Toxicity Evaluation of Potassium Sorbate In Vivo with Drosophila Melanogaster
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks
2.2. Immunochemistry Staining
2.3. Lifespan Assay
2.4. Fecundity Assay
2.5. Fly Gut Acidity Assay
2.6. Trypan Blue Staining
2.7. ROS Detection
2.8. Notch Signaling Detection
2.9. Real-Time Quantitative PCR
2.10. Statistical Analysis
3. Results
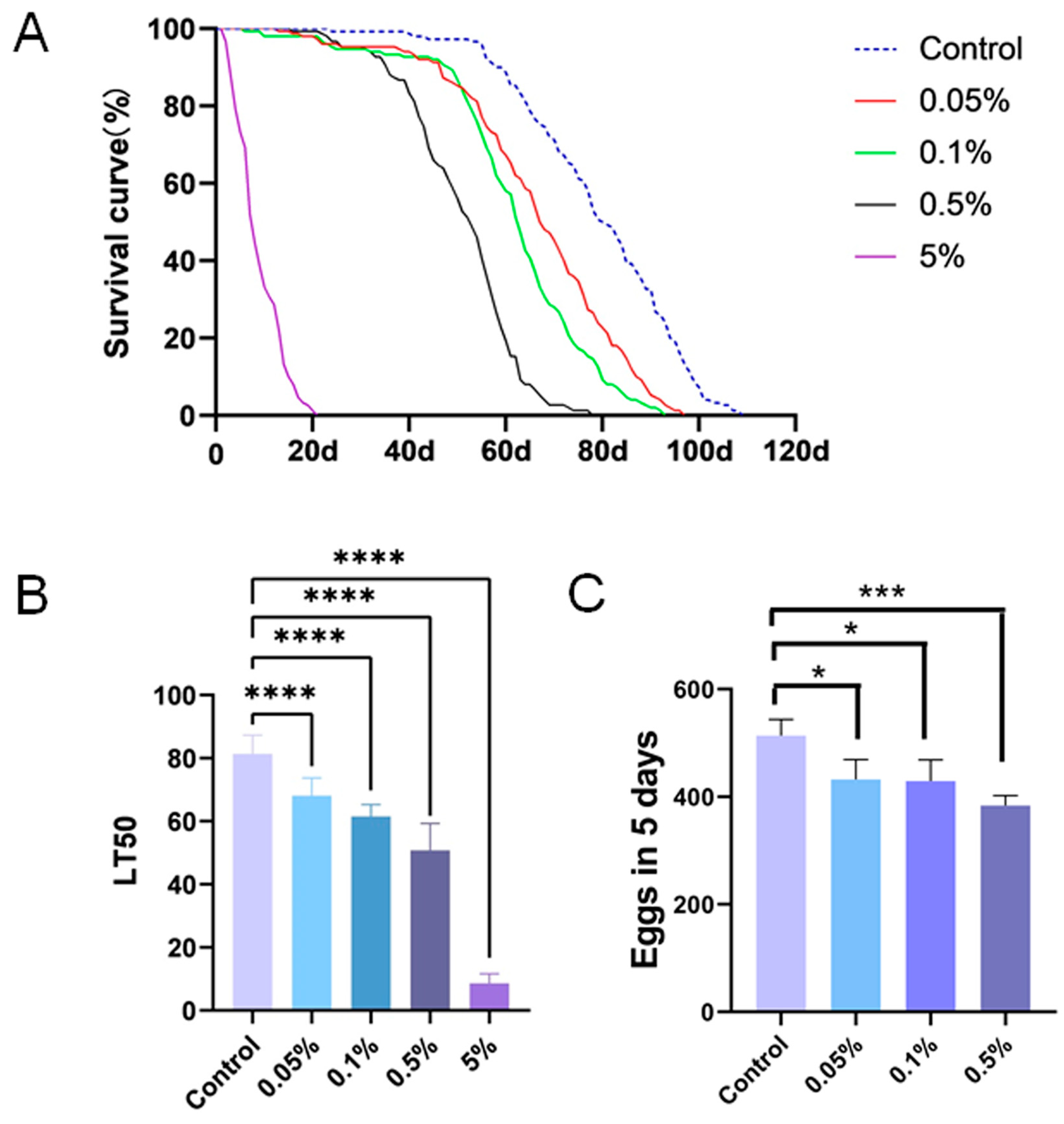
3.1. PS Ingestion Reduced the Lifespan and Fecundity of Drosophila
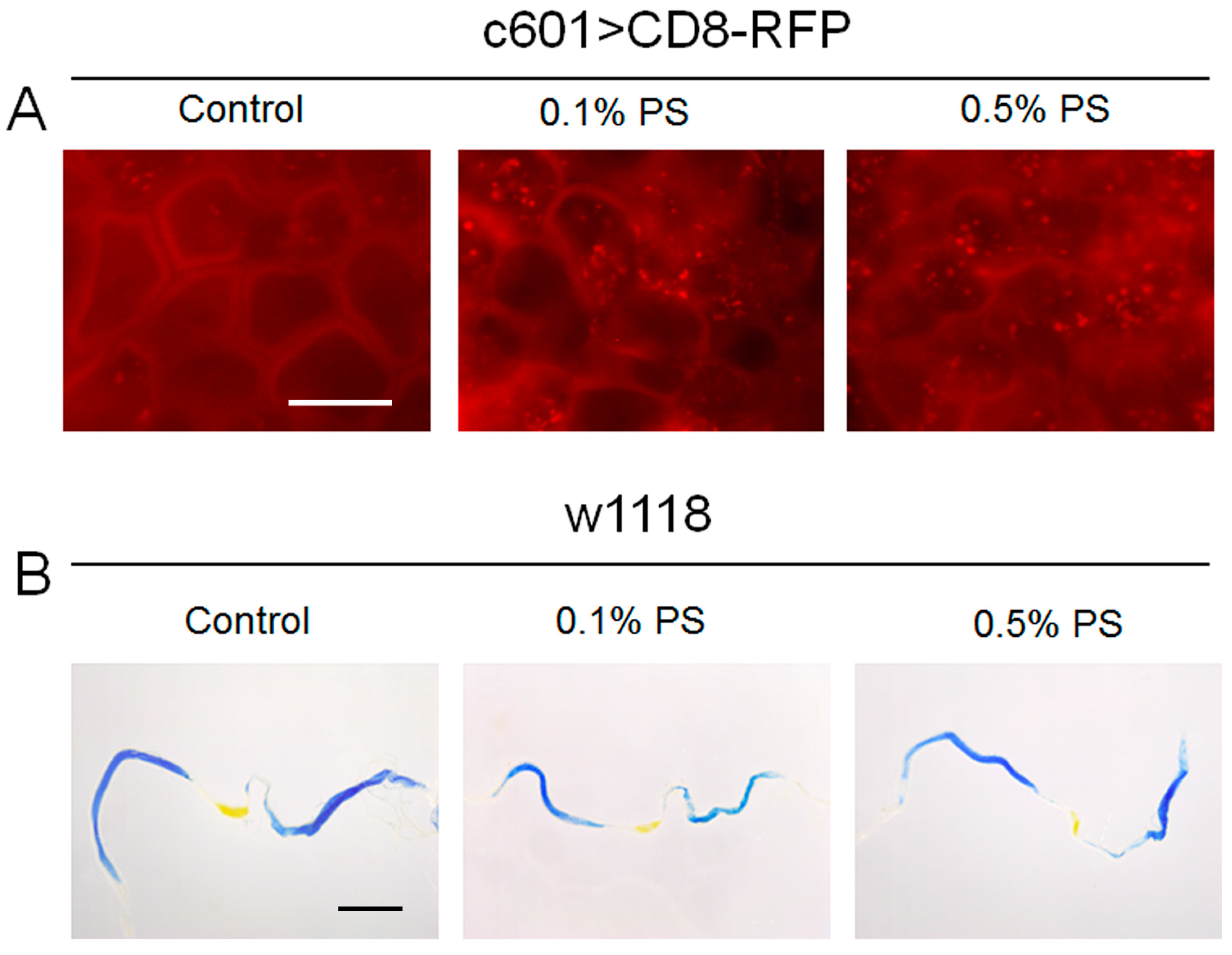
3.2. The PS Ingestion Disrupted Cell Membrane Integrity but Did Not Alter Midgut pH
3.3. PS Ingestion Induced Cell Apoptosis in the Midgut
3.4. PS Ingestion Induced Elevated Level of ROS in the Midgut
3.5. PS Ingestion Enhanced Mitophagy in the Midgut
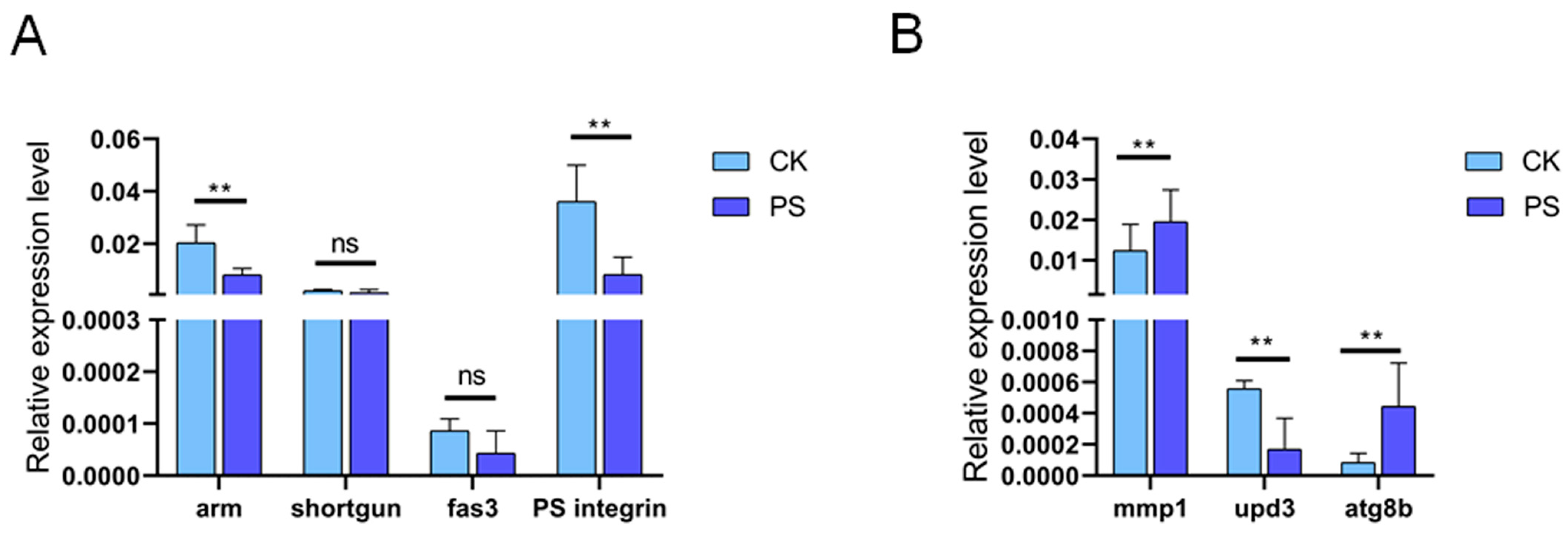
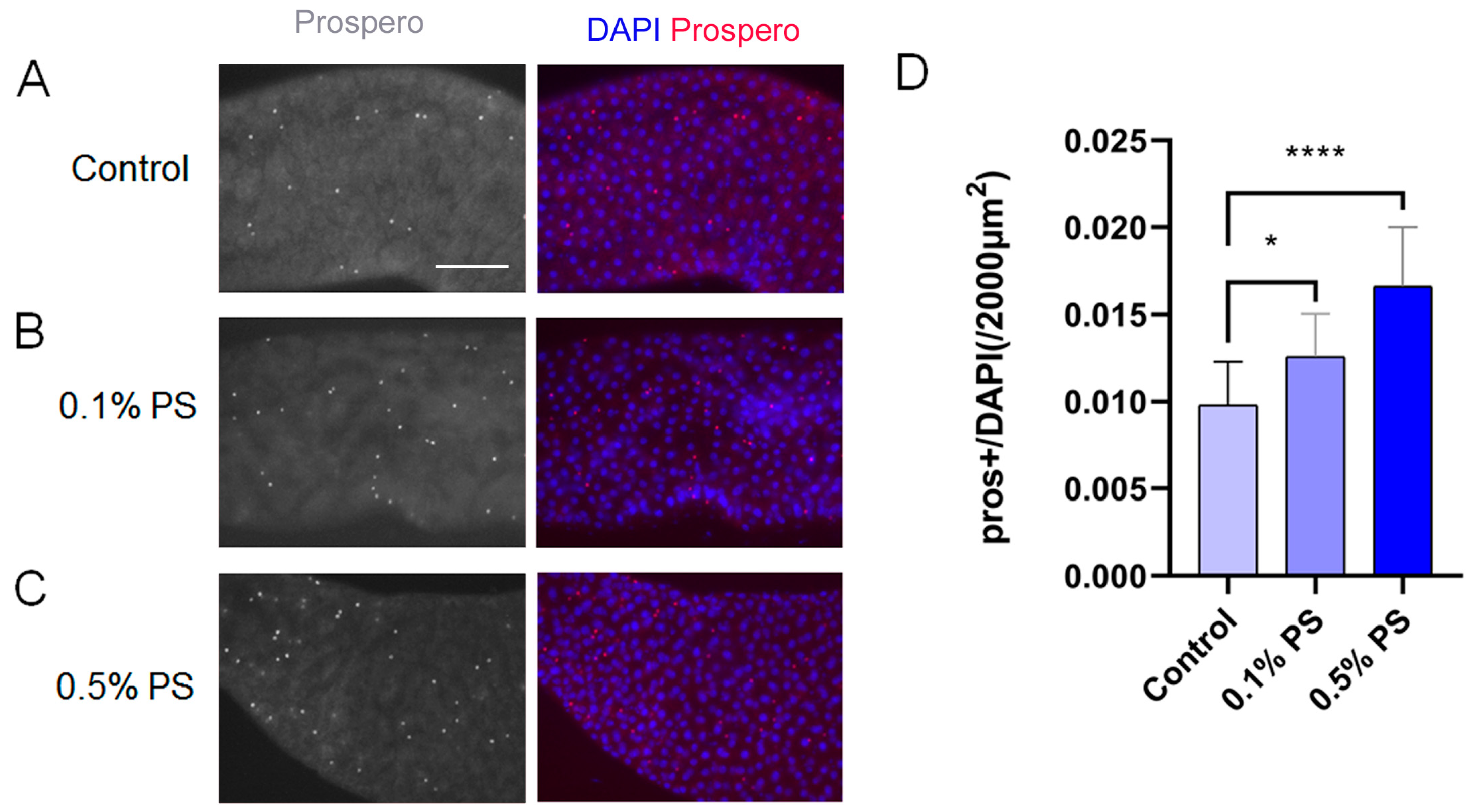
3.6. PS Ingestion Altered ISC Differentiation Trajectory
3.7. Decreased Notch Expression Was a Potential Cause of the Observed Changes in Cell Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Güngörmüş, C.; Kılıç, A. The Safety Assessment of Food Additives by Reproductive and Developmental Toxicity Studies. Food Addit. 2012, 31–48. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Zhou, P.; Mandal, J.; Srivastava, P.; Hou, D.; Edussuriya, R.; Vithanage, M.; Truong, V.K.; et al. The distribution, fate, and environmental impacts of food additive nanomaterials in soil and aquatic ecosystems. Sci. Total Environ. 2024, 916, 170013. [Google Scholar] [CrossRef] [PubMed]
