Imported Fire Ants Discard Cricket Eggs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
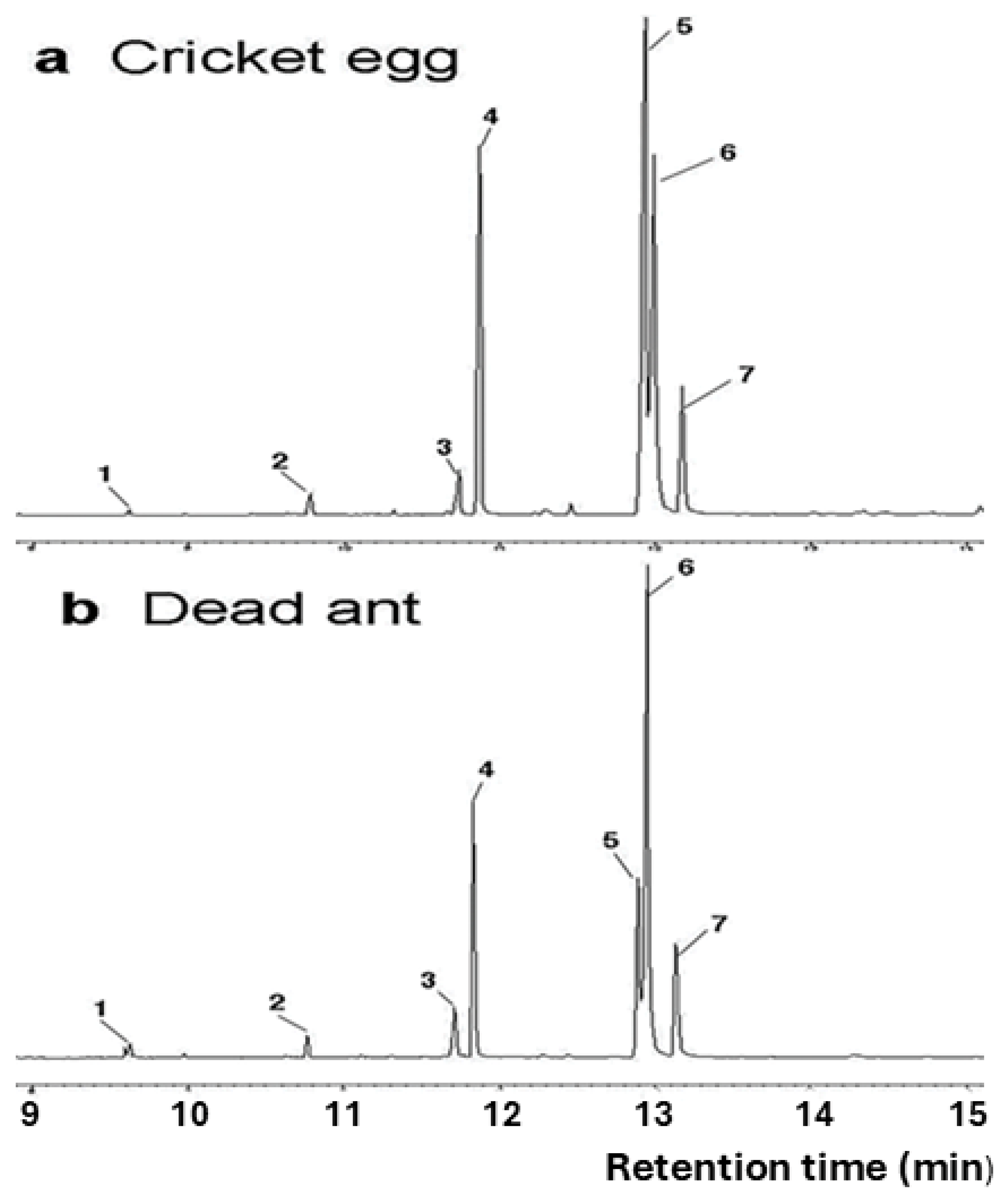
2.2. Fatty Acid Analysis
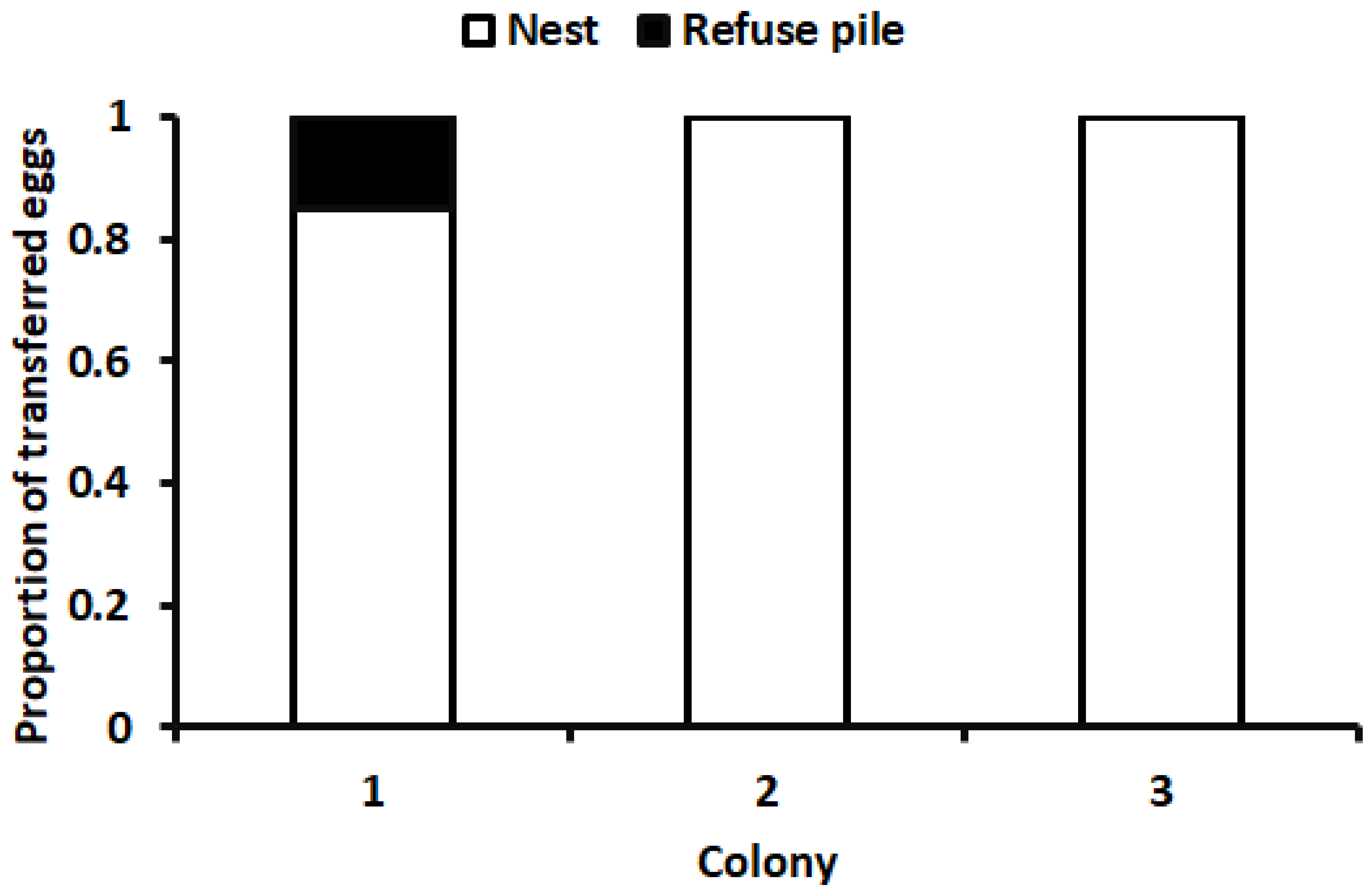
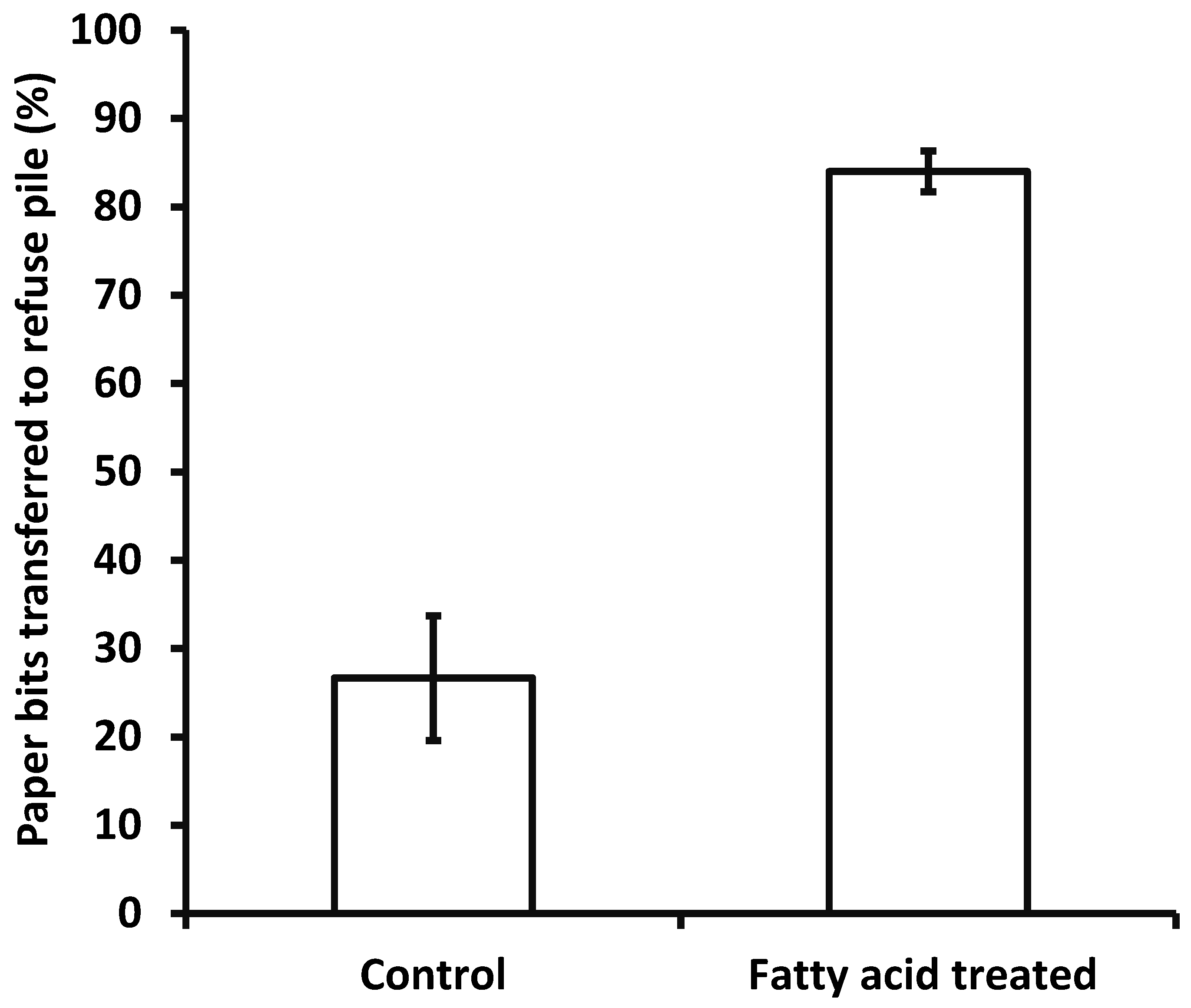
2.3. Bioassay
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ascunce, M.S.; Yang, C.C.; Oakey, J.; Calcaterra, L.; Wu, W.J.; Shih, C.J.; Goudet, J.; Ross, K.G.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Gland, Switzerland, 2000; pp. 1–12. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990; xii + 732p. [Google Scholar]
- Pfannenstiel, R.S. Nocturnal predators and their impact lepidopteran eggs in annual crops: What we don’t see does help us! In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 12–16 September 2005; Hoddle, M.E., Ed.; Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2005; pp. 463–471. [Google Scholar]
- Nuessly, G.S.; Sterling, W.L. Mortality of Helicoverpa zea (Lepidoptera: Noctuidae) eggs in cotton as a function of oviposition sites, predator species, and desiccation. Environ. Entomol. 1994, 23, 1189–1202. [Google Scholar] [CrossRef]
- McDaniel, S.G.; Sterling, W.L. Predation of Heliothis virescens (F.) eggs on cotton in East Texas. Environ. Entomol. 1982, 11, 60–66. [Google Scholar] [CrossRef]
