Endosymbiotic Fungal Diversity and Dynamics of the Brown Planthopper across Developmental Stages, Tissues, and Sexes Revealed Using Circular Consensus Sequencing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
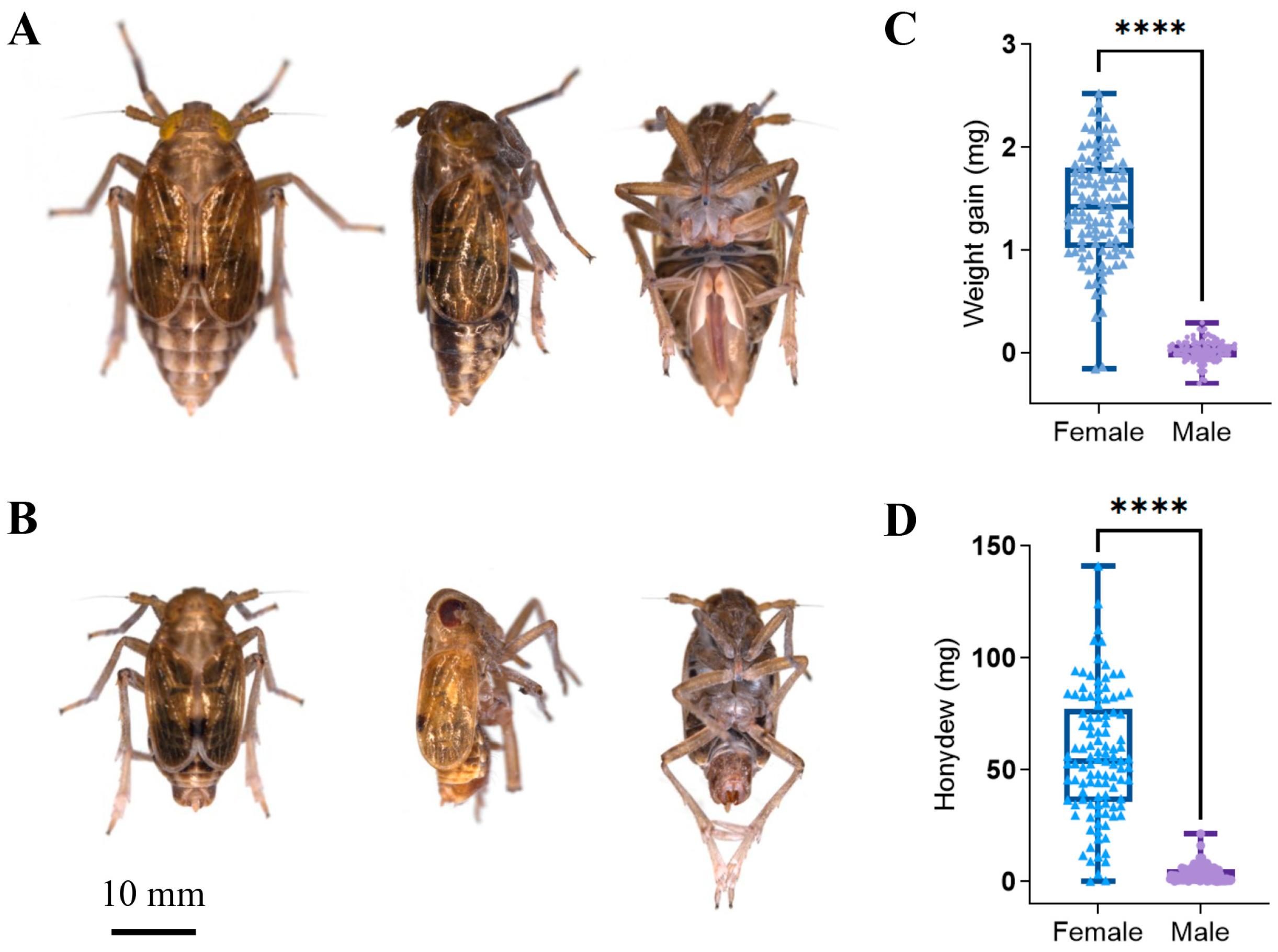
2.1. Insects
2.2. Sample Collection
2.3. BPH Virulence Identification
2.4. DNA Extraction and PCR Amplification
2.5. Library Construction and Sequencing
2.6. Statistical and Bioinformatics Analysis
3. Results
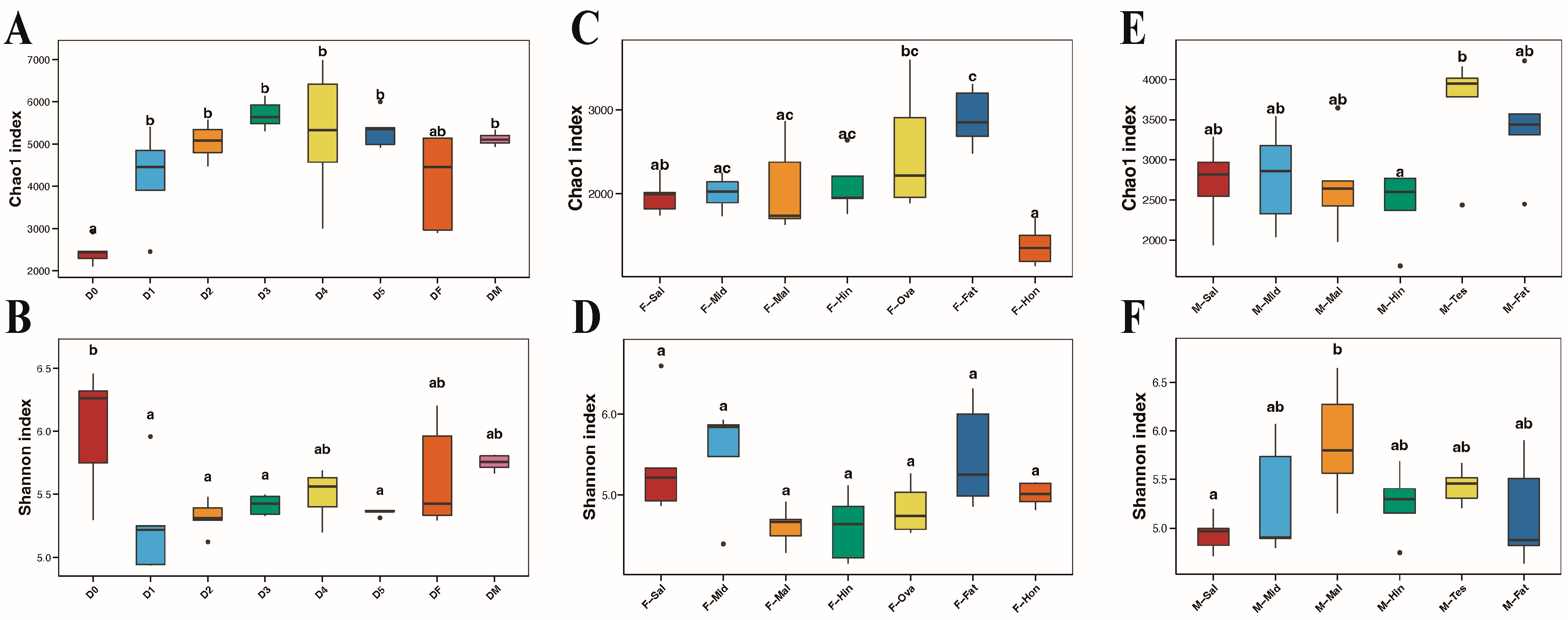
3.1. General Profile of ITS Amplicon Sequencing
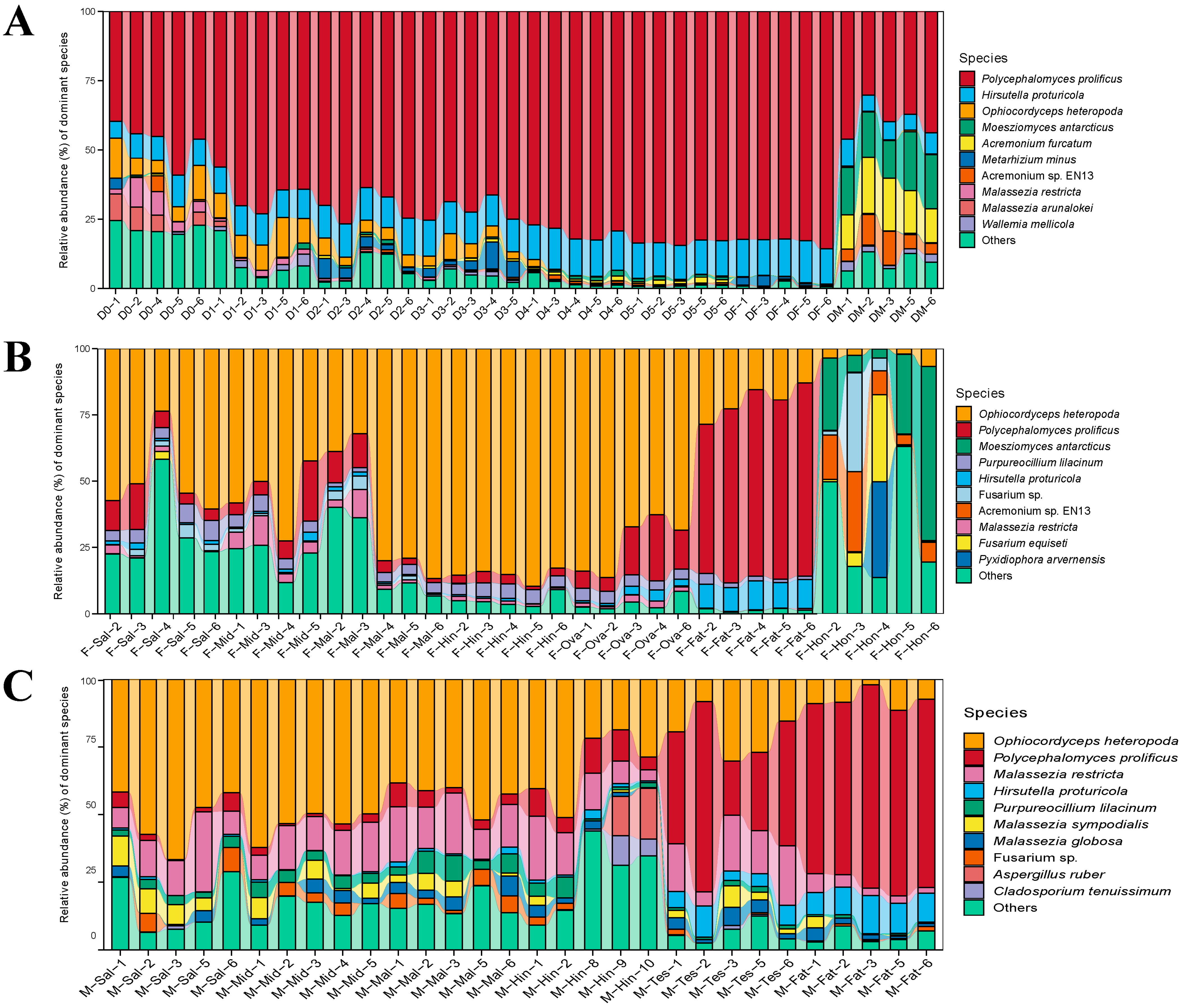
3.2. The Composition of Fungal Communities
3.3. Fungal Community Structure Comparisons
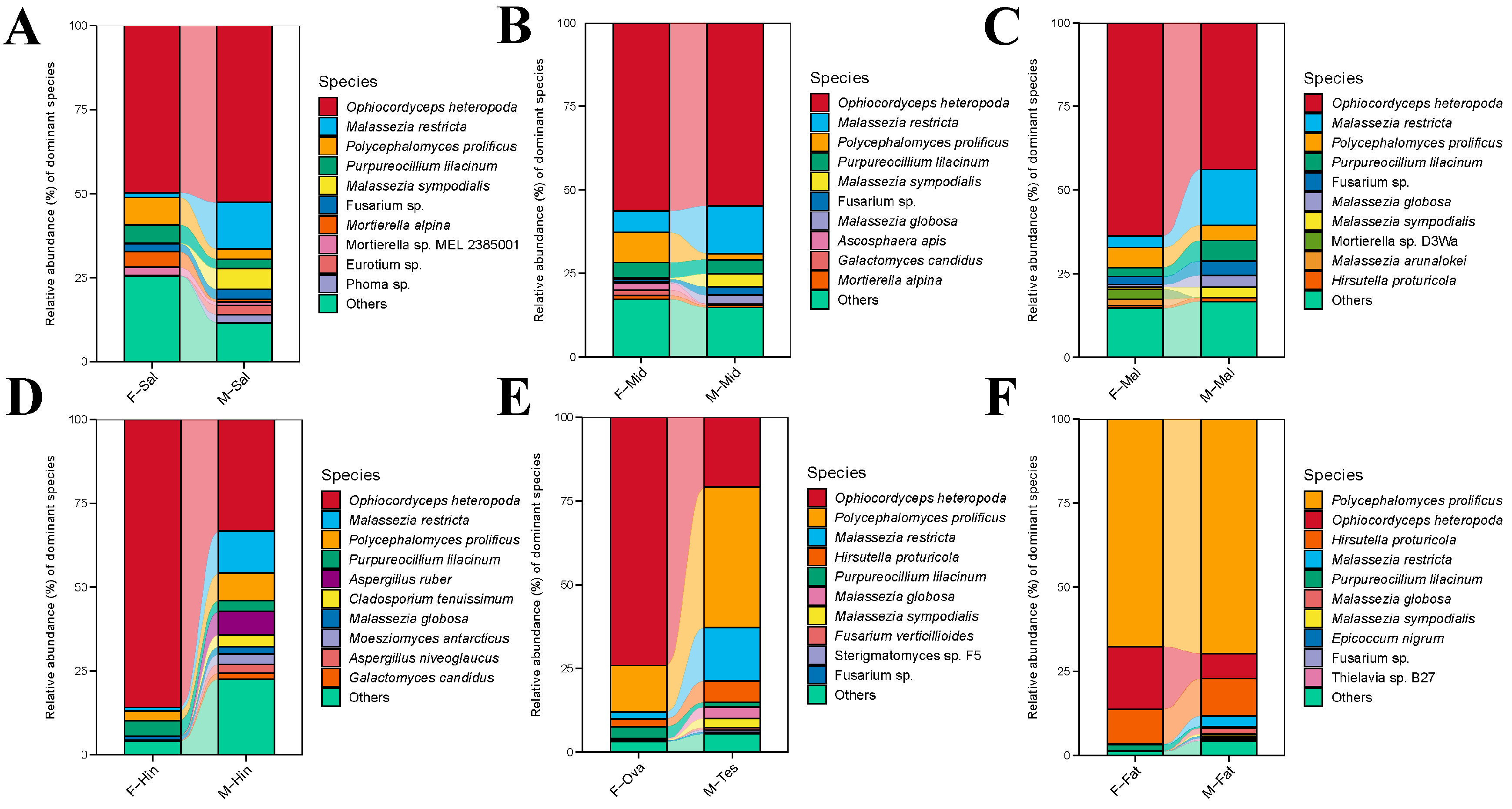
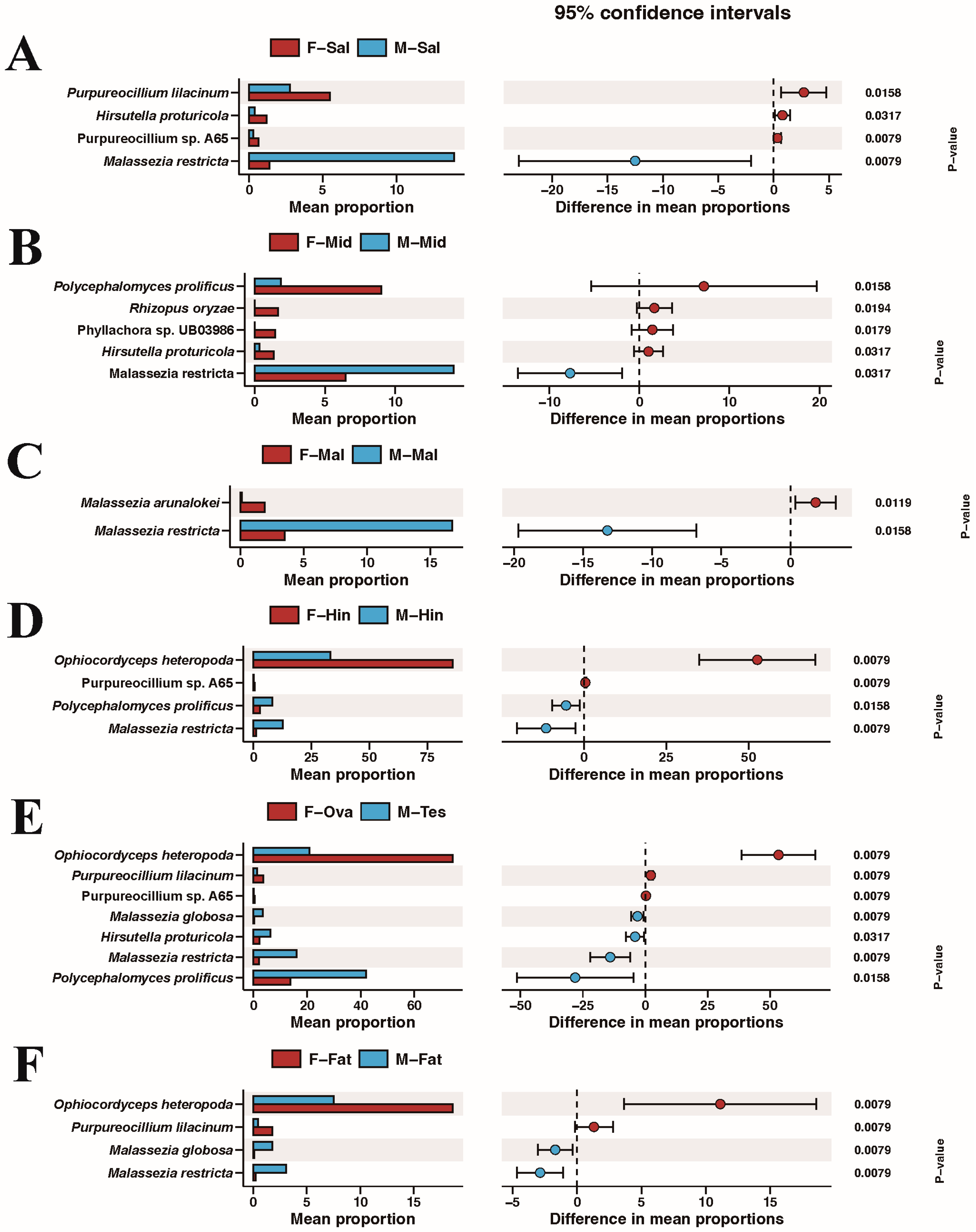
3.4. Comparison of Species Composition and Diversity between Female and Male Adults in Different Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Douglas, A.E. Nutritional interactions in insect–microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Brinker, P.; Fontaine, M.C.; Beukeboom, L.W.; Salles, J.F. Host, symbionts, and the microbiome: The missing tripartite interaction. Trends Microbiol. 2019, 27, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.Y.; Griggs, R.; Chicana, B.; Miller, C.; Swei, A. Vertical vs. horizontal transmission of the microbiome in a key disease vector, Ixodes pacificus. Mol. Ecol. 2017, 26, 6578–6589. [Google Scholar] [CrossRef] [PubMed]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 2013, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Cansado-Utrilla, C.; Zhao, S.Y.; McCall, P.J.; Coon, K.L.; Hughes, G.L. The microbiome and mosquito vectorial capacity: Rich potential for discovery and translation. Microbiome 2021, 9, 111. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.P.; Zhou, C.Y.; Zhao, D.S.; Zhu, Y.X.; Bing, X.L.; Huang, H.J.; Hong, X.Y. Antibiotic exposure perturbs the bacterial community in the small brown planthopper Laodelphax striatellus. Insect Sci. 2020, 27, 895–907. [Google Scholar] [CrossRef]
