The Effect of Soil Type and Moisture on the Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Soils
2.3. Observation on the Development of Necrophagous Phorid Flies
2.4. Measurement of Larval Body Length
2.5. Statistical Analysis
3. Results
3.1. Effects of Soil Type and Moisture on the Survival of Larvae and Pupae
3.2. Effects of Soil Type and Moisture on the Development Duration of Larvae and Pupae
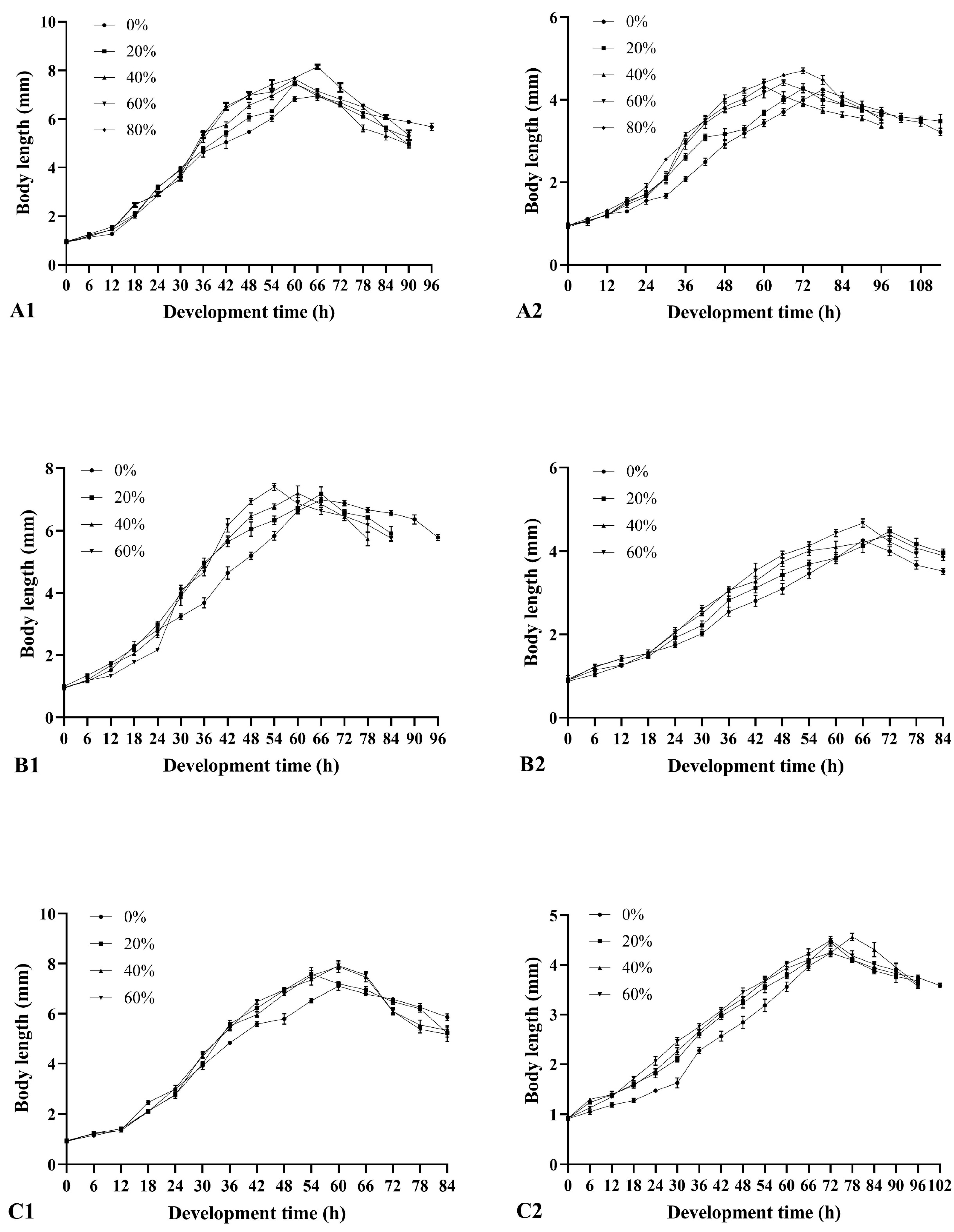
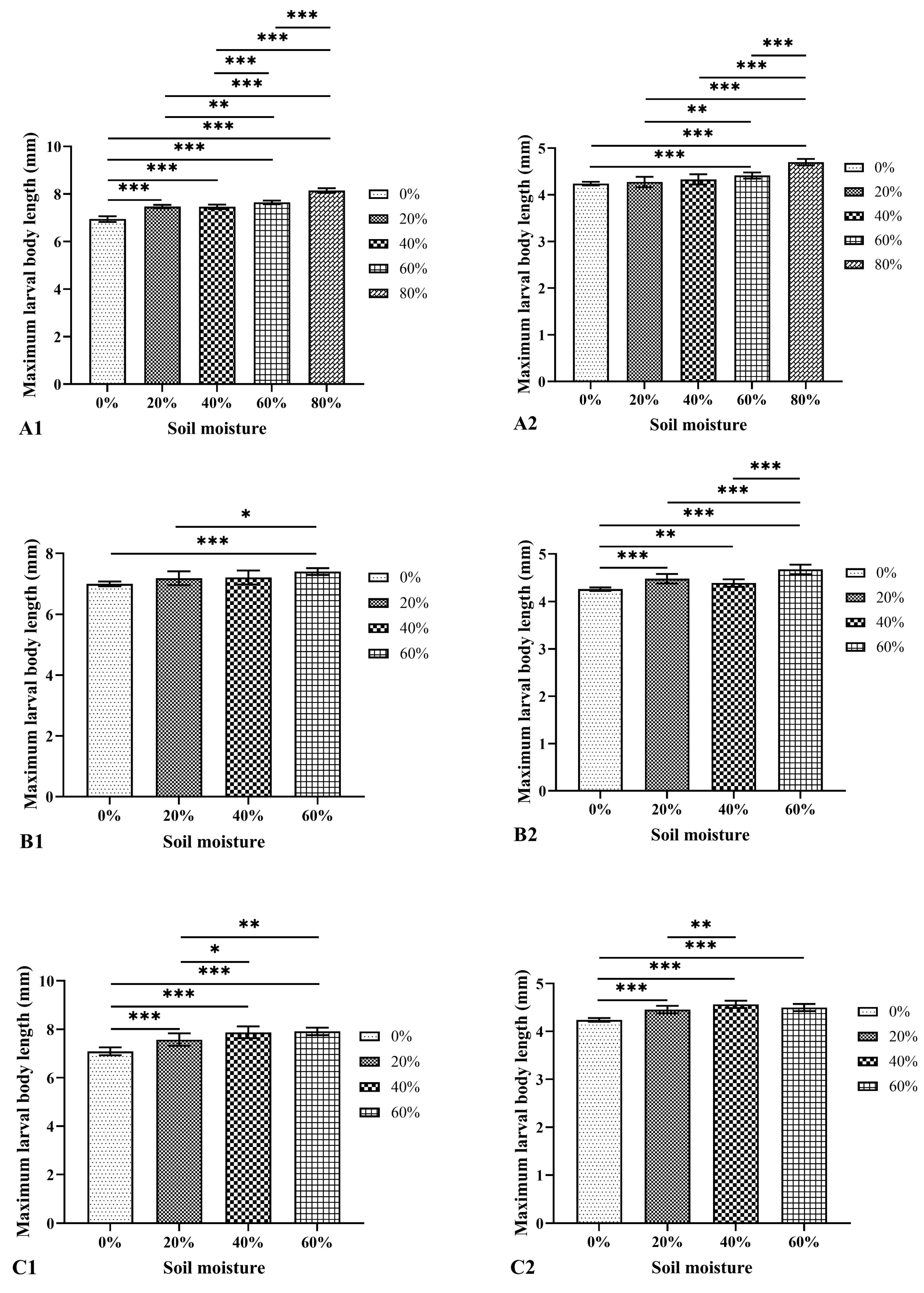
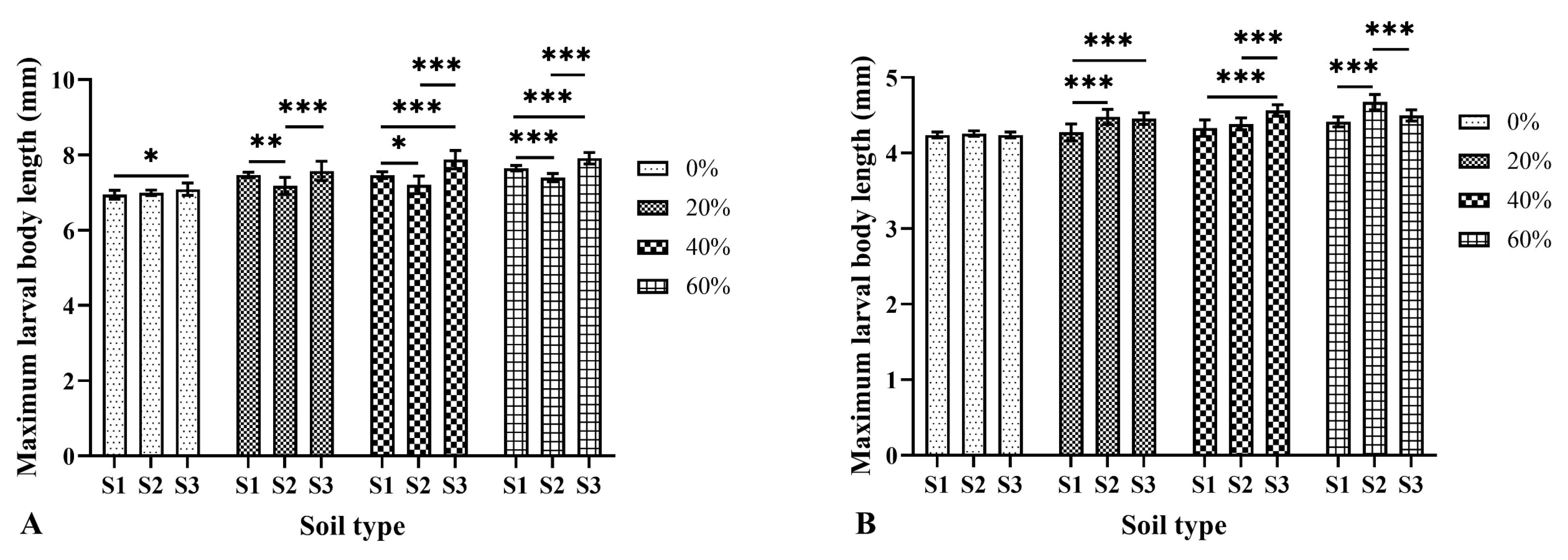
3.3. Effects of Soil Type and Moisture on the Larval Body Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on development of the forensically important holarctic blow fly Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae). Forensic. Sci. Int. 2002, 128, 177–182. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic. Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]
- Warren, J.A.; Anderson, G.S. The development of Protophormia terraenovae (Robineau-Desvoidy) at constant temperatures and its minimum temperature threshold. Forensic. Sci. Int. 2013, 233, 374–379. [Google Scholar] [CrossRef]
- Zuha, R.M.; Omar, B. Developmental rate, size, and sexual dimorphism of Megaselia scalaris (Loew) (Diptera:Phoridae): Its possible implications in forensic entomology. Parasitol. Res. 2014, 113, 2285–2294. [Google Scholar] [CrossRef]
- Kotzé, Z.; Villet, M.H.; Weldon, C.W. Effect of temperature on development of the blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Int. J. Legal. Med. 2015, 129, 1155–1162. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.N.; Liu, C.; Hu, G.L.; Wang, M.; Yang, L.J.; Chu, J.; Wang, J.F. Development of Aldrichina grahami (Diptera: Calliphoridae) at Constant Temperatures. J. Med. Entomol. 2018, 55, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, G.L.; Zhang, Y.N.; Wang, M.; Amendt, J.; Wang, J.F. Development of Muscina stabulans at constant temperatures with implications for minimum postmortem interval estimation. Forensic. Sci. Int. 2019, 298, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.N.; Hu, G.L.; Wang, M.; Zhu, R.; Zhai, Y.S.; Sun, J.; Li, X.F.; Wang, L.H.; Wu, M.W.; et al. Development of Megaselia spiracularis (Diptera: Phoridae) at different constant temperatures. J. Therm. Biol. 2020, 93, 102722. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, Y.; Li, X.B.; Zhang, J.S.; Wang, J.F. Development of Dermetses Maculatus at a constant temperature and its larval instar determination. Fa Yi Xue Za Zhi 2021, 37, 175–180. [Google Scholar]
