Nielsen, F.K.; Hansen, R.J.; Muurmann, A.T.; Bahrndorff, S.; Eriksen, N.T. Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products. Animals 2025, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, K.; Ur Rehman, R.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Ur Rehman, A.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced Bioconversion of Dairy and Chicken Manure by the Interaction of Exogenous Bacteria and Black Soldier Fly Larvae. J. Environ. Manag. 2019, 237, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xie, C.; Zhang, Z.; Huang, Z.; Zhou, J.; Wang, C. Microbial Fermentation and Black Soldier Fly Feeding to Enhance Maize Straw Degradation. Chemosphere 2024, 353, 141498. [Google Scholar] [CrossRef]
- Memon, F.U.; Zhu, Y.; Cui, Y.; Feng, X.; Ahmad, S.; Zeng, P.; Nabi, F.; Hao, D.; Huang, Z.; Tettamanti, G.; et al. Gut Microbial Communities and Transcriptional Profiles of Black Soldier Fly (Hermitia illucens) Larvae Fed on Fermented Sericulture Waste. Waste Manag. 2025, 194, 158–168. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of Substrate Moisture Content on Growth and Metabolic Performance of Black Soldier Fly Larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Gold, M.; Loh, R.K.; Chu, C.X.; Haberkorn, I.; Puniamoorthy, N.; Mathys, A. Physical Properties of Food Waste Influence the Efficiency of Black Soldier Fly Larvae Bioconversion via Microbial Activity. J. Environ. Manag. 2025, 387, 125777. [Google Scholar] [CrossRef]
- Knudsen, A.K.S.; Jespersen, E.E.; Markwardt, M.J.; Johansen, A.; Ortind, A.P.; Terp, M.; Eriksen, N.T. Effects of Fish Oil on Growth Kinetics and Lipid Accumulation in Black Soldier Fly Larve. J. Insects Food Feed 2022, 8, 237–244. [Google Scholar] [CrossRef]
- Eriksen, N.T. Metabolic Performance and Feed Efficiency of Black Soldier Fly Larvae. Front. Bioeng. Biotechnol. 2024, 12, 1397108. [Google Scholar] [CrossRef]
- Heussler, C.D.; Insam, H.; Walter, A.; Steiner, B.C.S.; Steiner, F.M.; Klammsteiner, T. Life-History Traits of Black Soldier Fly Reared on Agro-Industrial By-Products Subjected to Three Pre-Treatments: A Pilot-Scale Study. J. Insects Food Feed 2023, 9, 545–556. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Heussler, C.D.; Insam, H.; Schlick-Steiner, B.C.; Steiner, F.M. Larval Density Drives Thermogenesis and Affects Microbiota and Substrate Properties in Black Soldier Fly Trials. iScience 2025, 28, 112794. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Malik, T. Exploring the Untapped Potential of Naturally Occurring Antimicrobial Compounds: Novel Advancements in Food Preservation for Enhanced Safety and Sustainability. Front. Sustain. Food Syst. 2024, 8, 1307210. [Google Scholar] [CrossRef]
- Eklund, T. Inhibition of Microbial Growth at Different pH Levels by Benzoic and Propionic Acids and Esters of P-Hydroxybenzoic Acid. Int. J. Food Microbiol. 1985, 2, 159–167. [Google Scholar] [CrossRef]
- Krebs, H.A.; Wigginst, D.; Stubbs, M.; Sols, A.; Bedoyat, F. Studies on the Mechanism of the Antifungal Action of Benzoate. Biochem. J. 1983, 214, 657–663. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic Acid and Its Derivatives as Naturally Occurring Compounds in Foods and as Additives: Uses, Exposure, and Controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.D.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and Efficacy of Benzoic Acid for Pigs and Poultry. EFSA J. 2018, 16, e05210. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M.K.; Lopez-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and Efficacy of Benzoic Acid as a Technological Feed Additive for Weaned Piglets and Pigs for Fattening. EFSA J. 2019, 17, e05527. [Google Scholar] [CrossRef]
- Libanori, M.C.M.; Santos, G.G.; Pereira, S.A.; Ferrarezi, J.V.S.; Ferreira, M.B.; Cardoso, L.; Costa, D.S.; Fernandes, M.; Gomes, K.A.; Tedesco, M.; et al. Organic Benzoic Acid Modulates Health and Gut Microbiota of Oreochromis niloticus. Aquaculture 2023, 570, 739409. [Google Scholar] [CrossRef]
- Amaechi, N.; Anueyiagu, C. The effect of dietary benzoic acid supplementation on growth performance and intestinal wall morphology of broilers. Online J. Anim. Feed Res. 2012, 2, 401–404. [Google Scholar]