- Lempart-Rapacewicz, A.; Kudlek, E.; Brukało, K.; Rapacewicz, R.; Lempart, Ł.; Dudziak, M. The Threat of Food Additive Occurrence in the Environment—A Case Study on the Example of Swimming Pools. Foods 2023, 12, 1188. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Partridge, D.; Lloyd, K.A.; Rhodes, J.M.; Walker, A.W.; Johnstone, A.M.; Campbell, B.J. Food additives: Assessing the impact of exposure to permitted emulsifiers on bowel and metabolic health–introducing the FADiets study. Nutr. Bull. 2019, 44, 329–349. [Google Scholar] [CrossRef]
- Frye, M.A.; Dickinson, M.H. A signature of salience in the Drosophila brain. Nat. Neurosci. 2003, 6, 544–546. [Google Scholar] [CrossRef]
- Alaraby, M.; Annangi, B.; Hernández, A.; Creus, A.; Marcos, R. A comprehensive study of the harmful effects of ZnO nanoparticles using Drosophila melanogaster as an in vivo model. J. Hazard. Mater. 2015, 296, 166–174. [Google Scholar] [CrossRef]
- McParland, A.; Moulton, J.; Brann, C.; Hale, C.; Otis, Y.; Ganter, G. The brinker repressor system regulates injury-induced nociceptive sensitization in Drosophila melanogaster. Mol. Pain 2021, 17, 17448069211037401. [Google Scholar] [CrossRef]
- Jovanović, B.; Jovanović, N.; Cvetković, V.J.; Matić, S.; Stanić, S.; Whitley, E.M.; Mitrović, T.L. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster-a 20 generation dietary exposure experiment. Sci. Rep. 2018, 8, 17922. [Google Scholar] [CrossRef]
