1. Introduction
The cotton aphid,
Aphis gossypii, is a devastating cotton pest with resistance to a variety of insecticides, including pyrethroids, carbamates, organophosphates, and neonicotinoids [
1,
2]. In recent years, sulfoxaflor, classified as Group 4C by the Insecticide Resistance Action Committee [
3], has been widely utilized to control cotton aphids in China, and it has been an important insecticide for IPM programs registered in 92 countries in the world to control many sucking insects [
4]. Moreover, because sulfoxaflor has a special action mechanism that is different from other neonicotinoid insecticides, it has been extensively used to control resistant sucking insect pests, such as cotton aphids [
5,
6,
7]. However, with excessive application of sulfoxaflor in the field as an essential alternative insecticide, some populations in different regions of
A. gossypii have developed a high level of resistance to it [
8,
9]. By reducing the use of insecticides, rational rotation of different types of insecticides, and other comprehensive control measures, the development of resistance can be delayed [
10]. However, after the application of insecticides is reduced, whether the sensibility of insects can return to close to the original level and all biological parameters in declined resistant strain can be restored to the same level as the susceptible strain has been unknown till now.
The biological and physiological characteristics, such as fitness and behavioral responses of
A. gossypii, have changed while they had resistance to sulfoxaflor [
11]. With insecticide selection pressure, the pest can evolve resistance to insecticides. Normally, pests respond to this pressure at the expense of fitness costs, as evidenced by high energy costs or a significant disadvantage in survival [
12]. This decreased fitness has been reported in sulfoxaflor-resistant, thiamethoxam-resistant, clothianidin-resistant, and acetamiprid-resistant strains of
A. gossypii [
13,
14,
15,
16]. The fitness costs associated with insecticide resistance can result in resistant populations being less competitive than susceptible populations, and pests can regain susceptibility to insecticides without insecticide pressure [
17]. After resistance declines, the chlorpyrifos-resistant
Phenacoccus solenopsis [
18], sulfoxaflor-resistant
Nilaparvata lugens [
19], and acetamiprid-resistant
A. gossypii [
20] regain susceptibility to the screened insecticides, as well as to other insecticides to which they have previously been cross-resistant. However, it remains unclear whether this fitness cost associated with the resistance disappears after
A. gossypii regains susceptibility to insecticides along with losing the selective pressure in the resistant strain.
The electrical penetration graph (EPG) has been widely used to study resistance characteristics in host plants, transmission mechanisms of plant pathogens, and modes of action of insecticides [
21,
22,
23]. Insecticides may have an effect on aphid feeding behavior, and many studies have investigated the effects of insecticides on the feeding behavior of
Myzus persicae [
22] and
A. gossypii [
24,
25,
26] through EPG in recent years. Using this method, a previous report suggested that the sulfoxaflor-resistant
A. gossypii were more active in finding an appropriate site for feeding than the susceptible aphids [
24]. The sulfoxaflor could provoke more rapid and effective phloem feeding in the susceptible and sulfoxaflor-resistant aphids [
25]. However, little is known about the aphid feeding behavior after its resistance declines.
In order to explore the fitness, cross-resistance, and feeding behavior changes in cotton aphids after their resistance level declined to sulfoxaflor, a series of experiments were conducted in this study. First, the stability of resistance in cotton aphids to sulfoxaflor was investigated. Second, the life table parameters of aphids in the sulfoxaflor-resistant and resistance-decline strains were compared with the susceptible strain by the age-stage, two-sex life table theory [
27]. Finally, the feeding behaviors of three strains were analyzed by EPGs. The results of this study will contribute to understanding the risk assessment of resistance to sulfoxaflor in cotton aphids and provide a reference to the rational optimization of sulfoxaflor in the control strategy for cotton aphids.
