Chemical Composition of Essential Oil from Citrus reticulata Blanco cv. Chachiensis (Chachi) and Its Anti-Mosquito Activity against Pyrethroid-Resistant Aedes albopictus
Abstract
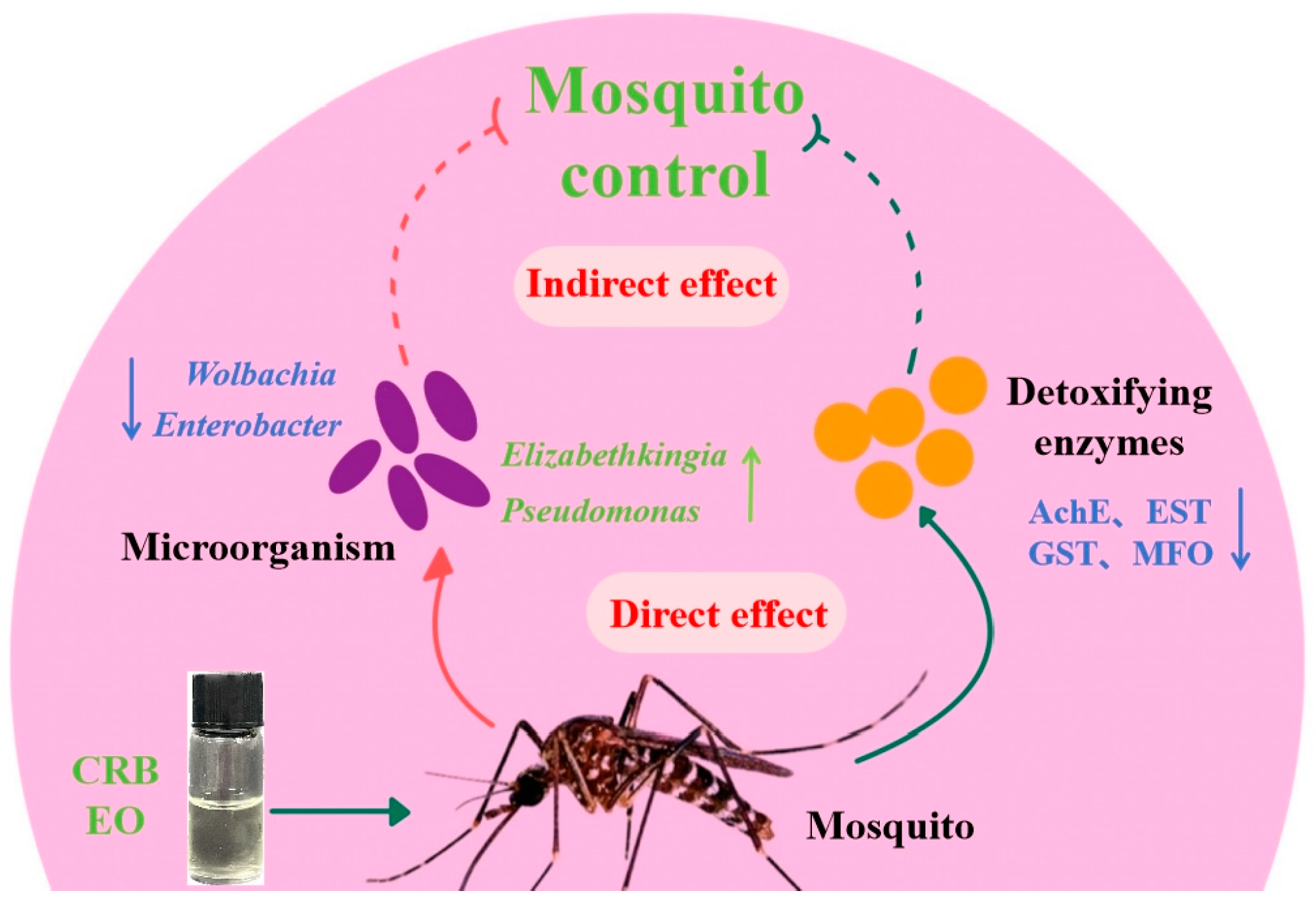
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Preparation of CRB EO
2.3. Mosquitoes
2.4. Chemical Analysis of CRB EO
2.5. Measurement of Insecticide Susceptibility
2.6. Larvicidal Assay
2.7. Mosquito-Adulticidal Activity
2.8. Enzymatic Activity
2.9. Internal Microbiota Community
2.9.1. DNA Extraction
2.9.2. Amplicon Sequencing
2.9.3. Bioinformatic Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition of CRB EO
3.2. Insecticide Susceptibility
3.3. Larvicidal Activity of CRB EO and Its Main Chemical Components
3.4. Adulticidal Activity of CRB EO
3.5. Enzymatic Activity
3.6. Internal Microbiota Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging arboviruses in the new world. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lourenço de Oliveira, R.; Vazeille, M.; de Filippis, A.M.; Failloux, A.B. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003, 69, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, S.; Fontenille, D.; Schuffenecker, I.; et al. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.S.J.; Li, M.Z.I.; Chong, C.S.; Ng, L.C.; Tan, C.H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Neglected Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef] [PubMed]
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PloS Neglected Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Santos, G.K.N.; Dutra, K.A.; Barros, R.A.; da Câmara, C.A.G.; Lira, D.D.; Gusmão, N.B.; Navarro, D.M.A.F. Essential oils from Alpinia purpurata (Zingiberaceae): Chemical composition, oviposition deterrence, larvicidal and antibacterial activity. Ind. Crops Prod. 2012, 40, 254–260. [Google Scholar] [CrossRef]
- Abd Elghani, E.M.; El Sayed, A.M.; Abdel-Aziz Emam, M.M.; Al-Mahallawi, A.M.; Tadros, S.H.; Soliman, F.M.; Youssef, F.S. Seasonal metabolic profiling of Valencia orange leaf essential oil using GC coupled with chemometrics, nano-formulation, and insecticidal evaluation: In vivo and in silico. RSC Adv. 2023, 13, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.; Johnson, J.; Gross, A.; Bartholomay, L.; Coats, J. Plant essential oils enhance diverse pyrethroids against multiple strains of mosquitoes and inhibit detoxification enzyme processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-CoV-2. Front. Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Dassanayake, M.K.; Chong, C.H.; Khoo, T.J.; Figiel, A.; Szumny, A.; Choo, C.M. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: A review. Foods 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.L.; Yu, K.Y.; Guo, L.; Bai-Zhong, C.; Li, P.; Liu, E.H. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017, 234, 254–261. [Google Scholar] [CrossRef]