- Hemingway, J.; Karunaratne, S.H.P.P.; Claridge, M.F. Insecticide resistance spectrum and underlying resistance mechanisms in tropical populations of the brown planthopper (Nilaparvata lugens) collected from rice and the wild grass Leersia hexandra. Int. J. Pest Manag. 1999, 45, 215–223. [Google Scholar] [CrossRef]
- Du, B.; Chen, R.Z.; Guo, J.P.; He, G.C. Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Mol. Breeding. 2020, 40, 24. [Google Scholar] [CrossRef]
- Noda, H.; Omura, T. Purification of yeast-like symbiotes of planthoppers. J. Invertebr. Pathol. 1992, 59, 104–105. [Google Scholar] [CrossRef]
- Tang, M.; Lv, L.; Jing, S.L.; Zhu, L.L.; He, G.C. Bacterial symbionts of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl. Environ. Microbiol. 2010, 76, 1740–1745. [Google Scholar] [CrossRef]
- Liu, M.Y.; Hong, G.J.; Li, H.J.; Bing, X.L.; Chen, Y.M.; Jing, X.F.; Gershenzon, J.; Lou, Y.G.; Baldwin, I.T.; Li, R. Sakuranetin protects rice from brown planthopper attack by depleting its beneficial endosymbionts. Proc. Natl. Acad. Sci. USA 2023, 120, e2305007120. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Interscience Inc.: New York, NY, USA, 1965. [Google Scholar]
- Sasaki, T.; Kawamura, M.; Ishikawa, H. Nitrogen recycling in the brown planthopper, Nilaparvata lugens: Involvement of yeast-like endosymbionts in uric acid metabolism. J. Insect Physiol. 1996, 42, 125–129. [Google Scholar] [CrossRef]
- Noda, H.; Koizumi, Y. Sterol biosynthesis by symbiotes: Cytochrome P450 sterol C-22 desaturase genes from yeastlike symbiotes of rice planthoppers and anobiid beetles. Insect Biochem. Mol. Biol. 2003, 33, 649–658. [Google Scholar] [CrossRef]