4. Discussion
Chemical control is a crucial aspect of cotton pest management strategy, aimed at mitigating the negative impact of pest damage on cotton yield. This has led to an over-reliance on insecticides for cotton pest control, resulting in high levels of insecticide resistance in cotton pests, particularly cotton aphids [
1,
2,
9]. Sulfoxaflor, the first sulfoximine insecticide [
5], has been widely used for stinging pest control in recent years because it has high efficacy against resistant pests, which have high levels of resistance to many chemical insecticides. But while providing good control, field populations of different pests have developed varying degrees of resistance to sulfoxaflor due to non-scientific use of this insecticide [
8,
35,
36]. Discontinuation or rotation of using insecticide can delay the development of pest resistance, and the physiological and behavioral response in the pest after the resistance level is reduced is critical to whether this insecticide can continue to be used. Therefore, in this study, we tested the changes in cross-resistance, fitness, and feeding behavior in
A. gossypii, along with the decline in resistance to sulfoxaflor. The results will contribute to providing the reference to rational optimize sulfoxaflor in control strategy against cotton aphids.
Understanding the stability of resistance in pests after lost exposure selection to insecticides is of great practical importance. When pest resistance to an insecticide is unstable, then eliminating this insecticide from the spray program can reduce the pest resistance and thus prolong the effectiveness of this insecticide [
37]. Unstable insecticide resistance has also been reported for chlorpyrifos-resistant
P. solenopsis [
18], acetamiprid-resistant
Bemisia tabaci [
37],
P. solenopsis [
38], and
A.
gossypii [
20]. In this study, sulfoxaflor resistance was unstable, the resistance ratio in
A.
gossypii reduced from 51.57 to 1.11-fold with a DR of −0.08 after 22 generations without any insecticide pressure. Moreover, as sulfoxaflor resistance declined, cross-resistance in the Sul-D strain to acetamiprid and imidacloprid also declined.
