Phylogeography of the Invasive Fruit Fly Species Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae) in South America
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Data Analysis
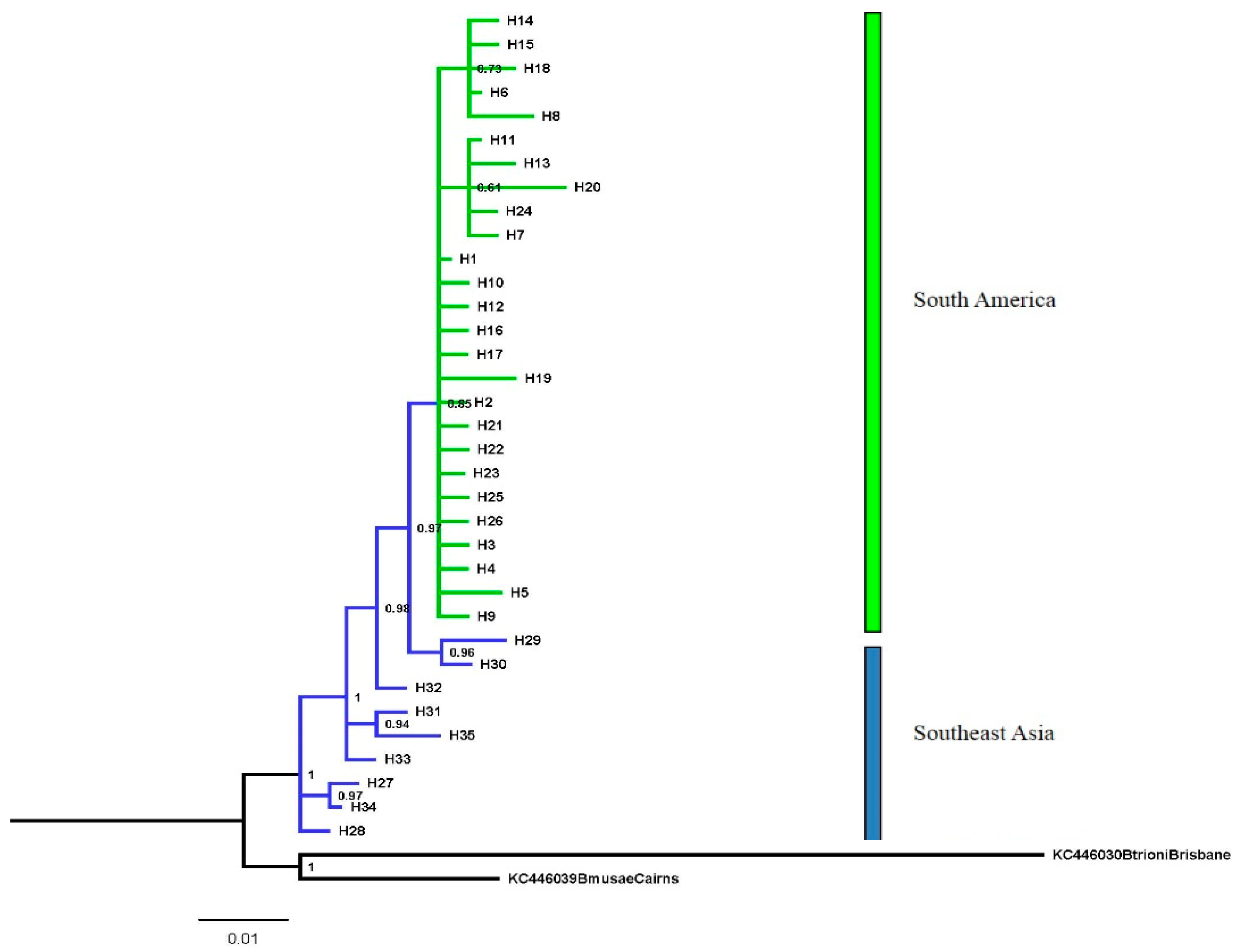
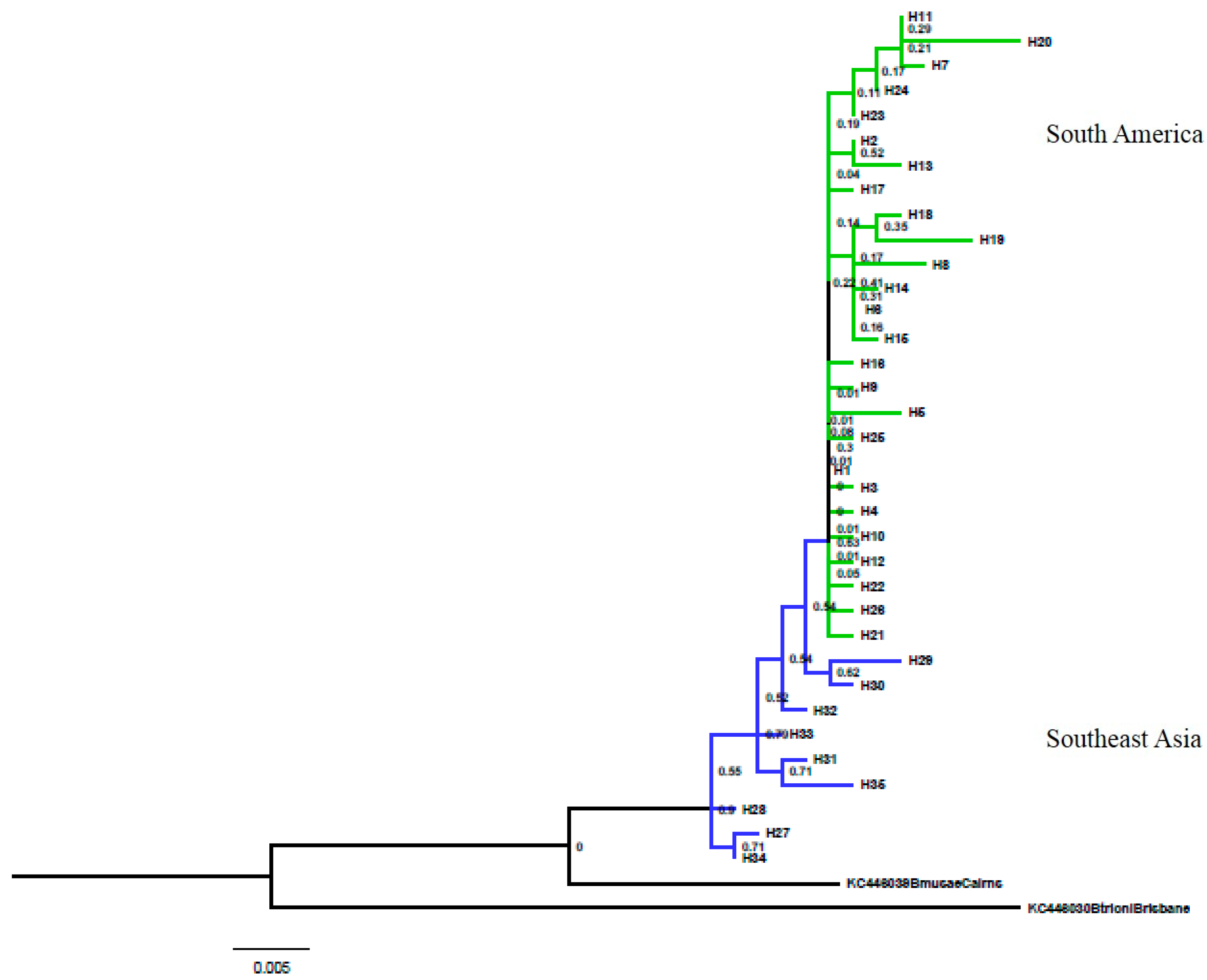
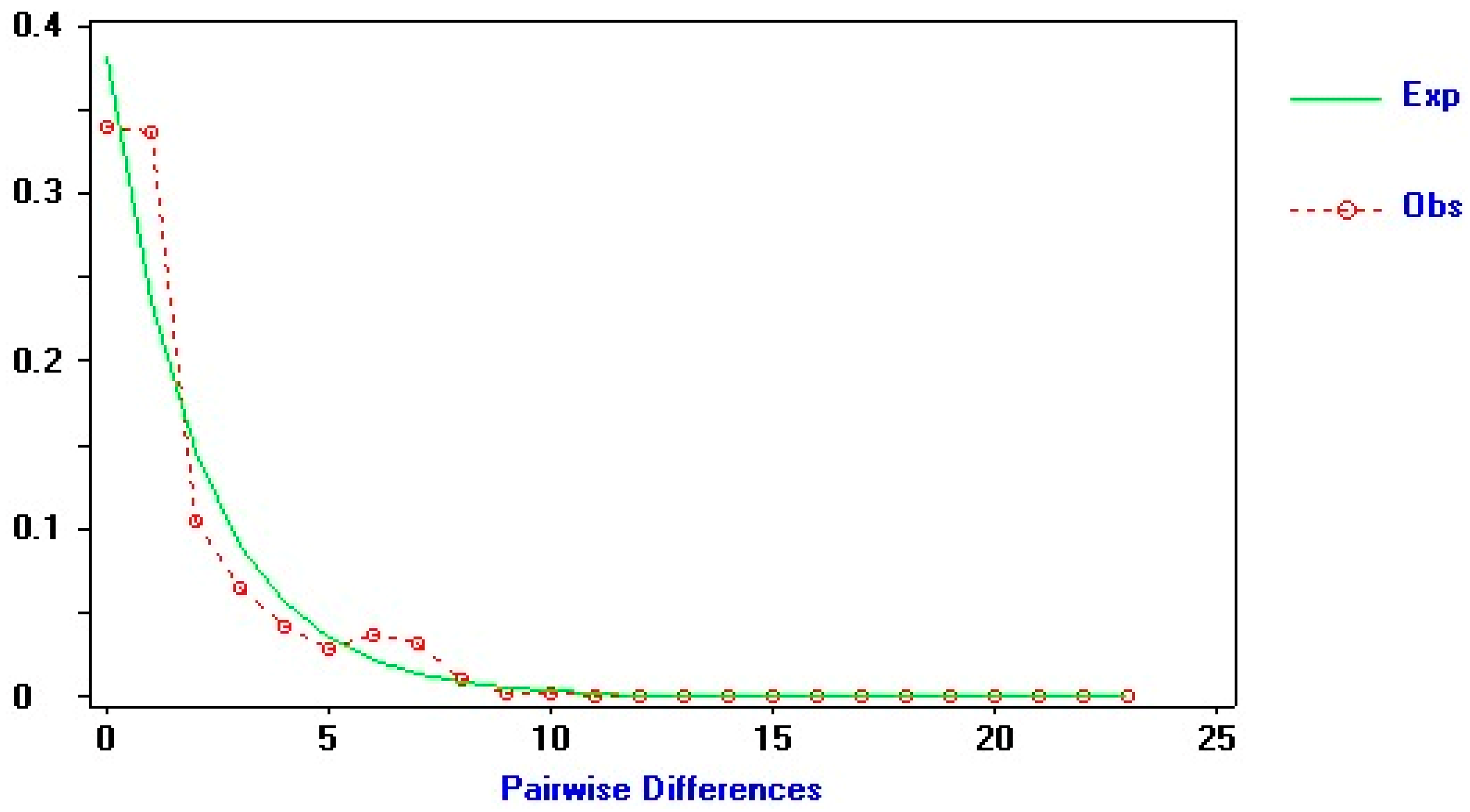
3. Results
Population Structure
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aluja, M. Bionomics and Management of Anastrepha. Annu. Rev. Entomol. 1994, 39, 155–178. [Google Scholar] [CrossRef]
- Zhao, Z.; Carey, J.R.; Li, Z. The Global Epidemic of Bactrocera Pests: Mixed-Species Invasions and Risk Assessment. Annu. Rev. Entomol. 2024, 69, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.A. Mosca-do-mediterrâneo, Ceratitis capitata (Diptera: Tephritidae). In Histórico e Impacto de Pragas Introduzidas no Brasil, 1st ed.; Vilela, E.F., Zucchi, R.A., Cantor, F., Eds.; Holos: Ribeirão Preto, Brazil, 2001; pp. 13–24. [Google Scholar]
- Vayssières, J.F.; Cayol, J.P.; Caplong, P.; Séguret, J.; Midgarden, D.; van Sauers Muller, A.; Zucchi, R.; Uramoto, K.; Malavasi, A. Diversity of fruit fly (Diptera: Tephritidae) species in French Guiana: Their main host plants and associated parasitoids during the period 1994–2003 and prospects for management. Fruits 2013, 68, 219–243. [Google Scholar] [CrossRef]
- Lemos, L.N.; Adaime, R.; Jesus-Barros, C.R.; Deus, E.G. New hosts of Bactrocera carambolae (Diptera: Tephritidae) in Brazil. Fla. Entomol. 2014, 97, 694–704. [Google Scholar] [CrossRef]
- Qui, Y.; Paini, D.R.; Wang, C.; Fang, Y.; Li, Z. Global establishment risk of economically important fruit fly species (Tephritidae). PLoS ONE 2015, 10, e0116424. [Google Scholar] [CrossRef]
- Drew, R.A.I. Biogeography and speciation in the Dacini (Diptera: Tephritidae: Dacinae). Bish. Mus. Bull. Entomol. 2004, 12, 165–178. [Google Scholar]
- Boykin, L.M.; Schutze, M.K.; Krosch, M.N.; Chomic, A.; Chapman, T.A.; Englezou, A.; Armstrong, K.F.; Clarke, A.R.; Hailstones, D.; Cameron, S.L. Multi gene phylogenetic analysis of the south-east Asian pest members of the Bactrocera dorsalis species complex (Diptera: Tephritidae) does not support current taxonomy. J. Appl. Entomol. 2014, 138, 235–253. [Google Scholar] [CrossRef]