- Liang, P.L.; Chen, X.L.; Gong, M.J.; Xu, Y.; Tu, H.S.; Zhang, L.; Liao, B.S.; Qiu, X.H.; Zhang, J.; Huang, Z.H.; et al. Guang Chen Pi (the pericarp of Citrus reticulata Blanco’s cultivars ‘Chachi’) inhibits macrophage-derived foam cell formation. J. Ethnopharmacol. 2022, 293, 115328. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus reticulata Blanco peel extract ameliorates hepatic steatosis, oxidative stress and inflammation in HF and MCD diet-induced NASH C57BL/6 J mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, B.; Ma, Z.; Feng, J.; Yan, H. Reticine A, A new potent natural elicitor: Isolation from the fruit peel of Citrus reticulate and induction of systemic resistance against tobacco mosaic virus and other plant fungal diseases. Pest Manag. Sci. 2021, 77, 354–364. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Taktak, N.E.M.; El-Aswad, A.F. Chemical composition of the essential oils isolated from peel of three citrus species and their mosquitocidal activity against Culex pipiens. Nat. Prod. Res. 2017, 32, 2829–2834. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Chen, B.; Zheng, W.; Yan, Z.; Zhang, Z.; Li, J.; Su, K.; Ang, S.; Wu, R.; et al. Chemical compositions and anti-mosquito activity of essential oils from Pericarpium Citri Reticulataes of different aging years. Ind. Crops Prod. 2022, 188, 115701. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant properties of pulp, peel and seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at different stages of fruit ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Vall-llaura, N.; Fernández-Cancelo, P.; Nativitas-Lima, I.; Echeverria, G.; Teixidó, N.; Larrigaudière, C.; Torres, R.; Giné-Bordonaba, J. ROS-scavenging-associated transcriptional and biochemical shifts during nectarine fruit development and ripening. Plant Physiol. Biochem. 2022, 141, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, pathogenicity, and fungicide sensitivity of Alternaria isolates associated with preharvest fruit drop in California citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.H.; Huong, L.T.; Chung, N.T.; Thuong, N.T.H.; Satyal, P.; Dung, N.A.; Tai, T.A.; Setzer, W.N. Callicarpa Species from Central Vietnam: Essential Oil Compositions and Mosquito Larvicidal Activities. Plants 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tang, X.; Jian, R.; Li, J.; Lin, M.; Dai, H.; Wang, K.; Sheng, Z.; Chen, B.; Xu, X.; et al. Chemical composition, antimicrobial and insecticidal activities of essential oils of discarded perfume lemon and leaves (Citrus Limon (L.) Burm. F.) as possible sources of functional botanical agents. Front. Chem. 2021, 9, 679116. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Polson, K.A.; Rawlins, S.C.; Brogdon, W.G.; Chadee, D.D. Characterisation of DDT and pyrethroid resistance in Trinidad and Tobago populations of Aedes aegypti. Bull. Entomol. Res. 2011, 101, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Azratul-Hizayu, T.; Chen, C.D.; Lau, K.W.; Azrizal-Wahid, N.; Tan, T.K.; Lim, Y.A.L.; Sofian-Azirun, M.; Low, V.L. Bioefficacy of mosquito mat vaporizers and associated metabolic detoxication mechanisms in Aedes aegypti (Linnaeus) in Selangor, Malaysia: A statewide assessment. Trop. Biomed. 2021, 38, 327–337. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crops Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Murugan, K.; Mahesh Kumar, P.; Kovendan, K.; Amerasan, D.; Subrmaniam, J.; Hwang, J.S. Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2012, 111, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, W.; Zheng, J.; Xu, J.; Wang, H.; Du, J.; Zhou, D.; Sun, Y.; Shen, B. Toxic effects of Perilla frutescens (L.) Britt. essential oil and its main component on Culex pipiens pallens (Diptera: Culicidae). Plants 2023, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Budiman; Ishak, H.; Stang; Ibrahim, E.; Daud, A.; Amiruddin, R. Essential oil as a new tool for larvicidal Aedes aegypti: A systematic review. Gac. Sanit. 2021, 35, S459–S462. [Google Scholar]
- Intirach, J.; Junkum, A.; Lumjuan, N.; Chaithong, U.; Somboon, P.; Jitpakdi, A.; Riyong, D.; Champakaew, D.; Muangmoon, R.; Chansang, A.; et al. Biochemical effects of Petroselinum crispum (Umbellifereae) essential oil on the pyrethroid resistant strains of Aedes aegypti (Diptera: Culicidae). Insects 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McComic, S.E.; Rault, L.C.; Swale, D.R.; Anderson, T.D. Bioinsecticidal activity of cajeput oil to pyrethroid-susceptible and -resistant mosquitoes. Pestic. Biochem. Physiol. 2023, 193, 105458. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Ramadan, G.R.M.; Zhu, K.Y.; Phillips, T.W. Synergism of deltamethrin with a mixture of short chain fatty acids for toxicity against pyrethroid-resistant and susceptible strains of Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2022, 184, 105132. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Peng, H.; Li, Y.; Meng, B.; Wang, S.; Bi, S.; Zhao, X. Pyrethroids exposure alters the community and function of the internal microbiota in Aedes albopictus. Ecotoxicol. Environ. Saf. 2023, 252, 114579. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Lagos-Kutz, D.; Dunlap, C.; Ramirez, J.L.; Rooney, A.P.; Hartman, G.L.; Fields, C.J.; Rendon, G.; Kim, C.-H. Mosquito microbiota cluster by host sampling location. Parasites Vectors 2018, 11, 468. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 109, E23–E31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Shen, R.-X.; Xing, D.; Zhao, C.-P.; Gao, H.-T.; Wu, J.-H.; Zhang, N.; Zhang, H.-D.; Chen, Y.; Zhao, T.