Insects cope with different survival environments with degrees of fitness. To deal with insecticides, insects use a variety of behavioral, physiological, and genetic strategies, such as mutations in the target sites or overexpression of detoxification enzymes, resulting in costly consequences that may affect insect reproduction, dispersal ability, and fitness [
12], Fitness costs associated with insecticide resistance have been reported in thiamethoxam-resistant
A.
gossypii [
16], pymetrozine-resistant
N.
lugens [
17], and methoxyfenozide-resistant
Musca domestica [
39]. Moreover, fitness cost varies due to the difference in pest populations and insecticide types [
11]. The current study has shown that the sulfoxaflor-resistant strains of pests also have fitness costs, such as
M.
persicae [
40] and
N.
lugens [
41]. Our present study showed that the Sul-R strain of
A.
gossypii had a significant decrease in fecundity,
r,
λ, R0,
T, and GRR, and its relative fitness declined to 0.87 compared with susceptible aphids. This result is similar to the findings of Ma et al. [
14] and our previous study [
11]. In addition, the susceptibility in the Sul-D strain to sulfoxaflor had recovered close to that in Sus strains after 22 generations without insecticide pressure. However, the relative fitness of the Sul-D strain was not recovered and still had a decreased fitness of 0.84, with a significant decrease in the adult longevity, total longevity, fecundity,
r,
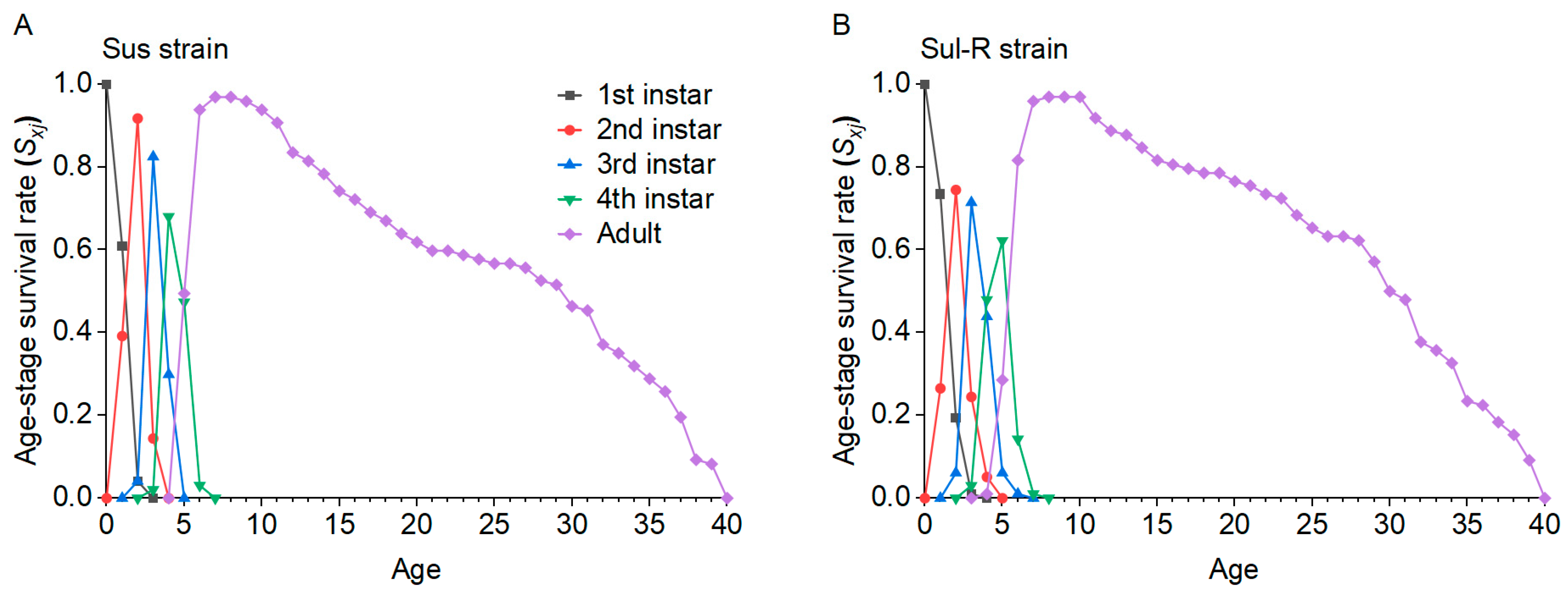
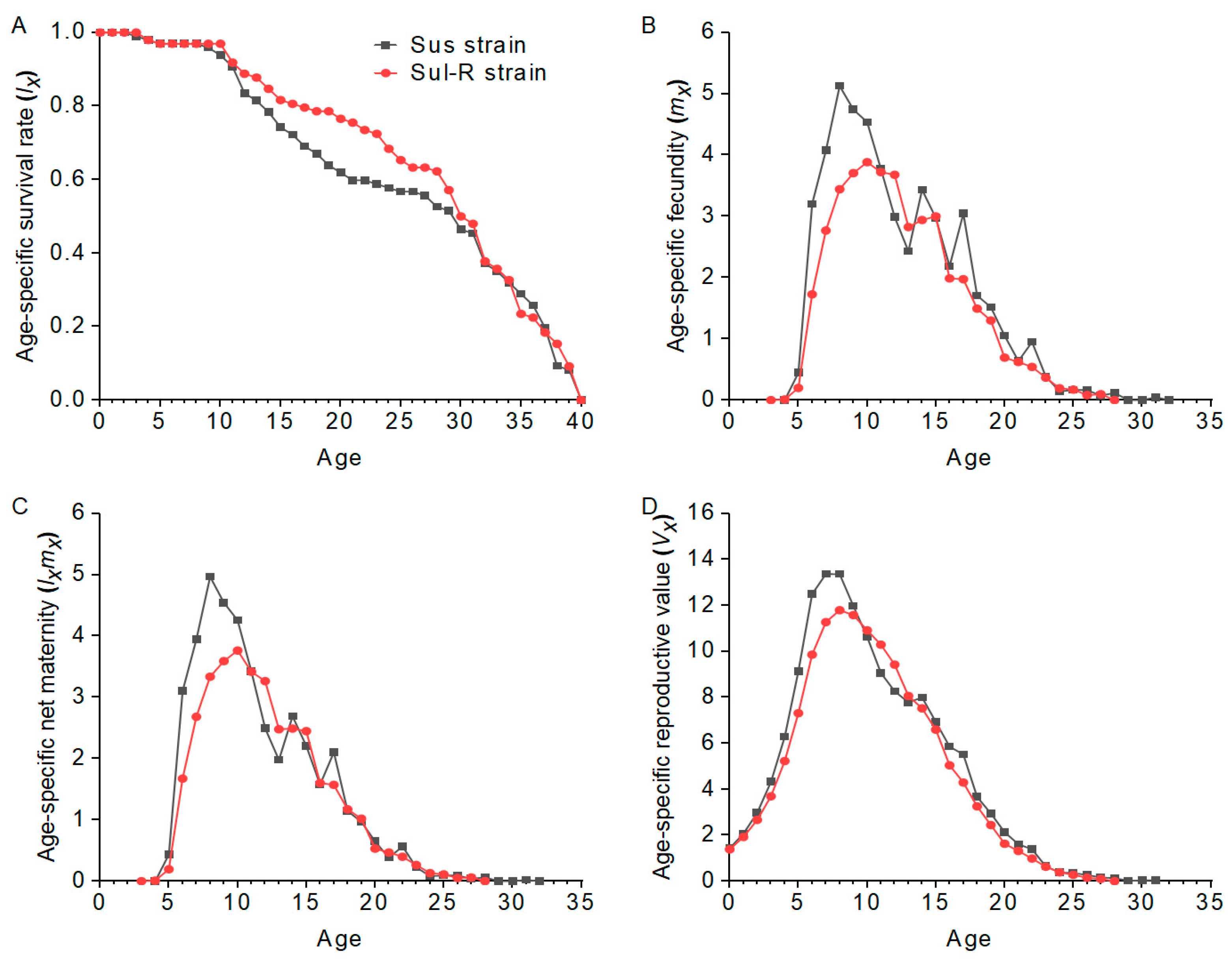
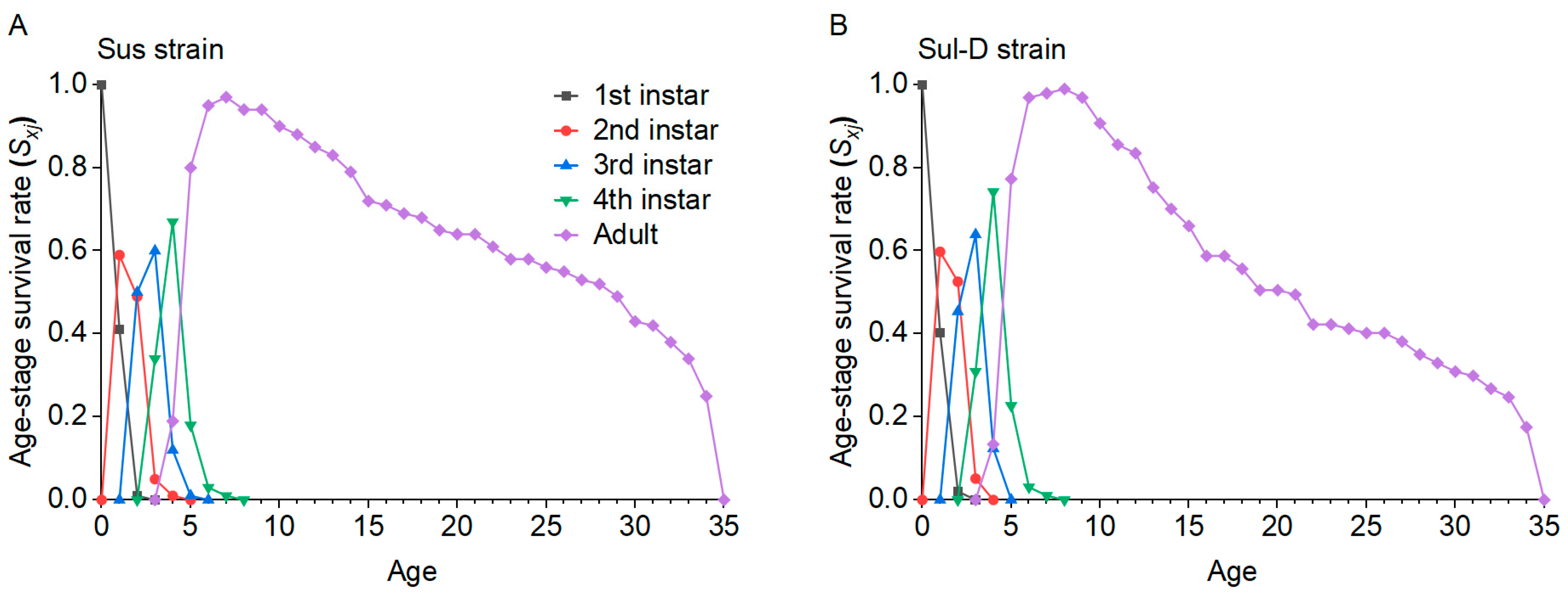
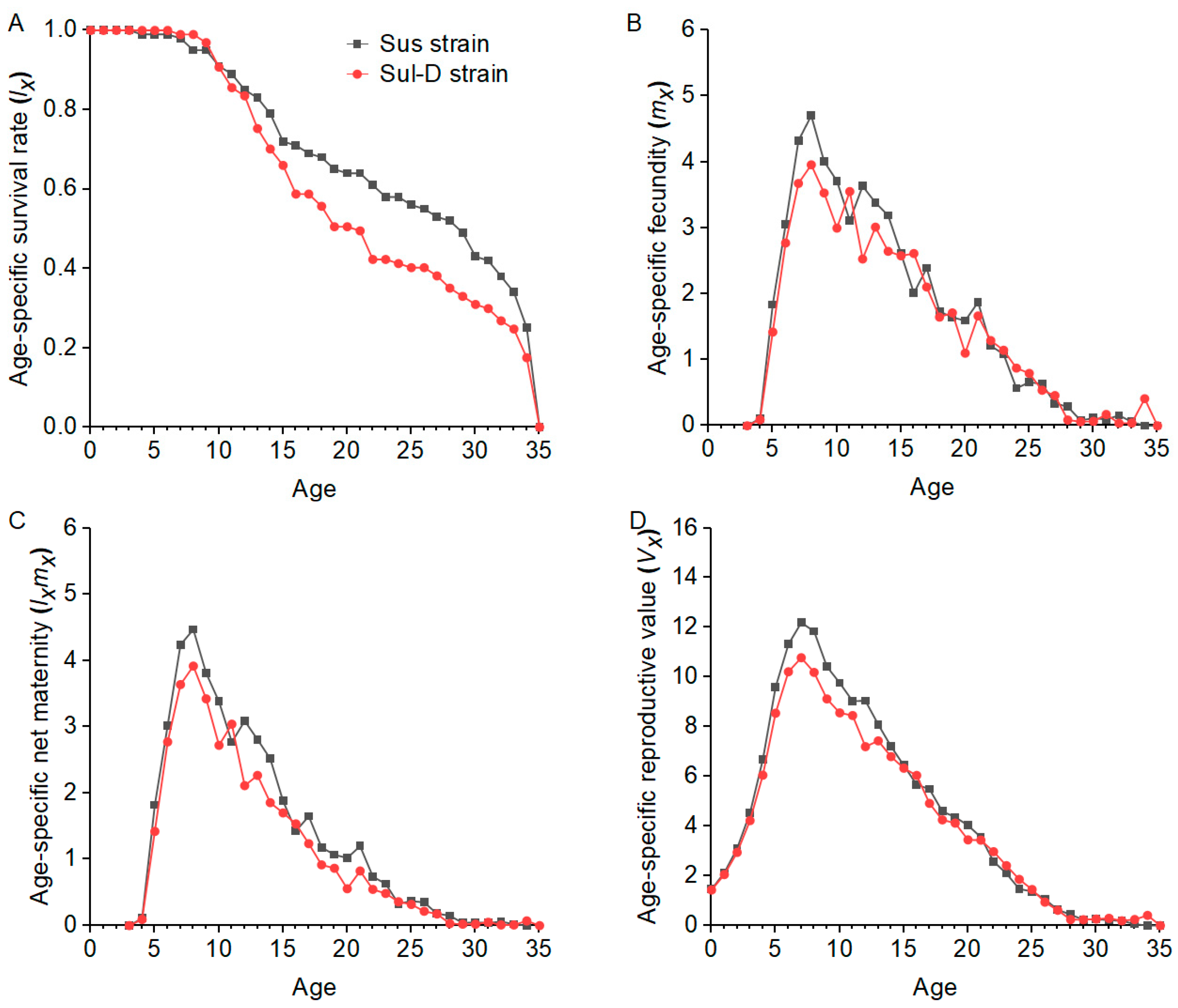
λ, R0, and GRR. This indicates that there may still be a fitness cost in resistant cotton aphid populations even if they recover susceptibility to insecticides, and this is beneficial for the implementation of resistance management strategies by reducing insecticide use or insecticide rotation.