- Wang, X.Q.; Guo, J.S.; Li, D.T.; Yu, Y.; Hagoort, J.; Moussian, B.; Zhang, C.X. Three-dimensional reconstruction of a whole insect reveals its phloem sap-sucking mechanism at nano-resolution. eLife 2021, 10, e62875. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.Y.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.W.; Lu, J.B.; Ye, Y.X.; Yu, X.P.; Zhang, C.X. Characteristics of the draft genome of “Candidatus Arsenophonus nilaparvatae”, a facultative endosymbiont of Nilaparvata lugens. Insect Sci. 2016, 23, 478–486. [Google Scholar] [CrossRef]
- Pang, R.; Chen, M.; Yue, L.; Xing, K.; Li, T.C.; Kang, K.; Liang, Z.K.; Yuan, L.Y.; Zhang, W.Q. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.J.; Zhang, Y.H.; Cai, T.W.; Mao, K.K.; Xu, Y.; Li, C.Y.; He, S.; Li, J.H.; Wan, H. Dynamics of Microbial Communities across the Life Stages of Nilaparvata lugens (Stål). Microb. Ecol. 2022, 83, 1049–1058. [Google Scholar] [CrossRef]
- Wang, Z.L.; Cheng, Y.Q.; Wang, Y.D.; Yu, X.P. Topical Fungal Infection Induces Shifts in the Gut Microbiota Structure of Brown Planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Insects 2022, 13, 528. [Google Scholar] [CrossRef]
- Jiao, X.L.; Zheng, X.; Ma, L.; Kutty, G.; Gogineni, E.; Sun, Q.; Sherman, B.T.; Hu, X.J.; Jones, K.; Raley, C.; et al. A Benchmark Study on Error Assessment and Quality Control of CCS Reads Derived from the PacBio RS. J. Data Min. Genom. Proteom. 2013, 4, 16008. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Go, M.J.; Lee, S.Y.; Kim, S.C.; Lee, C.H.; Cho, B.K. Analysis of the mouse gut microbiome using full-length 16S rRNA amplicon sequencing. Sci. Rep. 2016, 6, 29681. [Google Scholar] [CrossRef]
- Wang, H.L.; Lei, T.; Xia, W.Q.; Cameron, S.L.; Liu, Y.Q.; Zhang, Z.; Gowda, M.M.N.; De Barro, P.; Navas-Castillo, J.; Omongo, C.A.; et al. Insight into the microbial world of Bemisia tabaci cryptic species complex and its relationships with its host. Sci. Rep. 2019, 9, 6568. [Google Scholar] [CrossRef] [PubMed]