- Nentwig, W. Pathways in Animal Invasions. In Biological Invasions, 1st ed.; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 11–27. [Google Scholar] [CrossRef]
- Khamis, F.M.; Masiga, D.K.; Mohamed, S.A.; Salifu, D.; De Meyer, M.; Ekesi, S. Taxonomic identity of the invasive fruit fly pest, Bactrocera invadens: Concordance in morphometry and DNA barcoding. PLoS ONE 2012, 7, e44862. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics, 1st ed.; CAB International in Association with ACIAR: Wallingford, UK, 1992; 624p. [Google Scholar]
- van Sauers-Muller, A. An overview of the carambola fruit fly Bactrocera species (Diptera: Tephritidae) found recently in Suriname. Fla. Entomol. 1991, 74, 432–440. [Google Scholar] [CrossRef]
- Malavasi, A.; Zucchi, R.A.; Sugayama, R.L. Biogeografia. In Moscas-Das-Frutas de Importância Econômica no Brasil: Conhecimento Básico e Aplicado; Malavasi, A., Zucchi, R.A., Eds.; Holos: Ribeirão Preto, Brazil, 2000; pp. 93–98. [Google Scholar]
- Godoy, M.J.S.; Pacheco, W.S.P.; Portal, R.R.; Pires Filho, J.M.; Moraes, L.M.M. Programa Nacional de Erradicação da Mosca-da-carambola. In Moscas-Das-Frutas na Amazônia Brasileira: Diversidade, Hospedeiros e Inimigos Naturais; Silva, R.A., Lemos, W.P., Zucchi, R.A., Eds.; Embrapa Amapá: Macapá, Brazil, 2011; pp. 134–158. [Google Scholar]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento. Superintendência Federal no Estado do Pará. Portaria No 183, 9 November 2012. [Google Scholar]
- Malavasi, A.; Midgarden, D.; De Meyer, M. Bactrocera species that pose a threat to Florida: B. carambolae and B. invadens. In Potential Invasive Pests of Agricultural Crops; Peña, J.E., Ed.; CAB International: Wallingford, UK, 2013; pp. 214–227. [Google Scholar]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento. Superintendência Federal no Estado do Pará. Portaria No 55, 15 April 2014. [Google Scholar]
- Castilho, A.P.; Pasinato, J.; Santos, J.E.V.D.; Nava, D.E.; Jesus, C.R.; Adaime, R. Biology of Bactrocera carambolae (Diptera: Tephritidae) on four hosts. Rev. Bras. Entomol. 2019, 63, 302–307. [Google Scholar] [CrossRef]