-Y.; et al. Metagenome sequencing reveals the midgut microbiota makeup of Culex pipiens quinquefasciatus and its possible relationship with insecticide resistance. Front. Microbiol. 2021, 12, 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Ma, C.; Wu, N.; Li, C.; Yang, X. Simultaneous biodegradation of bifenthrin and chlorpyrifos by Pseudomonas sp. CB2. J. Environ. Sci. Health 2018, 53, 304–312. [Google Scholar] [CrossRef]
- Buck, M.; Nilsson, L.K.J.; Brunius, C.; Dabiré, R.K.; Hopkins, R.; Terenius, O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci. Rep. 2016, 6, 22806. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M.; Wang, L.; Wang, G. Polysaccharides and glycosides from Aralia echinocaulis protect rats from arthritis by modulating the gut microbiota composition. J. Ethnopharmacol. 2021, 269, 113749. [Google Scholar] [CrossRef]






| No. | Component | RI | Content (%) 3 | |||
|---|---|---|---|---|---|---|
| Exp. 1 | Lit. 2 | Immature | Semi-Mature | Mature | ||
| 1 | α-Thujene | 923 | 924 | 0.29 ± 0.01 | 0.31 ± 0.01 | 0.34 ± 0.02 |
| 2 | α-Pinene | 929 | 929 | 0.87 ± 0.03 | 1.05 ± 0.00 | 0.96 ± 0.00 |
| 3 | β-Pinene | 971 | 971 | 0.92 ± 0.00 | 0.92 ± 0.02 | 0.85 ± 0.00 |
| 4 | β-Myrcene | 990 | 990 | 1.18 ± 0.02 | 1.19 ± 0.01 | 1.27 ± 0.02 |
| 5 | Octanal | 1001 | 1001 | 0.64 ± 0.02 | 0.28 ± 0.00 | - |
| 6 | α-Terpinene | 1013 | 1014 | 0.11 ± 0.02 | 0.16 ± 0.03 | 0.17 ± 0.03 |
| 7 | p-Cymene | 1022 | 1024 | 5.35 ± 0.03 | 2.60 ± 0.05 | 1.39 ± 0.00 |
| 8 | D-Limonene | 1030 | 1030 | 62.35 ± 1.10 | 76.72 ± 0.79 | 73.15 ± 0.09 |
| 9 | γ-Terpinene | 1062 | 1062 | 14.26 ± 0.35 | 11.04 ± 0.65 | 11.27 ± 0.12 |
| 10 | Terpinolene | 1086 | 1086 | 0.65 ± 0.03 | 0.50 ± 0.05 | 0.43 ± 0.00 |
| 11 | Linalool | 1099 | 1099 | 0.91 ± 0.02 | 0.40 ± 0.02 | 0.17 ± 0.01 |
| 12 | Nonanal | 1103 | 1102 | 0.08 ± 0.02 | 0.01 ± 0.01 | - |
| 13 | trans-2,8-p-Mentha -dien-1-ol | 1119 | 1120 | 0.10 ± 0.01 | - | - |
| 14 | cis-(-)-1,2-Epoxy -p-menth-8-ene | 1131 | 1136 | 0.14 ± 0.01 | - | - |
| 15 | (E)-Limonene oxide | 1136 | 1137 | 0.16 ± 0.01 | - | - |
| 16 | Citronellal | 1152 | 1151 | 0.08 ± 0.02 | - | - |
| 17 | Terpinen-4-ol | 1175 | 1175 | 1.15 ± 0.03 | 0.61 ± 0.01 | 0.27 ± 0.03 |
| 18 | p-Cymen-8-ol | 1183 | 1184 | 0.06 ± 0.02 | - | - |
| 19 | α-Terpineol | 1188 | 1188 | 2.42 ± 0.09 | 1.26 ± 0.02 | 0.46 ± 0.04 |
| 20 | Carveol | 1198 | 1200 | - | - | 0.01 ± 0.01 |
| 21 | Decanal | 1203 | 1203 | 0.17 ± 0.01 | - | - |
| 22 | cis-Carveol | 1217 | 1216 | 0.06 ± 0.00 | - | - |
| 23 | Citronellol | 1232 | 1232 | 0.23 ± 0.01 | - | - |
| 24 | Perillaldehyde | 1271 | 1271 | - | 0.18 ± 0.02 | - |
| 25 | 1-Perillalcohol | 1297 | 1297 | 0.27 ± 0.02 | 0.12 ± 0.02 | 0.02 ± 0.00 |
| 26 | p-Cymen-2-ol | 1301 | 1300 | - | 0.01 ± 0.00 | - |
| 27 | Methyl 2-(methylamino) benzoate | 1405 | 1402 | 4.95 ± 0.05 | 1.93 ± 0.01 | 2.15 ± 0.20 |