Nowadays, the electrical penetration graph (EPG) technique is widely used to explore the differences in probing and feeding behavior in sucking pests. Previous findings suggest that insecticide pressure affects aphid probing and feeding behavior [
22,
24,
26,
42]. Insects often require multiple explorations to find suitable feeding sites [
43]. Wang et al. showed that the imidacloprid-resistant and sulfoxaflor-resistant
A. gossypii were more active in searching for suitable feeding sites before their stylets arrived at the phloem, as evidenced by an increase in the number and duration of C waveform [
24,
26]. Similar results were also observed in the present study. In this study, the Sus, Sul-R, and Sul-D strains showed significant differences in probing and feeding behaviors when aphids fed on common plants. The number and duration of the C waveform were increased in both Sul-R and Sul-D strains despite no significant difference from the Sus strain. Moreover, the number of the Np waveform was reduced in both Sul-R and Sul-D strains. These results indicate that the Sul-R and Sul-D strains not only actively look for suitable feeding sites but also probe more quickly. However, the Sul-R and Sul-D strains had less ability in phloem feeding. They ingested from the phloem (time from the 1st C to 1st, and E1 and E2) more slowly than the Sus strain. Furthermore, parameters for the E1 and E2 waveform associated with phloem activity decreased in the Sul-R and Sul-D strains. For example, the number and duration of E1 and E2 were significantly decreased in the Sul-D strain, the duration of E1 was significantly decreased in the Sul-R strain compared with the Sus strain, and the percentage of E1 and E2 was significantly decreased in the Sul-R and Sul-D strains. The phloem sap is a vital source of energy for insects as it contains amino acids, saccharides, and various nutrients, which are crucial for insect growth and development [
44]. The reduced ability of the Sul-R and Sul-D strains to feed makes them less efficient at obtaining energy from the phloem sap, which may lead to a reduction in their fitness.
The sublethal effects of insecticides on development, sex ratio, reproductive capacity, lifespan, feeding behavior, oviposition behavior, chemical communication, and other aspects of insects are known [
45]. A previous study found that the hormesis effects of sulfoxaflor on phloem feeding are observed in susceptible and sulfoxaflor-resistant
A. gossypii, with a significant increase in the duration and percentage of E2 [
24]. However, in this study, negative sublethal effects of sulfoxaflor on phloem feeding were observed in the Sus strain. The number and duration of E1, the duration of E2, and the percentage of E1 and E2 were significantly reduced in the Sus strain when exposed to sulfoxaflor-treated plants (LC
25 value on Sus strain). This result suggests that there is an uncertain effect of sulfoxaflor on the phloem-feeding of
A. gossypii. This may be related to many factors affecting the sublethal effect of insecticides, such as insecticides, pest species, genetic backgrounds and generations of pests, and the use of sublethal treatment concentrations [
45,
46]. In addition, significant changes in phloem feeding were not observed in Sul-R and Sul-D strains under the same treatment. This indicates that this concentration of sulfoxaflor (LC
25 value on Sus strain) could not affect the feeding in the Sul-R strain due to its high resistance to sulfoxaflor (51.57-fold). Previous studies have found that the P450 genes involving high resistance to sulfoxaflor are still overexpressed in sulfoxaflor resistance-decline
N. lugens [
19], which would result in an energy trade-off based on resource trade-offs theory [
47,
48]. In this study, the decrease in relative fitness of the Sul-D strain may be related to the fact that genes involved in sulfoxaflor resistance are still overexpressed in the Sul-D strain and that overexpression of such genes may have eliminated the effect of sulfoxaflor on Sul-D strain. Therefore, the expression of genes involved in sulfoxaflor resistance in the Sul-D strain needs to be investigated in the future.