- Montoya-Molina, S.; Jakubec, P.; Qubaiová, J.; Novák, M.; Šuláková, H.; Růžička, J. Developmental Models of the Forensically Important Carrion Beetle, Thanatophilus sinuatus (Coleoptera: Silphidae). J. Med. Entomol. 2021, 58, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Li, L.L.; Liao, M.Q.; Kang, C.T.; Hu, G.W.; Guo, Y.; Wang, Y.; Wang, J.F. Development of Megaselia scalaris at constant temperatures and its significance in estimating the time of death. Int. J. Legal. Med. 2024, 138, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Alyokhin, A.V.; Mille, C.; Messing, R.H.; Duan, J.J. Selection of pupation habitats by oriental fruit fly larvae in the laboratory. J. Insect. Behav. 2001, 14, 57–67. [Google Scholar] [CrossRef]
- Hou, B.H.; Xie, Q.; Zhang, R.J. Depth of pupation and survival of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) pupae at selected soil moistures. Appl. Entomol. Zool. 2006, 41, 515–520. [Google Scholar] [CrossRef]
- Hulthe, A.D.; Clarke, A.R. The influence of soil type and moisture on pupal survival of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust. J. Entomol. 2006, 45, 16–19. [Google Scholar] [CrossRef]
- Chen, M.; Shelton, A.M. Impact of soil type, moisture, and depth on Swede Midge (Diptera: Cecidomyiidae) pupation and emergence. Environ. Entomol. 2007, 36, 1349–1355. [Google Scholar] [CrossRef]
- Simelane, D.O. Influence of soil texture, moisture, and surface cracks on the performance of a root-feeding flea beetle, Longitarsus bethae (Coleoptera: Chrysomelidae), a biological control agent for Lantana camara (Verbenaceae). Environ. Entomol. 2007, 36, 512–517. [Google Scholar] [CrossRef]
- Han, Y.; Tang, L.D.; Fu, B.L.; Qiu, H.Y.; Wu, J.H.; Liu, Q. The influences of different soil moisture and type on pupation and eclosion of Megalurothrips usitatus. J. Environ. Entomol. 2015, 37, 710–714. [Google Scholar]
- Wen, Y.Z.; Jin, X.F.; Zhu, C.Q.; Chen, X.; Ma, T.; Zhang, S.N.; Zhang, Y.; Zeng, S.C.; Chen, X.Y.; Sun, Z.H.; et al. Effect of substrate type and moisture on pupation and emergence of Heortia vitessoides (Lepidoptera: Crambidae): Choice and no-choice studies. J. Insect. Behav. 2016, 29, 473–489. [Google Scholar] [CrossRef]
- Li, L.K.; Wang, X.H.; Liu, J.W.; Wang, Y.H.; Chen, F.J. Effect of soil moisture on the pupation and emergence of the armyworm, Mythimna separata. J. Appl. Entomol. 2019, 56, 1324–1330. [Google Scholar]
- Amaral, E.J.; Sousa, M.D.; Santos, L.M.; Costa, L.M.; Melem Junior, N.J.; Toledo, J.J.; Adaime, R. Effect of soil class and moisture on the depth of pupation and pupal viability of Bactrocera carambolae Drew & Hancock (1994). Rev. Bras. Entomol. 2021, 65, e20200075. [Google Scholar]