- Pecundo, M.H.; Chang, A.C.G.; Chen, T.; Dela Cruz, T.E.E.; Ren, H.; Li, N. Full-Length 16S rRNA and ITS Gene Sequencing Revealed Rich Microbial Flora in Roots of Cycas spp. in China. Evol. Bioinform. 2021, 17, 1176934321989713. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.K.D.; Nierychlo, M.; Andersen, K.S.; Rudkjøbing, V.; Knutsson, S.; MiDAS Global Consortium; Albertsen, M.; Nielsen, P.H. MiDAS 4: A global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 2022, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, X.X.; Zhang, J.; Liu, B.F.; Zhao, Y.; Wang, H.Y.; Wang, Z.Z.; Guo, J.P.; Rao, W.W.; Jing, S.L.; Guan, W.; et al. A mucin-like protein of Planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Yu, N.; Xu, X.X.; Liu, Z.W. Community structure, dispersal ability and functional profiling of microbiome existing in fat body and ovary of the brown planthopper, Nilaparvata lugens. Insect Sci. 2019, 26, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhao, Y.; Wang, H.Y.; Song, C.P.; Shangguan, X.X.; Zhu, L.L.; He, G.C. Comparative metabolomics of the interaction between rice and the brown planthopper. Metabolomics 2016, 12, 132. [Google Scholar] [CrossRef]
- Shentu, X.P.; Xiao, Y.; Song, Y.; Cao, Z.Y.; Fan, J.X.; Yu, X.P. Comparative Analysis of the Diversity of the Microbial Communities between Non-Fertilized and Fertilized Eggs of Brown Planthopper, Nilaparvata lugens Stål. Insects 2020, 11, 49. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Brunson, J.C. ggalluvial: Layered Grammar for Alluvial Plots. J. Open Source Softw. 2020, 5, 2017. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non–parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Cheng, D.; Hou, R. Histological observations on transovarial transmission of a yeast-like symbiote in Nilaparvata lugens Stål (Homoptera, Delphacidae). Tissue Cell 2001, 33, 273–279. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Nasu, S.; Kusumi, T.; Suwa, Y.; Kita, H. Symbiotes of planthoppers: II. isolation of intracellular symbiotic microorganisms from the brown planthopper, Nilaparata lugens Stål, and immunological comparison of the symbiotes associated with rice planthoppers (Hemiptera: Delphacidae). Appl. Entomol. Zool. 1981, 16, 88–93. [Google Scholar] [CrossRef]
- Suh, S.O.; Noda, H.; Blackwell, M. Insect symbiosis: Derivation of yeast-like endosymbionts within an entomopathogenic filamentous lineage. Mol. Biol. Evol. 2001, 18, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011, 13, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.A. A Monograph of Stilbella and Some Allied hyphomycetes; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 1985. [Google Scholar]
- Kepler, R.M.; Ban, S.; Nakagiri, A.; Bischoff, J.F.; Hywel-Jones, N.L.; Owensby, C.A.; Spatafora, J.W. The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: An application of one fungus one name. Fungal Biol. 2013, 117, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Doehlemann, G.; Kemen, E.; Thines, M. Asexual and sexual morphs of Moesziomyces revisited. IMA Fungus. 2017, 8, 117–129. [Google Scholar] [CrossRef]
- Jicinska, E. Dimorphic and yeast like mutants of the genus Cephalosporium Cda. Folia Microbiol. 1974, 19, 1–4. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Schroers, H.J. Analysis of phylogenetic relationship of Cylidrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and review of similarities in the spectrum of opportunistic infections caused by these fungi. J. Clin. Microbiol. 2002, 40, 2866–2875. [Google Scholar] [CrossRef]