- Negm, A.A.; Hensley, S.D. Evaluation of certain biological control agents of the sugarcane borer in Louisiana. J. Econ. Entomol. 1969, 62, 1008–1013. [Google Scholar] [CrossRef]
- Mitchell, P.L.; Paysen, E.S.; Muckenfuss, A.E.; Schaffer, M.; Shepard, B.M. Natural mortality of leaffooted bug (Hemiptera: Heteroptera: Coreidae) eggs in cowpea. J. Agric. Urban Entomol. 1999, 16, 25–36. [Google Scholar]
- Lee, D.K. The Effects of Certain Physical and Biological Factors on the Survival and the Hatching Rates of Psorophora Columbiae (Dyar and Knab) (Diptera: Culicidae) Eggs. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1989; 292p. [Google Scholar]
- Diez, L.; Moquet, L.; Detrain, C. Post-mortem changes in chemical profile and their influence on corpse removal in ants. J. Chem. Ecol. 2013, 39, 1424–1432. [Google Scholar] [CrossRef]
- Blum, M.S. The chemical basis of insect sociality. In Chemicals Controlling Insect Behavior; Beroza, M., Ed.; Academic Press: New York, NY, USA, 1970; pp. 61–94. [Google Scholar]
- Oi, D.H.; Pereira, R.M. Ant Behavior and Microbial Pathogens (Hymenoptera: Formicidae). Fla. Entomol. 1993, 76, 63–73. Available online: http://www.fcla.edu/FlaEnt (accessed on 25 November 2024). [CrossRef]
- Wilson, E.O.; Durlach, N.I.; Roth, L.M. Chemical releasers of necrophoric behavior in ants. Psyche 1958, 65, 108–114. [Google Scholar] [CrossRef]