- Allwood, A.J.; Chinajariyawong, A.; Drew, R.A.I.; Hamacek, E.L.; Hancock, D.L.; Hengsawad, C.; Jinapin, J.C.; Jirasurat, M.; Kong Krong, C.; Kritsaneepaiboon, S.; et al. Host plant records for fruit flies (Diptera:Tephritidae) in South-East Asia. Raffles Bull. Zool. 1999, 47, 1–92. [Google Scholar]
- van Sauers-Muller, A. Host Plants of the Carambola Fruit Fly, Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae), in Suriname, South America. Neotrop. Entomol. 2005, 34, 203–214. [Google Scholar] [CrossRef]
- Adaime, R.; Pereira, J.D.B.; Sousa, M.S.M.; Jesus, C.R.; Souza-Filho, M.F.; Zucchi, R.A. Moscas-das-frutas, suas plantas hospedeiras e parasitoides no Estado do Amapá. In Moscas das Frutas no Brasil: Conhecimento Básico e Aplicado; Zucchi, R.A., Malavasi, A., Adaime, R., Nava, D.E., Eds.; Fealq: Piracicaba, Brazil, 2023; pp. 51–68. [Google Scholar]
- Costa, J.V.T.A.; Sousa, M.S.M.; Jesus, C.R.; Souza-Filho, M.F.; Costa, V.A.; Silva, B.M.S.; Oliveira, J.P.M.; Adaime, R. New Findings on Carambola Fruit Fly Hosts in South America. Fla. Entomol. 2023, 106, 161–174. [Google Scholar] [CrossRef]
- Costa, J.V.T.A.; Sousa, M.S.M.; Souza-Filho, M.F.; Adaime, R. Chrysophyllum cainito L. (Sapotaceae): Novo hospedeiro da mosca-da-carambola no Brazil. Agrotrópica 2023, 35, 161–164. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Schutze, M.K.; Aketarawong, N.; Amornsak, W.; Armstrong, K.F.; Augustinos, A.A.; Barr, N.; Bo, W.; Bourtzis, K.; Boykin, L.M.; Caceres, C.; et al. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Syst. Entomol. 2015, 40, 456–471. [Google Scholar] [CrossRef]
- Taddei, A.; Reisenzein, H.; Mouttet, R.; Lethmayer, C.; Egartner, A.; Gottsberger, R.A.; Blümel, B.; Heiss, C.; Pohn, C.; Reynaud, P. Morphological and Molecular identification protocols for Bactrocera dorsalis: A joint validation study. PhytoFrontiers™ 2023, 3, 186–198. [Google Scholar] [CrossRef]
- Schutze, M.; Mahmood, K.; Pavasovic, A.; Bo, W.; Newman, J.; Clark, A.R.; Krosch, M.N.; Cameron, S.L. One and the same: Integrative taxonomic evidence that Bactrocera invadens (Diptera: Tephritidae) is the same species as the Oriental fruit fly Bactrocera dorsalis. Syst. Entomol. 2014, 40, 472–486. [Google Scholar] [CrossRef]