- Shi, Y.; Li, L.Y.; Shahid, S.; Smagghe, G.; Liu, T.X. Effect of soil moisture on pupation behavior and inhabitation of Spodoptera frugiperda (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2021, 56, 69–74. [Google Scholar] [CrossRef]
- Kökdener, M.; Şahin Yurtgan, M. The Effect of soil type and moisture level on the development of Lucilia sericata (Diptera: Calliphoridae). J. Med. Entomol. 2022, 59, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.D.; Li, P.Z.; Han, D.Y.; Fu, Y.G.; Zhan, C.L.; Li, L. Effect of soil and water content on the development and eclosion of Frankliniella Intonsa Pseudopupa. Chin. Agric. Sci. Bull. 2023, 39, 138–143. [Google Scholar]
- Xu, T.T.; Hu, F.; Hu, B.J.; Bi, S.J.; Wang, Z.Y.; Xu, L.N. Effect of soil moisture and flooding on the emergence and reproduction of Spodoptera frugiperda. J. Appl. Entomol. 2023, 60, 1133–1140. [Google Scholar]
- Reibe, S.; Madea, B. Use of Megaselia scalaris (loew) (Diptera: Phoridae) for post-mortem interval estimation indoors. Parasitol. Res. 2010, 106, 637–640. [Google Scholar] [CrossRef]
- Zuha, R.M.; See, H.W.; Disney, R.H.; Omar, B. First record of genus Puliciphora Dahl (Diptera: Phoridae) associated with rabbit carcasses placed in concealed environments in Malaysia. Forensic. Sci. Int. 2014, 245, 36–37. [Google Scholar] [CrossRef]
- Disney, R.H.L.; Manlove, J.D. First report of Triphleba nudipalpis (Becker) (Diptera: Phoridae) in a forensic case. Forensic. Sci. Int. 2009, 191, 1–3. [Google Scholar] [CrossRef]
- Pastula, E.C.; Merritt, R.W. Insect arrival pattern and succession on buried carrion in Michigan. J. Med. Entomol. 2013, 50, 432–439. [Google Scholar] [CrossRef]
- Rysavy, N.M.; Goff, M.L. Preliminary observations of arthropods associated with buried carrion on Oahu. J. Forensic. Sci. 2015, 60, 462–467. [Google Scholar] [CrossRef]
- Iancu, L.; Junkins, E.N.; Necula-Petrareanu, G.; Purcarea, C. Characterizing forensically important insect and microbial community colonization patterns in buried remains. Sci. Rep. 2018, 8, 15513. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Gómez-Gómez, A.; Baz, A. The “coffin fly” Conicera tibialis (Diptera: Phoridae) breeding on buried human remains after a postmortem interval of 18 years. J. Forensic Sci. 2011, 56, 1654–1656. [Google Scholar] [CrossRef]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Inda, A.M. Entomofauna of a buried body: Study of the exhumation of a human cadaver in Buenos Aires, Argentina. Forensic Sci. Int. 2014, 237, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Kierbel, I. New records of forensic entomofauna in legally buried and exhumed human infants