- Lemaitre, B.; Miguel-Aliaga, I. The Digestive Tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.S.; Baldwin, K.; Smagghe, G. Regulation of Midgut Growth, Development, and Metamorphosis. Annu. Rev. Entomol. 2010, 55, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.-J.; Hu, Y.; Kirchner, R.; Liu, Y.; Xu, C.; Comjean, A.; Tattikota, S.G.; Li, F.; Song, W.; Sui, S.H.; et al. A cell atlas of the adult Drosophila midgut. Proc. Natl. Acad. Sci. USA 2020, 117, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Veenstra, A.; Perrimon, N. Control of Lipid Metabolism by Tachykinin in Drosophila. Cell Rep. 2014, 9, 40–47. [Google Scholar] [CrossRef]
- Guo, Z.; Ohlstein, B. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 2015, 350, aab09881–aab09888. [Google Scholar] [CrossRef]
- Micchelli, C.A.; Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef]
- Perdigoto, C.N.; Schweisguth, F.; Bardin, A.J. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 2011, 138, 4585–4595. [Google Scholar] [CrossRef]
- Téfit, M.A.; Gillet, B.; Joncour, P.; Hughes, S.; Leulier, F. Stable association of a Drosophila-derived microbiota with its animal partner and the nutritional environment throughout a fly population’s life cycle. J. Insect Physiol. 2018, 106, 2–12. [Google Scholar] [CrossRef]
- Hedin, P.A.; Thompson, A.C.; Gueldner, C.; Henson, R.D. Analysis of the Antimicrobial Agents, Potassium Sorbate and Methy1-p-Hydroxybenzoate, in Boll Weevil Diets123. J. Econ. Entomol. 1974, 67, 147–149. [Google Scholar] [CrossRef]
- Andow, D.A.; Stodola, T.J. Detecting Subtle Effects of Diet Preservatives on European Corn Borer (Lepidoptera: Crambidae). J. Entomol. Sci. 2001, 36, 285–296. [Google Scholar] [CrossRef]