- Wan, X.; Nardi, F.; Zhang, B.; Liu, Y. The Oriental Fruit Fly, Bactrocera dorsalis, in China: Origin and Gradual Inland Range Expansion Associated with Population Growth. PLoS ONE 2011, 6, 25238. [Google Scholar] [CrossRef]
- Prabhakar, C.S.; Mehta, P.K.; Sood, P.; Singh, S.K.; Sharma, P.; Sharma, P.N. Population genetic structure of the melon fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) based on mitochondrial cytochrome oxidase (COI) gene sequences. Genetica 2012, 140, 83–91. [Google Scholar] [CrossRef]
- Wu, Y.; McPheron, B.A.; Wu, J.J.; Li, Z.H. Genetic relationship of the melon fly, Bactrocera curcubitae (Diptera: Tephritidae) inferred from mitochondrial DNA. Insect Sci. 2012, 19, 195–204. [Google Scholar] [CrossRef]
- Aketarawong, N.; Isasawin, S.; Sojikul, P.; Thanaphum, S. Gene flow and genetic structure of Bactrocera carambolae (Diptera, Tephritidae) among geographical differences and sister species, B. dorsalis, inferred from microsatellite DNA data. Zookeys 2015, 540, 239–272. [Google Scholar] [CrossRef] [PubMed]
- Schutze, M.K.; Bourtzis, K.; Cameron, S.L.; Clarke, A.R.; De Meyer, M.; Hee, A.K.W.; Hendrichs, J.; Krosch, M.N.; Mwatawala, M. Integrative taxonomy versus taxonomic authority without peer review: The case of the Oriental fruit fly, Bactrocera dorsalis (Tephritidae): Integrative taxonomy versus authority. Syst. Entomol. 2017, 42, 609–620. [Google Scholar] [CrossRef]
- Meixner, M.D.; McPheron, B.A.; Silva, J.G.; Gasparich, G.E.; Sheppard, W.S. The Mediterranean fruit fly in California: Evidence for multiple introductions and persistent populations based on microsatellite and mitochondrial DNA variability. Mol. Ecol. 2002, 11, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.T.; Kambhampati, S.; Armstrong, K.A. Phylogenetic relationships among Bactrocera species (Diptera: Tephritidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Chen, H.; Wu, J.; Li, Z. Population structure and colonization of Bactrocera dorsalis (Diptera: Tephritidae) in China, inferred from mtDNA COI sequences. J. Appl. Entomol. 2012, 136, 241–251. [Google Scholar] [CrossRef]
- Ruiz-Arce, R.; Barr, N.B.; Owen, C.L.; Thomas, D.B.; McPheron, B.A. Phylogeography of Anastrepha obliqua inferred with mtDNA sequencing. J. Econ. Entomol. 2012, 105, 2147–2158. [Google Scholar] [CrossRef]