remains in Buenos Aires, Argentina. J. Forensic Leg. Med. 2017, 52, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Pittner, S.; Bugelli, V.; Benbow, M.E.; Ehrenfellner, B.; Zissler, A.; Campobasso, C.P.; Oostra, R.J.; Aalders, M.C.G.; Zehner, R.; Lutz, L.; et al. The applicability of forensic time since death estimation methods for buried bodies in advanced decomposition stages. PLoS ONE 2020, 15, 243395. [Google Scholar] [CrossRef]
- Disney, R.H.L.; Garcia-Rojo, A.; Lindström, A.; Manlove, J.D. Further occurrences of Dohrniphora cornuta (Bigot) (Diptera, Phoridae) in forensic cases indicate likely importance of this species in future cases. Forensic Sci. Int. 2014, 241, 20–22. [Google Scholar] [CrossRef]
- Montoya, P.; Flores, S.; Toledo, J. Effect of rainfall and soil moisture on survival of adults and immature stages of Anastrepha ludens and A. obliqua (Diptera: Tephritidae) under semi-field conditions. Fla. Entomol. 2008, 91, 643–650. [Google Scholar]
- Eskafi, F.M.; Fernandez, A. Larval–pupal mortality of Mediterranean fruit fly (Diptera: Tephritidae) from interaction of soil, moisture, and temperature. Environ. Entomol. 1990, 19, 1666–1670. [Google Scholar] [CrossRef]
- Bento, F.M.; Marques, R.N.; Costa, M.L.; Walder, J.M.; Silva, A.P.; Parra, J.R. Pupal development of Ceratitis capitata (Diptera: Tephritidae) and Diachasmimorpha longicaudata (Hymenoptera: Braconidae) at different moisture values in four soil types. Environ. Entomol. 2010, 39, 1315–1322. [Google Scholar] [CrossRef]
- Yan, S.Q.; Lv, B.Q.; Tang, J.H.; Lu, H.; Tang, X.; Su, H.; Xiang, K.P. Influence of simulated rainfall on the emergence of Spodoptera frugiperda. J. Environ. Entomol. 2022, 44, 18–26. [Google Scholar]
- Zheng, X.L.; Wang, P.; Lei, C.L.; Lu, W.; Xian, Z.H.; Wang, X.P. Effect of soil moisture on overwintering pupae in Spodoptera exigua (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2013, 48, 365–371. [Google Scholar] [CrossRef]
- Wu, J.; Feng, D.X.; Zou, T.L.; Wang, X.H. Larval growth and development of Doprniphora cornuta (bigot) under different temperature conditions. J. Shenyang Univ. 2017, 29, 457–460. [Google Scholar]
- Thomas, J.K.; Sanford, M.R.; Longnecker, M.; Tomberlin, J.K. Effects of temperature and tissue type on the development of Megaselia scalaris (Diptera: Phoridae). J. Med. Entomol. 2016, 53, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Tomberlin, J.K.; Magni, P.A. Effect of type of tissue on the development of Chrysomya rufifacies (Diptera: Calliphoridae) in Sri Lanka. J. Med. Entomol. 2021, 58, 1673–1679. [Google Scholar] [CrossRef] [PubMed]