- Zhang, W.; Sun, S.; Zhang, Y.; Zhang, Y.; Wang, J.; Liu, Z.; Yang, K. Benzoic Acid Supplementation Improves the Growth Performance, Nutrient Digestibility and Nitrogen Metabolism of Weaned Lambs. Front. Vet. Sci. 2024, 11, 1351394. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Effects of Dietary Supplementation with Benzoic Acid on Intestinal Morphological Structure and Microflora in Weaned Piglets. Livest. Sci. 2014, 167, 249–256. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. The Impact of Acids Approved for Use in Foods on the Vitality of Haemonchus contortus and Strongyloides papillosus (Nematoda) Larvae. Helminthologia 2019, 56, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ding, Z.; Song, L.; Zhang, D.; Xie, C.; Zhang, S.; Feng, L.; Liu, H.; Pang, Q. Sodium Benzoate Delays the Development of Drosophila melanogaster Larvae and Alters Commensal Microbiota in Adult Flies. Front. Microbiol. 2022, 13, 911928. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ghosh, S.K.; Paik, S.; Chakravarty, A.; Basak, A.K. Benzoic Acid Treated Drosophila melanogaster: The Genetic Disruption of Larval Brain Stem Cells and Non-Neural Cells during Metamorphosis. Toxicol. Environ. Health Sci. 2021, 13, 215–223. [Google Scholar] [CrossRef]
- Sookar, P.; Alleck, M.; Ahseek, N.; Permalloo, S.; Bhagwant, S.; Chang, C.L. Artificial Rearing of the Peach Fruit Fly Bactrocera zonata (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S99–S107. [Google Scholar] [CrossRef]
- Aslam, I.; Ahsan Khan, M.; Ahmad, T.; Gul, T.; Nazeer, N.; Aslam, F.; Shehzadi, K. Feeding Potential of Fruit Fly (Diptera: Tephritidae) on Different Artificial Diets under Laboratory Conditions. Acta Sci. Agric. 2019, 3, 109–113. [Google Scholar] [CrossRef]
- Ma, J.; Lei, Y.; Rehman, K.U.; Yu, Z.; Zhang, J.; Li, W.; Li, Q.; Tomberlin, J.K.; Zheng, L. Dynamic Effects of Initial pH of Substrate on Biological Growth and Metamorphosis of Black Soldier Fly (Diptera: Stratiomyidae). Environ. Entomol. 2018, 47, 159–165. [Google Scholar] [CrossRef]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and Feeding System on Black Soldier Fly (Hermetia illucens, L; Diptera: Stratiomyidae) Larval Development. PLoS ONE 2018, 13, e0202591. [Google Scholar] [CrossRef]
- Deruytter, D.; Gasco, L.; Yakti, W.; Katz, H.; Coudron, C.L.; Gligorescu, A.; Frooninckx, L.; Noyens, I.; Meneguz, M.; Grosso, F.; et al. Standardising Black Soldier Fly Larvae Feeding Experiments: An Initial Protocol and Variability Estimates. J. Insects Food Feed. 2024, 10, 1685–1696. [Google Scholar] [CrossRef]
- Roels, J.A. Application of Macroscopic Principles to Microbial Metabolism. Biotechnol. Bioeng. 1980, 22, 2457–2514. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Hansen, R.J.; Nielsen, S.H.M.; Johansen, M.; Nielsen, F.K.; Dragsbæk, F.B.; Sørensen, O.S.B.; Eriksen, N.T. Metabolic Performance of Black Soldier Fly Larvae during Entomoremediation of Brewery Waste. J. Appl. Entomol. 2023, 147, 423–431. [Google Scholar] [CrossRef]
- Eriksen, N.T. Dynamic Modelling of Feed Assimilation, Growth, Lipid Accumulation, and CO2 Production in Black Soldier Fly Larvae. PLoS ONE 2022, 17, e0276605. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.H.; MacCluer, C.R. Hyperbolic Saturation. Bull. Math. Biol. 2010, 72, 1315–1322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christiansen, T.B.; Eriksen, N.T. Effects of Low Benzoic Acid Concentrations on Growth and Substrate Utilization in Black Soldier Fly Larvae. Insects 2025, 16, 1155. https://doi.org/10.3390/insects16111155
Christiansen TB, Eriksen NT. Effects of Low Benzoic Acid Concentrations on Growth and Substrate Utilization in Black Soldier Fly Larvae. Insects. 2025; 16(11):1155. https://doi.org/10.3390/insects16111155
Chicago/Turabian StyleChristiansen, Thor Brødsted, and Niels Thomas Eriksen. 2025. "Effects of Low Benzoic Acid Concentrations on Growth and Substrate Utilization in Black Soldier Fly Larvae" Insects 16, no. 11: 1155. https://doi.org/10.3390/insects16111155
APA StyleChristiansen, T. B., & Eriksen, N. T. (2025). Effects of Low Benzoic Acid Concentrations on Growth and Substrate Utilization in Black Soldier Fly Larvae. Insects, 16(11), 1155. https://doi.org/10.3390/insects16111155