- Barr, N.B.; Ledezma, L.A.; Leblanc, L.; San Jose, M.; Rubinoff, D.; Geib, S.M.; Fujita, B.; Bartels, D.W.; Garza, D.; Kerr, P.; et al. Genetic diversity of Bactrocera dorsalis (Diptera: Tephritidae) on the Hawaiian islands: Implications for an introduction pathway into California. J. Econ. Entomol. 2014, 107, 1946–1958. [Google Scholar] [CrossRef]
- Ruiz-Arce, R.; Owen, C.L.; Thomas, D.B.; Barr, N.B.; McPheron, B.A. Phylogeographic structure in Anastrepha ludens (Diptera: Tephritidae) populations inferred with mtDNA sequencing. J. Econ. Entomol. 2015, 108, 1324–1336. [Google Scholar] [CrossRef]
- Smith-Caldas, M.R.B.; McPheron, B.A.; Silva, J.G.; Zucchi, R.A. Phylogenetic relationships among species of the fraterculus group (Anastrepha: Diptera: Tephritidae) inferred from DNA sequence of the mitochondrial cytocrome oxidase I. Neotrop. Entomol. 2001, 30, 565–573. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse Metazoan Invertebrate. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–297. [Google Scholar] [PubMed]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. MCMC Trace Analysis Tool, v1.6.0; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Rambaut, A. Tree Figure Drawing Tool, Version 1.3.1; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2009. [Google Scholar]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin v3.11: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Tajima, F. Statistical methods for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 1447, 915–925. [Google Scholar] [CrossRef]
- Ramos-Osins, S.E.; Rozas, J. Statistical Properties of New Neutrality Testes Against Population Growth. Mol. Biol. Evol. 2002, 12, 2092–2100. [Google Scholar] [CrossRef]
- Dlugosch, K.M.; Parker, M. Founding events in species invasions: Genetic variation, adaptive evolution, and role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef]
- Lynch, M. (Ed.) The Origins of Genome Architecture; Sinauer Associates: Sunderland, MA, USA, 2007; pp. 60–100. [Google Scholar]