- Karl, A.R.; Indira, K.; Vinson, S.B.; Behmer, S.T. Evaluation of a microbial inhibitor in artificial diets of a generalist caterpillar, Heliothis virescens. J. Insect Sci. 2010, 10, 197. [Google Scholar]
- Stopforth, J.; Sofos, J.; Busta, F. Sorbic Acid and Sorbates. Food Sci. Technol. 2005, 145, 49–90. [Google Scholar]
- Mamur, S.; Yüzbaşıoğlu, D.; Ünal, F.; Yılmaz, S. Does potassium sorbate induce genotoxic or mutagenic effects in lymphocytes? Toxicol. In Vitro 2010, 24, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Fukukawa, T.; Ohtsuji, Y.; Masuda, T.; Yamaguchi, H. Mutagenicity and DNA-damaging activity caused by decomposed products of potassium sorbate reacting with ascorbic acid in the presence of Fe salt. Food Chem. Toxicol. 2002, 40, 1589–1594. [Google Scholar] [CrossRef]
- Pongsavee, M.; Mishra, R. Potassium Sorbate Induces Oxidative Stress and Genotoxicity in Human Lymphocytes. Indian J. Forensic Med. Toxicol. 2021, 15, 2795. [Google Scholar] [CrossRef]
- Benlİ, D.; TÜRkoĞLu, Ş. The Effect of Some Food Preservatives on Percentage of Survival and Longevity in Drosophila melanogaster. Cumhur. Sci. J. 2017, 38, 461–472. [Google Scholar] [CrossRef][Green Version]
- Peng, Q.; Chang, H.; Wang, R.; You, Z.; Jiang, S.; Ma, C.; Huo, D.; Zhu, X.; Zhang, J. Potassium sorbate suppresses intestinal microbial activity and triggers immune regulation in zebrafish (Danio rerio). Food Funct. 2019, 10, 7164–7173. [Google Scholar] [CrossRef]