- Haskins, C.P.; Haskins, E.F. Notes on the necrophoric behaviour in the archaic ant Myrmecia vindex. Psyche 1974, 81, 258–267. [Google Scholar] [CrossRef]
- Gordon, D.M. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 1983, 9, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.F.; Tschinkel, W.R. Aspects of necrophoric behavior in the red imported fire ant. Solenopsis invicta. Behaviour 1976, 56, 157–180. [Google Scholar] [CrossRef]
- Renucci, M.; Tirard, A.; Provost, E. Complex undertaking behavior in Temnothorax lichtensteini ant colonies: From corpse-burying behavior to necrophoric behavior. Insectes Sociaux 2011, 58, 9–16. [Google Scholar] [CrossRef]
- Choe, D.H. Necrophoric Behavior of the Argentine Ant, Linepithema Humile (Mayr) (Hymenoptera: Formicidae), and Its Implications for Horizontal Transfer of Slow-Acting iNsecticides. Ph.D. Thesis, Department of Entomology, University of California, Riverside, CA, USA, 2009. [Google Scholar]
- Fefferman, N.H.; Traniello, J.F.A.; Rosengaus, R.B.; Calleri, I.D.V. Disease prevention and resistance in social insects: Modeling the survival consequences of immunity, hygienic behavior, and colony organization. Behav. Ecol. Sociobiol. 2007, 61, 565–577. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, X. Corpse management in social insects. Int. J. Biol. Sci. 2013, 9, 313–321. [Google Scholar] [CrossRef]