- Passos, J.F.; Nascimento, D.B.; Menezes, R.S.T.; Adaime, R.; Araujo, E.L.; Lima, K.M.; Roberto Zucchi, R.A.; Ronchi Teles, B.; Nascimento, R.R.; Ruiz-Arce, R.; et al. Genetic structure and diversity in Brazilian populations of Anastrepha obliqua (Diptera: Tephritidae). PLoS ONE 2018, 13, e0208997. [Google Scholar] [CrossRef]
- Gasparich, G.E.; Silva, J.G.; Han, J.Y.; McPheron, B.A.; Steck, G.J.; Sheppard, W.S. Population genetic structure of Mediterranean fruit fly (Diptera: Tephritidae) and implications for worldwide colonization patterns. Ann. Entomol. Soc. Am. 1997, 90, 790–797. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Zheng, L.; Guglielmino, C.R. Microsatellite analysis of medfly bioinfestation in California. Mol. Ecol. 2001, 10, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.G.; Meixner, M.D.; McPheron, B.A.; Steck, G.J.; Sheppard, W.S. Recent Mediterranean fruit fly (Diptera: Tephritidae) infestations in Florida—A genetic perspective. J. Econ. Entomol. 2003, 96, 1711–1718. [Google Scholar] [CrossRef]
- Aketarawong, N.; Bonizzoni, M.; Thanaphum, S.; Gomulski, L.M.; Gasperi, G.; Malacrida, A.R.; Guglielmino, C.R. Inferences on the population structure and colonization process of the invasive oriental fruit fly, Bactrocera dorsalis (Hendel). Mol. Ecol. 2007, 16, 3522–3532. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.L.; Nardi, F.; Zhang, R.J. Population genetic structure of the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae), from China and Southeast Asia. Genetica 2008, 134, 319–324. [Google Scholar] [CrossRef]


| Country | District/State | Locality | N | Coordinates | Host | Haplotypes |
|---|---|---|---|---|---|---|
| Suriname | Coronie | Jenny | 07 | 56°05′ W 05°80′ N | Averrhoa carambola | H1 (1), H22 (1), H23 (4), H24 (1) |
| Totness | 06 | 56°33′ W 05°87′ N | Averrhoa carambola | H1 (6) | ||
| Brokopondo | Berg en Dal | 06 | 55°07′ W 05°13′ N | Averrhoa carambola | H1 (6) | |
| Wanica | Houttuin | 07 | 55°10′ W 05°43′ N | Averrhoa carambola | H1 (4), H6 (3) | |
| Domburg | 05 | 55°04′ W 05°41′ N | Averrhoa carambola | H1 (2), H6 (1), H11 (1), H21 (1) | ||
| Saramacca | Dirkshoop | 07 | 55°47′ W 05°77′ N | Averrhoa carambola | H1(3), H23 (1), H25 (2), H26 (1) | |
| Paramaribo † | - | 11 | 55°10′ W 05°48′ N | Averrhoa carambola | H1 (7) | |