- Majumbar, A.; Boetel, M.A.; Jaronski, T.S. Discovery of Fusarium solani as a naturally occurring pathogen of sugarbeet root maggot (Diptera: Ulidiidae) pupae: Prevalence and baseline suceptibility. J. Invertebr. Pathol. 2008, 97, 1–8. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; Donnell, K.O.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Tian, J.; Lai, D.W.; Zhou, L.G. Secondary Metabolites from Acremonium Fungi: Diverse Structures and Bioactivities. Mini Rev. Med. Chem. 2017, 17, 603–632. [Google Scholar] [CrossRef]
- Wari, D.; Kabir, M.A.; Mujiono, K.; Hojo, Y.; Shinya, T.; Tani, A.; Nakatani, H.; Galis, I. Honeydew-associated microbes elicit defense responses against brown planthopper in rice. J. Exp. Bot. 2019, 70, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Wisecaver, J.H.; Lind, A.L. The birth, evolution and death of metabolic gene clusters in fungi. Nat. Rev. Microbiol. 2018, 16, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.S.; Adler, P.H.; Vogel, H.; Ping, L.Y. Gender-specific bacterial composition of black flies (Diptera: Simuliidae). FEMS Microbiol. Ecol. 2012, 80, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhang, Y.H.; Cai, T.W.; Deng, X.Q.; Liu, C.Y.; Li, J.M.; He, S.; Li, J.H.; Wan, H. Antibiotics increased host insecticide susceptibility via collapsed bacterial symbionts reducing detoxification metabolism in the brown planthopper, Nilaparvata lugens. J. Pest. Sci. 2021, 94, 757–767. [Google Scholar] [CrossRef]
- Shentu, X.P.; Li, D.T.; Xu, J.F.; She, L.; Yu, X.P. Effects of fungicides on the yeast-like symbiotes and their host, Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2016, 128, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.P.; Wang, X.L.; Xiao, Y.; Yu, X.P. Effects of Fungicide Propiconazole on the Yeast-Like Symbiotes in Brown Planthopper (BPH, Nilaparvata lugens Stål) and Its Role in Controlling BPH Infestation. Front. Physiol. 2019, 10, 89. [Google Scholar] [CrossRef]