| Soil | Site | Particle Size (%) | pH | Organic Matter (%) | ||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| Loamy sand | 123°7′31″ E, 41°38′39″ N | 83.03 | 15.79 | 1.17 | 6.50 | 0.15 |
| Sandy loam A | 123°26′49″ E, 41°54′39″ N | 49.01 | 48.24 | 2.75 | 6.01 | 1.77 |
| Sandy loam B | 114°13′51″ E, 38°29′8″ N | 58.67 | 39.16 | 2.17 | 5.05 | 1.13 |
| Soil | Moisture (%) | Feeding Period | Larval Period | Pupal Period | Pupation Rate (%) | Emergence Rate (%) |
|---|---|---|---|---|---|---|
| Loamy sand | 0 | 71.37 ± 2.47 c | 93.33 ± 4.37 c | - | 51.34 ± 0.08 a | - |
| 20 | 50.22 ± 4.04 a | 73.03 ± 3.52 ab | 241.05 ± 3.82 a | 91.00 ± 0.08 c | 93.50 ± 0.05 c | |
| 40 | 57.60 ± 2.90 b | 77.00 ± 4.66 b | 244.57 ± 4.10 a | 86.67 ± 0.06 c | 80.50 ± 0.07 b | |
| 60 | 48.27 ± 2.99 a | 76.96 ± 5.66 b | 241.17 ± 3.30 a | 86.34 ± 0.07 c | 68.17 ± 0.09 a | |
| 80 | 48.31 ± 2.05 a | 70.53 ± 2.76 a | 248.73 ± 3.10 b | 75.33 ± 0.08 b | 60.00 ± 0.11 a | |
| 100 | - | - | - | - | - | |
| Sandy loam A | 0 | 64.70 ± 2.63 b | 77.98 ± 1.69 c | - | 43.67 ± 0.07 a | - |
| 20 | 45.47 ± 1.74 a | 63.04 ± 3.45 a | 237.57 ± 3.41 ab | 92.01 ± 0.04 c | 81.50 ± 0.09 b | |
| 40 | 42.79 ± 2.59 a | 66.12 ± 3.83 a | 234.78 ± 3.85 a | 86.65 ± 0.08 c | 85.50 ± 0.08 b | |
| 60 | 43.11 ± 2.82 a | 71.26 ± 4.76 b | 238.52 ± 2.73 b | 72.33 ± 0.06 b | 46.50 ± 0.06 a | |
| 80 | - | - | - | - | - | |
| 100 | - | - | - | - | - | |
| Sandy loam B | 0 | 67.68 ± 3.84 b | 83.90 ± 3.68 b | - | 53.00 ± 0.03 a | - |
| 20 | 52.19 ± 4.32 a | 73.17 ± 3.14 a | 241.00 ± 2.15 b | 91.33 ± 0.05 c | 94.50 ± 0.04 b | |
| 40 | 50.36 ± 2.59 a | 70.93 ± 2.55 a | 237.55 ± 4.84 ab | 93.32 ± 0.04 c | 92.00 ± 0.04 b | |
| 60 | 48.80 ± 2.96 a | 70.02 ± 3.57 a | 237.02 ± 2.29 a | 76.67 ± 0.05 b | 77.50 ± 0.05 a | |
| 80 | - | - | - | - | - | |
| 100 | - | - | - | - | - |
| Soil | Moisture (%) | Feeding Period | Larval Period | Pupal Period | Pupation Rate (%) | Emergence Rate (%) |
|---|---|---|---|---|---|---|
| Loamy sand | 0 | 92.87 ± 5.15 b | 115.98 ± 7.61 b | - | 57.00 ± 0.05 a | - |
| 20 | 67.76 ± 3.41 a | 93.57 ± 7.07 a | 242.47 ± 2.29 a | 90.65 ± 0.06 c | 90.00 ± 0.05 d | |
| 40 | 68.57 ± 6.42 a | 91.86 ± 5.50 a | 256.23 ± 2.67 d | 89.99 ± 0.08 c | 79.00 ± 0.04 c | |
| 60 | 68.05 ± 2.85 a | 93.01 ± 3.89 a | 246.97 ± 3.79 b | 91.67 ± 0.05 c | 71.50 ± 0.03 b | |
| 80 | 68.79 ± 3.99 a | 89.97 ± 7.04 a | 250.42 ± 1.66 c | 70.01 ± 0.06 b | 60.50 ± 0.06 a | |
| 100 | - | - | - | - | - | |
| Sandy loam A | 0 | 86.57 ± 2.64 c | 95.85 ± 3.18 b | - | 24.34 ± 0.04 a | - |
| 20 | 62.53 ± 5.80 b | 86.04 ± 3.91 a | 236.52 ± 2.66 a | 90.00 ± 0.05 c | 88.00 ± 0.08 b | |