| Brazil | Amapá | Ilha de Santana | 10 | 51°10′ W 00°03′ S | Eugenia uniflora | H1 (7), H11 (1), H16 (1), H17 (1) |
| Macapá | 11 | 50°47′ W 00°46′ N | Syzygium malaccense | H1 (5), H6 (4), H7 (1), H8 (1) | ||
| Oiapoque | 9 | 51°49′ W 03°49′ N | Psidium guajava | H1 (7), H3 (2) | ||
| Mazagão | 05 | 51°16′ W 00°05′ S | Psidium guajava | H1 (2), H11 (1), H19 (1), H20 (1) | ||
| Porto Grande | 04 | 51°24′ W 00°42’ N | Psidium guajava | H1 (3), H18 (1) | ||
| Ferreira Gomes | 08 | 51°14′ W 00°51′ N | Psidium guajava | H1 (3), H6 (3), H9 (1), H10 (1) | ||
| Laranjal do Jari | 03 | 52°30′ W 00°50′ S | McPhail traps | H1 (2), H4 (1) | ||
| Roraima | Uiramutã | 09 | 60°10′ W 04°35′ N | Averrhoa carambola | H1 (7), H2 (1), H3 (1) | |
| Pacaraima | 10 | 61°07′ W 04°29′ N | Averrhoa carambola | H1 (3), H6 (2), H11 (1), H12 (1), H13 (1), H14 (1), H15 (1) | ||
| Normandia | 04 | 59°37′ W 03°52′ N | McPhail traps | H1 (3), H6 (1) | ||
| Pará | Monte Dourado | 05 | 52°34′ W 01°31′ S | McPhail traps | H1 (4), H5 (1) | |
| Indonesia | South Sumatra † | Lampung | 04 | 105°56′ E 5°40′ S | H27 (1), H28 (1), H29 (1), H30 (1) | |
| Thailand | Chiang Mai † | San Pa Tong | 01 | 98°53′ E 18°37′ N | H31 (1) | |
| Nakhon Si Thammarat † | Muang District | 04 | 99°53′ E 8°25′ N | H32 (1), H33 (1), H34 (1), H35 (1) |
| Population | N | H | Hd (±S.D.) | π (±S.D.) |
|---|---|---|---|---|
| Jenny-CO | 07 | 4 | 0.7143 (0.112) | 0.00163 (0.00054) |
| Totness-CO | 06 | 1 | - | - |
| Berg en Dal-BK | 06 | 1 | - | - |
| Houttuin-WA | 07 | 2 | 0.571 (0.119) | 0.00089 (0.00019) |
| Domburg-WA | 05 | 4 | 0.900 (0.161) | 0.00187 (0.00050) |
| Dirkshoop-SA | 07 | 4 | 0.810 (0.130) | 0.00163 (0.00040) |
| Paramaribo-PA | 11 | 2 | 0.182 (0.144) | 0.00028 (0.00022) |
| Ilha de Santana-AP | 10 | 4 | 0.533 (0.180) | 0.00093 (0.00037) |
| Macapá-AP | 11 | 4 | 0.709 (0.099) | 0.00227 (0.00079) |
| Oiapoque-AP | 9 | 2 | 0.389 (0.164) | 0.00061 (0.00026) |
| Mazagão-AP | 05 | 4 | 0.900 (0.161) | 0.00654 (0.00222) |
| Porto Grande-AP | 04 | 2 | 0.500 (0.265) | 0.00234 (0.00124) |
| Ferreira Gomes-AP | 08 | 4 | 0.786 (0.113) | 0.00161 (0.00037) |
| Laranjal do Jari-AP | 03 | 2 | 0.667 (0.314) | 0.00104 (0.00049) |
| Uiramutã-RR | 09 | 3 | 0.417 (0.191) | 0.00069 (0.00034) |