| Sample | Average Length (bp) 1 | OTUs | Chao1 1 | ACE 1 | Shannon 1 | Simpson 1 |
|---|---|---|---|---|---|---|
| D0 | 697.18 ± 64.70 | 8910 | 2438.46 ± 301.77 | 2685.45 ± 392.33 | 6.02 ± 0.49 | 0.97 ± 0.01 |
| D1 | 679.50 ± 7.40 | 7670 | 4211.15 ± 1128.26 | 4473.42 ± 1181.89 | 5.26 ± 0.42 | 0.91 ± 0.03 |
| D2 | 682.52 ± 5.24 | 7376 | 5050.25 ± 436.99 | 5387.22 ± 405.28 | 5.32 ± 0.13 | 0.91 ± 0.02 |
| D3 | 682.24 ± 3.11 | 7920 | 5694.85 ± 339.04 | 5991.18 ± 356.93 | 5.41 ± 0.08 | 0.91 ± 0.00 |
| D4 | 722.13 ± 19.83 | 8548 | 5258.31 ± 1571.99 | 5566.48 ± 1568.56 | 5.50 ± 0.20 | 0.91 ± 0.01 |
| D5 | 702.84 ± 7.32 | 7150 | 5328.88 ± 428.94 | 5653.63 ± 434.55 | 5.35 ± 0.02 | 0.90 ± 0.00 |
| DF | 694.77 ± 1.96 | 7560 | 4117.70 ± 1121.43 | 4410.26 ± 1053.31 | 5.64 ± 0.41 | 0.92 ± 0.02 |
| DM | 666.93 ± 11.08 | 7660 | 5119.20 ± 155.58 | 5641.36 ± 217.72 | 5.75 ± 0.06 | 0.96 ± 0.00 |
| F-Sal | 612.42 ± 12.27 | 7692 | 1967.43 ± 209.16 | 2116.07 ± 193.72 | 5.39 ± 0.70 | 0.92 ± 0.04 |
| F-Mid | 677.73 ± 59.68 | 7078 | 2003.80 ± 221.89 | 2162.75 ± 196.80 | 5.50 ± 0.74 | 0.92 ± 0.06 |
| F-Mal | 615.61 ± 14.93 | 4934 | 2058.24 ± 539.83 | 2208.23 ± 348.79 | 4.62 ± 0.23 | 0.88 ± 0.07 |
| F-Hin | 658.89 ± 66.65 | 6402 | 2098.50 ± 342.34 | 2247.86 ± 261.80 | 4.60 ± 0.41 | 0.83 ± 0.03 |
| F-Ova | 732.75 ± 82.09 | 5887 | 2511.27 ± 731.67 | 2660.79 ± 643.26 | 4.83 ± 0.31 | 0.89 ± 0.04 |
| F-Fat | 676.28 ± 7.26 | 7951 | 2903.54 ± 348.07 | 3091.26 ± 334.51 | 5.48 ± 0.64 | 0.93 ± 0.03 |
| F-Hon | 628.93 ± 56.37 | 4818 | 1378.55 ± 241.18 | 1573.77 ± 310.57 | 5.01 ± 0.15 | 0.96 ± 0.01 |
| M-Sal | 620.45 ± 17.57 | 5581 | 2709.21 ± 508.60 | 2987.41 ± 522.73 | 4.94 ± 0.18 | 0.92 ± 0.02 |
| M-Mid | 617.28 ± 4.61 | 7056 | 2787.83 ± 611.12 | 3046.64 ± 703.01 | 5.28 ± 0.58 | 0.93 ± 0.02 |