- Xiao, N.; Ruan, S.; Mo, Q.; Zhao, M.; Liu, T.; Feng, F. Effects of potassium sorbate on systemic inflammation and gut microbiota in normal mice: A comparison of continuous intake and washout period. Food Chem. Toxicol. 2024, 184, 114443. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.R.; González-Suárez, M.; Hernández-Camacho, C.J.; Young, J.K.; Sabo, J.L. The Cost of Male Aggression and Polygyny in California Sea Lions (Zalophus californianus). PLoS ONE 2010, 5, e12230. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Clark, T.M. Evolution and Adaptive Significance of Larval Midgut Alkalinization in the Insect Superorder Mecopterida. J. Chem. Ecol. 1999, 25, 1945–1960. [Google Scholar] [CrossRef]
- Johnson, K.S.; Felton, G.W. Potential influence of midgut pH and redox potential on protein utilization in insect herbivores. Arch. Insect Biochem. Physiol. 1996, 32, 85–105. [Google Scholar] [CrossRef]
- Li, H.; Qi, Y.; Jasper, H. Preventing Age-Related Decline of Gut Compartmentalization Limits Microbiota Dysbiosis and Extends Lifespan. Cell Host Microbe 2016, 19, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Spinnenhirn, V.; Demgenski, J.; Brunner, T. Death Receptor Interactions With the Mitochondrial Cell Death Pathway During Immune Cell-, Drug- and Toxin-Induced Liver Damage. Front. Cell Dev. Biol. 2019, 7, 72. [Google Scholar] [CrossRef]
- Avelar-Freitas, B.A.; Almeida, V.G.; Pinto, M.C.X.; Mourão, F.A.G.; Massensini, A.R.; Martins-Filho, O.A.; Rocha-Vieira, E.; Brito-Melo, G.E.A. Trypan blue exclusion assay by flow cytometry. Braz. J. Med. Biol. Res. 2014, 47, 307–315. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-κB and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Ugbode, C.; Garnham, N.; Fort-Aznar, L.; Evans, G.J.O.; Chawla, S.; Sweeney, S.T. JNK signalling regulates antioxidant responses in neurons. Redox Biol. 2020, 37, 101712. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Å.B.; Dorn, G.W. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev. 2018, 99, 853–892. [Google Scholar] [CrossRef] [PubMed]
- Song, S.B.; Jang, S.-Y.; Kang, H.T.; Wei, B.; Jeoun, U.-W.; Yoon, G.S.; Hwang, E.S. Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy. Mol. Cells 2017, 40, 503–514. [Google Scholar] [CrossRef]
- Lee, J.J.; Sanchez-Martinez, A.; Zarate, A.M.; Benincá, C.; Mayor, U.; Clague, M.J.; Whitworth, A.J. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J. Cell Biol. 2018, 217, 1613–1622. [Google Scholar] [CrossRef]
- Lidsky, P.V.; Lukyanov, K.A.; Misra, T.; Handke, B.; Mishin, A.S.; Lehner, C.F. A genetically encoded fluorescent probe for imaging of oxygenation gradients in living Drosophila. Development 2018, 145, dev156257. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, F.; Ui-Tei, K.; Kojima, T.; Saigo, K. Requirements of genetic interactions between Src42A, armadilloand shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development 2005, 132, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.E.; Barry, J.D.; Warrington, S.J.; Cuhlmann, S.; Evans, P.; Huber, W.; Strutt, D.; Zeidler, M.P. Control of tissue morphology by Fasciclin III-mediated intercellular adhesion. Development 2013, 140, 3858–3868. [Google Scholar] [CrossRef]