- Haskins, C.P. Researches in the biology and social behavior of primitive ants. In Development and Evolution of Behavior; Aronson, L.R., Tobach, E., Lehrman, D.S., Rosenblatt, J.S., Eds.; W. H. Freeman: San Francisco, CA, USA, 1970; pp. 355–388, xviii + 656p. [Google Scholar]
- Choe, D.H.; Millar, J.G.; Rust, M.K. Chemical signals associated with life inhibit necrophoresis in Argentine ants. Proc. Natl. Acad. Sci. USA 2009, 106, 8251–8255. [Google Scholar] [CrossRef]
- Compton, S.G.; Ware, A.B. Ants disperse the elaiosome-bearing eggs of an African stick insect. Psyche 1991, 98, 207–213. [Google Scholar] [CrossRef]
- Hughes, L.; Westoby, M. Capitula on stick insect eggs and elaiosomes on seeds: Convergent adaptations for burial by ants. Funct. Ecol. 1992, 6, 642–648. [Google Scholar]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Freese, E.; Shew, C.W.; Galliers, E. Function of lipophilic acids as antimicrobial food additives. Nature 1973, 241, 321–325. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia 2002, 73, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Rosenfeld, J.; Attridge, S.; Sidhu, S.; Aksenov, V.; Rollo, C.D. The ancient chemistry of avoiding risks of predation and disease. Evol. Biol. 2009, 36, 267–281. [Google Scholar] [CrossRef]