| 40 | 59.48 ± 5.25 b | 86.88 ± 3.88 a | 238.41 ± 2.29 a | 83.00 ± 0.07 c | 83.50 ± 0.08 b | |
| 60 | 48.58 ± 5.12 a | 91.33 ± 7.08 ab | 236.28 ± 3.53 a | 41.34 ± 0.09 b | 36.00 ± 0.06 a | |
| 80 | - | - | - | - | - | |
| 100 | - | - | - | - | - | |
| Sandy loam B | 0 | 96.74 ± 2.86 b | 115.65 ± 3.81 b | - | 41.67 ± 0.07 a | - |
| 20 | 72.47 ± 2.32 a | 97.62 ± 2.19 a | 238.73 ± 3.00 a | 94.00 ± 0.05 b | 88.50 ± 0.06 ab | |
| 40 | 70.38 ± 3.22 a | 95.60 ± 3.96 a | 237.08 ± 3.44 a | 93.00 ± 0.05 b | 92.50 ± 0.08 b | |
| 60 | 71.87 ± 2.33 a | 96.85 ± 2.86 a | 239.18 ± 2.61 a | 91.00 ± 0.06 b | 83.00 ± 0.05 a | |
| 80 | - | - | - | - | - | |
| 100 | - | - | - | - | - |
| Analysis Indicator | Factor | SSFactor | df | Mean Square | F | p | Contribution Rate (%) |
|---|---|---|---|---|---|---|---|
| Pupation rate | Soil type | 0.068 | 2 | 0.034 | 8.806 | <0.001 | 1.92 |
| Soil moisture | 3.362 | 4 | 0.840 | 217.573 | <0.001 | 95.05 | |
| Soil type × soil moisture | 0.115 | 6 | 0.019 | 4.944 | <0.001 | 3.25 | |
| Error | 0.452 | 117 | 0.004 | - | - | - | |
| Total | 3.989 | 129 | - | - | - | - | |
| Emergence rate | Soil type | 0.321 | 2 | 0.160 | 40.075 | <0.001 | 1.98 |
| Soil moisture | 15.548 | 4 | 3.887 | 971.335 | <0.001 | 95.83 | |
| Soil type × soil moisture | 0.356 | 6 | 0.059 | 14.840 | <0.001 | 2.19 | |
| Error | 0.468 | 117 | 0.004 | - | - | - | |
| Total | 16.693 | 129 | - | - | - | - | |
| Feeding period | Soil type | 1319.645 | 2 | 659.822 | 73.041 | <0.001 | 12.60 |
| Soil moisture | 9046.119 | 4 | 2261.530 | 250.346 | <0.001 | 86.40 | |
| Soil type × soil moisture | 436.876 | 6 | 72.813 | 8.060 | <0.001 | 4.17 | |
| Error | 1056.932 | 117 | 9.034 | - | - | - | |
| Total | 11,527.281 | 129 | - | - | - | - | |
| Larval period | Soil type | 2198.321 | 2 | 1099.160 | 76.198 | <0.001 | 30.05 |
| Soil moisture | 5142.241 | 4 | 1285.560 | 89.121 | <0.001 | 70.28 | |
| Soil type × soil moisture | 542.140 | 6 | 90.357 | 6.264 | <0.001 | 7.41 | |
| Error | 1687.721 | 117 | 14.425 | - | - | - | |
| Total | 9004.142 | 129 | - | - | - | - | |
| Pupal period | Soil type | 334.408 | 2 | 167.204 | 18.250 | <0.001 | 0.03 |
| Soil moisture | 1,323,844.856 | 4 | 330,961.214 | 36,123.271 | <0.001 | 99.32 | |
| Soil type × soil moisture | 342.404 | 6 | 57.067 | 6.229 | <0.001 | 0.03 | |
| Error | 1071.953 | 117 | 9.162 | - | - | - | |
| Total | 1,333,970.485 | 129 | - | - | - | - | |
| Maximum larval body length | Soil type | 3.547 | 2 | 1.773 | 67.537 | <0.001 | 21.25 |
| Soil moisture | 11.609 | 4 | 2.902 | 110.532 | <0.001 | 69.55 | |
| Soil type × soil moisture | 1.031 | 6 | 0.172 | 6.545 | <0.001 | 6.18 | |
| Error | 3.072 | 117 | 0.026 | - | - | - | |