| 28 | α-Farnesene | 1507 | 1507 | 0.06 ± 0.02 | - | - |
| Ae. albopictus | Strain | LC50 (95% CI)/mg/L | RR | Resistance Level |
|---|---|---|---|---|
| Larvae | Lab-S | 0.011 (0.01–0.02) | - | - |
| Pyr-R | 0.085 (0.05–0.13) | 7.73 | Low resistance | |
| Adult | Lab-S | 0.49 (0.45–0.58) | - | - |
| Pyr-R | 6.62 (4.11–9.30) | 13.51 | Mid resistance |
| Sample | LC50 (95% CI)/mg/L | LC90 (95% CI)/mg/L | χ2 | p-Value |
| Immature | 65.32 (61.63–68.89) | 92.29 (86.54–100.19) | 1.60 | 0.90 |
| Semi-mature | 61.47 (58.82–64.08) | 77.40 (73.97–81.96) | 6.05 | 0.20 |
| Mature | 65.91 (58.55–72.78) | 100.21 (89.30–119.32) | 8.50 | 0.13 n.s. |
| p-cymene | 72.31 (67.08–77.39) | 90.29 (83.52–102.96) | 6.92 | 0.14 n.s. |
| D-Limonene | 64.33 (54.75–71.90) | 104.67 (92.94–126.14) | 13.55 | 0.04 n.s. |
| γ-Terpinene | 75.78 (70.25–80.89) | 100.54 (93.27–111.99) | 10.75 | 0.10 n.s. |
| α-Terpineol | >155 | - | - | - |
| Methyl 2-(methyl- amino)benzoate | 109.75 (105.98–113.58) | 146.53 (139.43–156.22) | 6.05 | 0.42 |
| Sample | LC50 (95% CI)/mg/L | LC90 (95% CI)/mg/L | χ2 | p-Value |
|---|---|---|---|---|
| Immature | >2500 | - | - | - |
| Semi-mature | 2715.03 (2448.99–2991.23) | 3850.04 (3412.25–4825.69) | 11.20 | 0.02 n.s. |
| Mature | >2500 | - | - | - |
| Deltamethrin | 6.62 (4.11–9.30) | 18.14 (11.98–65.9) | 19.14 | 0.01 n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zheng, W.; Chen, B.; Yan, Z.; Tang, X.; Li, J.; Zhang, Z.; Ang, S.; Li, C.; Wu, R.; et al. Chemical Composition of Essential Oil from Citrus reticulata Blanco cv. Chachiensis (Chachi) and Its Anti-Mosquito Activity against Pyrethroid-Resistant Aedes albopictus. Insects 2024, 15, 345. https://doi.org/10.3390/insects15050345
Cao J, Zheng W, Chen B, Yan Z, Tang X, Li J, Zhang Z, Ang S, Li C, Wu R, et al. Chemical Composition of Essential Oil from Citrus reticulata Blanco cv. Chachiensis (Chachi) and Its Anti-Mosquito Activity against Pyrethroid-Resistant Aedes albopictus. Insects. 2024; 15(5):345. https://doi.org/10.3390/insects15050345
Chicago/Turabian StyleCao, Jifan, Wende Zheng, Baizhong Chen, Zhenping Yan, Xiaowen Tang, Jiahao Li, Zhen Zhang, Song Ang, Chen Li, Rihui Wu, and et al. 2024. "Chemical Composition of Essential Oil from Citrus reticulata Blanco cv. Chachiensis (Chachi) and Its Anti-Mosquito Activity against Pyrethroid-Resistant Aedes albopictus" Insects 15, no. 5: 345. https://doi.org/10.3390/insects15050345
APA StyleCao, J., Zheng, W., Chen, B., Yan, Z., Tang, X., Li, J., Zhang, Z., Ang, S., Li, C., Wu, R., Wu, P., & Chen, W.-H. (2024). Chemical Composition of Essential Oil from Citrus reticulata Blanco cv. Chachiensis (Chachi) and Its Anti-Mosquito Activity against Pyrethroid-Resistant Aedes albopictus. Insects, 15(5), 345. https://doi.org/10.3390/insects15050345