| Insect | Relative content of fatty acid (%, mean ± SE) | ||||
|---|---|---|---|---|---|
| palmitoleic acid | palmitic acid | linoleic acid | oleic acid | stearic acid | |
| Cricket eggs | 2.13 ± 0.48 | 25.39 ± 0.89 | 26.46 ± 0.78 | 39.97 ± 0.93 | 6.05 ± 0.55 |
| Dead ants | 7.42 ± 0.62 | 17.40 ± 1.28 | 17.75 ± 0.94 | 44.01 ± 1.70 | 13.43 ± 0.98 |
| Absolute concentration of fatty acid (µg/egg, mean ± SE) | |||||
| palmitoleic acid | palmitic acid | linoleic acid | oleic acid | stearic acid | |
| Cricket eggs | 0.089 ± 0.035 | 1.002 ± 0.347 | 1.031 ± 0.338 | 1.580 ± 0.522 | 0.254 ± 0.098 |
| Dead ants | 0.239 ± 0.099 | 0.861 ± 0.209 | 1.283 ± 0.380 | 1.784 ± 0.467 | 0.298 ± 0.132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ni, X.; Grodowitz, M.J. Imported Fire Ants Discard Cricket Eggs. Insects 2024, 15, 954. https://doi.org/10.3390/insects15120954
Chen J, Ni X, Grodowitz MJ. Imported Fire Ants Discard Cricket Eggs. Insects. 2024; 15(12):954. https://doi.org/10.3390/insects15120954
Chicago/Turabian StyleChen, Jian, Xinzhi Ni, and Michael J. Grodowitz. 2024. "Imported Fire Ants Discard Cricket Eggs" Insects 15, no. 12: 954. https://doi.org/10.3390/insects15120954
APA StyleChen, J., Ni, X., & Grodowitz, M. J. (2024). Imported Fire Ants Discard Cricket Eggs. Insects, 15(12), 954. https://doi.org/10.3390/insects15120954