- Worley, M.I.; Alexander, L.A.; Hariharan, I.K. CtBP impedes JNK- and Upd/STAT-driven cell fate misspecifications in regenerating Drosophila imaginal discs. eLife 2018, 7, e30391. [Google Scholar]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Kong, H.H. Identification of atg8 isoform in encysting Acanthamoeba. Korean J. Parasitol. 2013, 51, 497–502. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Matthias, J.; Meßling, S.; Eichinger, L. The two Dictyostelium autophagy eight proteins, ATG8a and ATG8b, associate with the autophagosome in succession. Eur. J. Cell Biol. 2016, 95, 15–25. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Q.; Song, X.; Yang, W.; Cheng, A.; Zhang, J.; Dong, W. Toxicity Evaluation of Potassium Sorbate In Vivo with Drosophila Melanogaster. Insects 2024, 15, 703. https://doi.org/10.3390/insects15090703
Zhang X, Zhang Q, Song X, Yang W, Cheng A, Zhang J, Dong W. Toxicity Evaluation of Potassium Sorbate In Vivo with Drosophila Melanogaster. Insects. 2024; 15(9):703. https://doi.org/10.3390/insects15090703
Chicago/Turabian StyleZhang, Xubo, Qian Zhang, Xiaoxuan Song, Wanchen Yang, Andi Cheng, Jianzhen Zhang, and Wei Dong. 2024. "Toxicity Evaluation of Potassium Sorbate In Vivo with Drosophila Melanogaster" Insects 15, no. 9: 703. https://doi.org/10.3390/insects15090703
APA StyleZhang, X., Zhang, Q., Song, X., Yang, W., Cheng, A., Zhang, J., & Dong, W. (2024). Toxicity Evaluation of Potassium Sorbate In Vivo with Drosophila Melanogaster. Insects, 15(9), 703. https://doi.org/10.3390/insects15090703