| Pacaraima-RR | 10 | 7 | 0.911 (0.077) | 0.00294 (0.00068) |
| Normandia-RR | 04 | 2 | 0.500 (0.265) | 0.00078 (0.00041) |
| Monte Dourado-PA | 05 | 2 | 0.400 (0.237) | 0.00187 (0.00111) |
| Lampung-SU | 04 | 4 | 1.000 (0.177) | 0.00961 (0.00231) |
| San Pa Tong-CM | 01 | 1 | - | - |
| Muang District-NT | 04 | 4 | 1.000 (0.177) | 0.00779 (0.00170) |
| Groups | ||||
| South America | 127 | 26 | 0.611 (0.050) | 0.00164 (0.00024) |
| Asian Southeast | 9 | 9 | 1.000 (0.052) | 0.00865 (0.00110) |
| Total | 136 | 35 | 0.6607 (0.046) | 0.00254 (0.00037) |
| Source of Variation | Variance Components | Percentage of Variation (%) | ɸST |
|---|---|---|---|
| Among populations | 0.28966 | 22.43 | 0.31075 |
| Within populations | 0.64247 | 85.44 |
| Source of Variation | Variance Components | Percentage of Variation (%) | ɸST |
|---|---|---|---|
| Among populations | 2.22336 | 65.29 | |
| Among populations within groups | 0.00796 | 0.42 | 0.65294 |
| Within populations | 0.64247 | 34.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Deus, E.; Passos, J.; van Sauers-Muller, A.; Jesus, C.; Silva, J.G.; Adaime, R. Phylogeography of the Invasive Fruit Fly Species Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae) in South America. Insects 2024, 15, 949. https://doi.org/10.3390/insects15120949
de Deus E, Passos J, van Sauers-Muller A, Jesus C, Silva JG, Adaime R. Phylogeography of the Invasive Fruit Fly Species Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae) in South America. Insects. 2024; 15(12):949. https://doi.org/10.3390/insects15120949
Chicago/Turabian Stylede Deus, Ezequiel, Joseane Passos, Alies van Sauers-Muller, Cristiane Jesus, Janisete Gomes Silva, and Ricardo Adaime. 2024. "Phylogeography of the Invasive Fruit Fly Species Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae) in South America" Insects 15, no. 12: 949. https://doi.org/10.3390/insects15120949
APA Stylede Deus, E., Passos, J., van Sauers-Muller, A., Jesus, C., Silva, J. G., & Adaime, R. (2024). Phylogeography of the Invasive Fruit Fly Species Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae) in South America. Insects, 15(12), 949. https://doi.org/10.3390/insects15120949