| M-Mal | 632.90 ± 17.83 | 8825 | 2685.96 ± 610.87 | 2946.71 ± 711.27 | 5.89 ± 0.59 | 0.96 ± 0.01 |
| M-Hin | 689.25 ± 74.71 | 8349 | 2437.76 ± 455.96 | 2857.79 ± 596.39 | 5.26 ± 0.35 | 0.96 ± 0.03 |
| M-Tes | 704.38 ± 25.59 | 8010 | 3668.89 ± 703.50 | 4079.21 ± 826.86 | 5.43 ± 0.18 | 0.95 ± 0.02 |
| M-Fat | 684.36 ± 7.27 | 7842 | 3399.84 ± 639.60 | 3697.81 ± 777.00 | 5.15 ± 0.54 | 0.90 ± 0.03 |
| All | 693.78 ± 94.97 | 87,131 | 3337.92 ± 1455.67 | 3608.31 ± 1524.12 | 5.31 ± 0.73 | 0.92 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Yang, J.; Li, T.; Li, J.; Ye, M.; Wang, J.; Chen, R.; Zhu, L.; Du, B.; He, G. Endosymbiotic Fungal Diversity and Dynamics of the Brown Planthopper across Developmental Stages, Tissues, and Sexes Revealed Using Circular Consensus Sequencing. Insects 2024, 15, 87. https://doi.org/10.3390/insects15020087
Cheng Y, Yang J, Li T, Li J, Ye M, Wang J, Chen R, Zhu L, Du B, He G. Endosymbiotic Fungal Diversity and Dynamics of the Brown Planthopper across Developmental Stages, Tissues, and Sexes Revealed Using Circular Consensus Sequencing. Insects. 2024; 15(2):87. https://doi.org/10.3390/insects15020087
Chicago/Turabian StyleCheng, Yichen, Jing Yang, Tianzhu Li, Jiamei Li, Meng Ye, Jing Wang, Rongzhi Chen, Lili Zhu, Bo Du, and Guangcun He. 2024. "Endosymbiotic Fungal Diversity and Dynamics of the Brown Planthopper across Developmental Stages, Tissues, and Sexes Revealed Using Circular Consensus Sequencing" Insects 15, no. 2: 87. https://doi.org/10.3390/insects15020087
APA StyleCheng, Y., Yang, J., Li, T., Li, J., Ye, M., Wang, J., Chen, R., Zhu, L., Du, B., & He, G. (2024). Endosymbiotic Fungal Diversity and Dynamics of the Brown Planthopper across Developmental Stages, Tissues, and Sexes Revealed Using Circular Consensus Sequencing. Insects, 15(2), 87. https://doi.org/10.3390/insects15020087