| Total | 19.764 | 129 | - | - | - | - |
| Analysis Indicator | Factor | SSFactor | df | Mean Square | F | p | Contribution Rate (%) |
|---|---|---|---|---|---|---|---|
| Pupation rate | Soil type | 1.239 | 2 | 0.619 | 165.633 | <0.001 | 17.42 |
| Soil moisture | 4.958 | 4 | 1.239 | 331.497 | <0.001 | 69.70 | |
| Soil type × soil moisture | 1.023 | 6 | 0.171 | 45.623 | <0.001 | 14.38 | |
| Error | 0.437 | 117 | 0.004 | - | - | - | |
| Total | 7.550 | 129 | - | - | - | - | |
| Emergence rate | Soil type | 0.403 | 2 | 0.201 | 71.567 | <0.001 | 2.44 |
| Soil moisture | 15.201 | 4 | 3.800 | 1350.396 | <0.001 | 92.13 | |
| Soil type × soil moisture | 0.894 | 6 | 0.149 | 52.970 | <0.001 | 5.42 | |
| Error | 0.329 | 117 | 0.003 | - | - | - | |
| Total | 16.828 | 129 | - | - | - | - | |
| Feeding period | Soil type | 3963.955 | 2 | 1981.977 | 113.408 | <0.001 | 18.76 |
| Soil moisture | 16,446.456 | 4 | 4111.614 | 235.264 | <0.001 | 77.83 | |
| Soil type × soil moisture | 860.151 | 6 | 143.358 | 8.203 | <0.001 | 4.07 | |
| Error | 2044.759 | 117 | 17.477 | - | - | - | |
| Total | 23,176.072 | 129 | - | - | - | - | |
| Larval period | Soil type | 2821.122 | 2 | 1410.561 | 54.517 | <0.001 | 26.59 |
| Soil moisture | 6899.243 | 4 | 1724.811 | 66.662 | <0.001 | 65.02 | |
| Soil type × soil moisture | 1070.054 | 6 | 178.342 | 6.893 | <0.001 | 10.09 | |
| Error | 3027.257 | 117 | 25.874 | - | - | - | |
| Total | 13,637.478 | 129 | - | - | - | - | |
| Pupal period | Soil type | 1786.091 | 2 | 893.045 | 141.661 | <0.001 | 0.13 |
| Soil moisture | 1,343,381.128 | 4 | 335,845.282 | 53,274.138 | <0.001 | 98.91 | |
| Soil type × soil moisture | 1293.651 | 6 | 215.608 | 34.201 | <0.001 | 0.10 | |
| Error | 737.579 | 117 | 6.304 | - | - | - | |
| Total | 1,358,925.937 | 129 | - | - | - | - | |
| Maximum larval body length | Soil type | 0.452 | 2 | 0.226 | 35.786 | <0.001 | 15.29 |
| Soil moisture | 2.411 | 4 | 0.603 | 95.555 | <0.001 | 81.56 | |
| Soil type × soil moisture | 0.457 | 6 | 0.076 | 12.080 | <0.001 | 15.46 | |
| Error | 0.738 | 117 | 0.006 | - | - | - | |
| Total | 3.694 | 129 | - | - | - | - |
| Soil | Moisture | Equation | R2 | F | p |
|---|---|---|---|---|---|
| Loamy sand | 0% | Y= −0.942X3 + 11.227X2 − 25.707X + 23.119 | 0.798 | 218.798 | <0.001 |
| 20% | Y= −0.681X3 + 7.429X2 − 10.562X + 8.489 | 0.745 | 151.552 | <0.001 | |
| 40% | Y= −0.642X3 + 6.114X2 − 2.661X − 0.016 | 0.709 | 126.867 | <0.001 | |
| 60% | Y= −0.786X3 + 8.457X2 − 13.258X + 10.808 | 0.733 | 142.508 | <0.001 | |
| 80% | Y= −0.371X3 + 3.528X2 + 3.365X − 3.770 | 0.735 | 143.965 | <0.001 | |
| Sandy loam A | 0% | Y= −0.628X3 + 7.315X2 − 11.195X + 9.749 | 0.861 | 342.223 | <0.001 |
| 20% | Y= −0.127X3 + 1.772X2 + 3.969X − 2.196 | 0.844 | 264.044 | <0.001 | |
| 40% | Y= −0.162X3 + 1.527X2 + 6.661X − 4.239 | 0.844 | 246.156 | <0.001 | |
| 60% | Y= −0.715X3 + 7.826X2 − 13.198X + 14.000 | 0.800 | 194.816 | <0.001 | |
| Sandy loam B | 0% | Y= −0.396X3 + 4.951X2 − 6.778X + 7.786 | 0.842 | 259.906 | <0.001 |
| 20% | Y= −0.467X3 + 4.530X2 − 0.334X + 0.429 | 0.757 | 151.967 | <0.001 | |
| 40% | Y= −0.341X3 + 3.228X2 + 3.202X − 2.026 | 0.732 | 133.169 | <0.001 | |
| 60% | Y= −0.194X3 + 0.932X2 + 13.204X − 11.438 | 0.715 | 122.336 | <0.001 |
| Soil | Moisture | Equation | R2 | F | p |
|---|---|---|---|---|---|
| Loamy sand | 0% | Y= −5.818X3 + 40.085X2 − 52.761X + 25.961 | 0.799 | 260.359 | <0.001 |
| 20% | Y= −7.410X3 + 56.258X2 − 100.740X + 59.603 | 0.765 | 213.046 | <0.001 | |
| 40% | Y= −4.718X3 + 30.775X2 − 34.325X + 13.267 | 0.744 | 161.197 | <0.001 | |
| 60% | Y= −2.499X3 + 17.586X2 − 14.257X + 5.266 | 0.780 | 196.268 | <0.001 | |
| 80% | Y= −3.026X3 + 22.684X2 − 28.493X + 13.790 | 0.755 | 170.884 | <0.001 | |
| Sandy loam A | 0% | Y= −2.382X3 + 16.458X2 − 10.868X + 2.146 | 0.908 | 479.697 | <0.001 |
| 20% | Y = 0.504X3 − 3.199X2 + 25.842X − 18.401 | 0.932 | 662.438 | <0.001 | |
| 40% | Y = 0.589X3 − 3.254X2 + 23.838X − 17.694 | 0.893 | 408.135 | <0.001 | |
| 60% | Y= −0.411X3 + 3.213X2 + 10.530X − 9.326 | 0.895 | 385.249 | <0.001 | |
| Sandy loam B | 0% | Y= −3.300X3 + 23.576X2 − 24.736X + 12.995 | 0.870 | 391.462 | <0.001 |
| 20% | Y= −2.978X3 + 23.037X2 − 29.246X + 13.929 | 0.866 | 358.989 | <0.001 | |
| 40% | Y= −1.730X3 + 13.852X2 − 9.944X + 1.555 | 0.863 | 347.975 | <0.001 | |
| 60% | Y= −3.748X3 + 30.246X2 − 49.957X + 28.646 | 0.840 | 290.376 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Feng, D.; Tang, Y. The Effect of Soil Type and Moisture on the Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae). Insects 2024, 15, 666. https://doi.org/10.3390/insects15090666
Han W, Feng D, Tang Y. The Effect of Soil Type and Moisture on the Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae). Insects. 2024; 15(9):666. https://doi.org/10.3390/insects15090666
Chicago/Turabian StyleHan, Wei, Dianxing Feng, and Yanan Tang. 2024. "The Effect of Soil Type and Moisture on the Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae)" Insects 15, no. 9: 666. https://doi.org/10.3390/insects15090666
APA StyleHan, W., Feng, D., & Tang, Y. (2024). The Effect of Soil Type and Moisture on the Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae). Insects, 15(9), 666. https://doi.org/10.3390/insects15090666





