Functional Role of Odorant-Binding Proteins in Response to Sex Pheromone Component Z8-14:Ac in Grapholita molesta (Busck)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Colony Maintenance
2.2. Chemicals
2.3. Electroantennogram Analysis
2.4. Determination of the Inhibitory Effects of Z8-14:Ac and Z10-14:Ac on OFM Males
2.5. Tissue Expression of GmolPBPs and GmolGOBPs in OFM Adults
2.6. Preparation of rGmolPBPs/rGmolGOBPs Proteins and Fluorescence Competitive Binding Assays
2.7. Homology Modeling and Molecular Docking
2.8. Molecular Dynamic Simulation and Calculation of Binding Free Energy of Each Amino Acid
2.9. Site-Directed Mutagenesis and the Binding Affinities of GmolPBP2 Mutants to Z8-14:Ac
3. Results
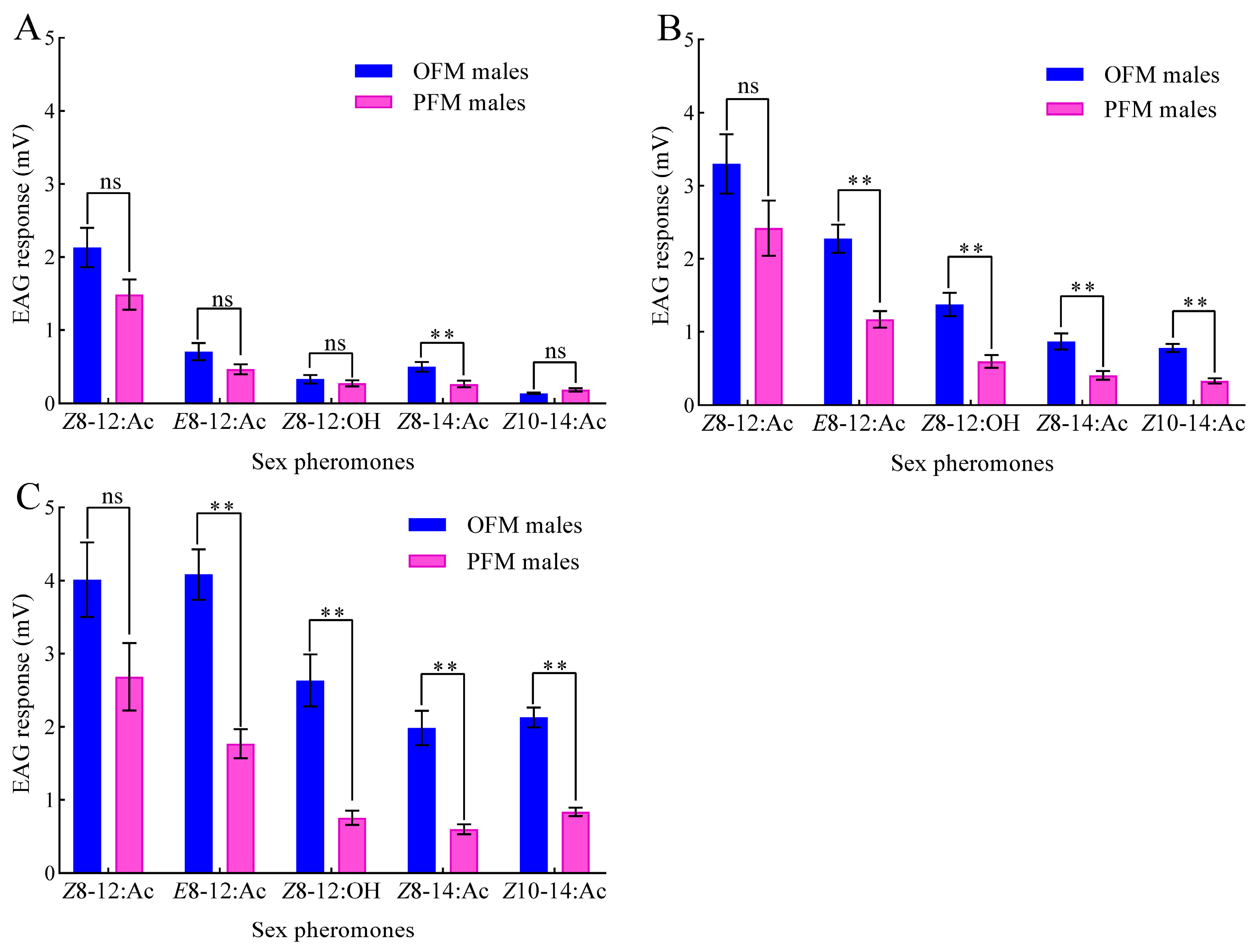
3.1. EAG Response of OFM and PFM Males to Different Doses of Sex Pheromones
3.2. Effects of Z8-14:Ac and Z10-14:Ac on Captured OFM and PFM Males
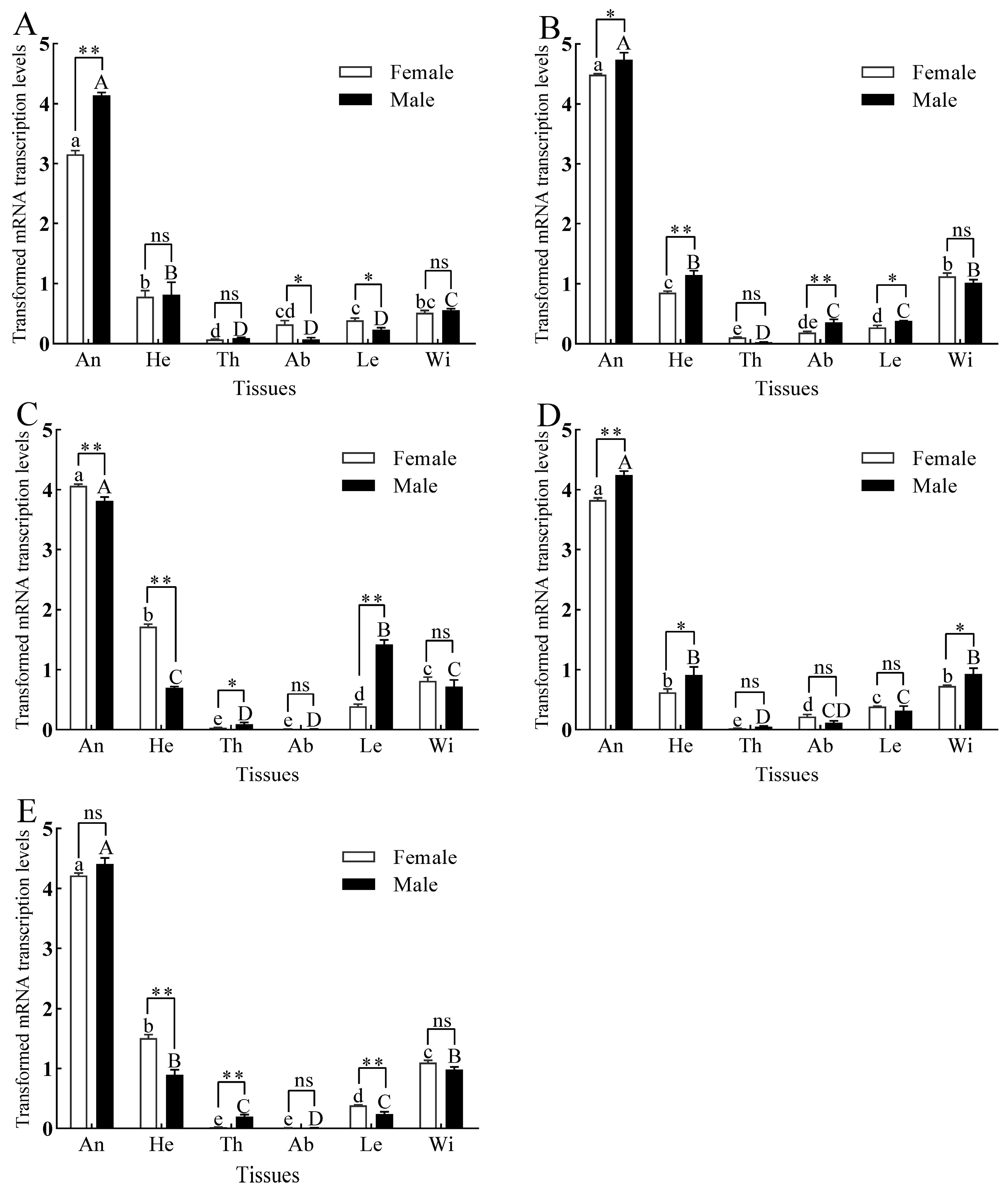
3.3. Tissue Expression of GmolPBPs and GmolGOBPs
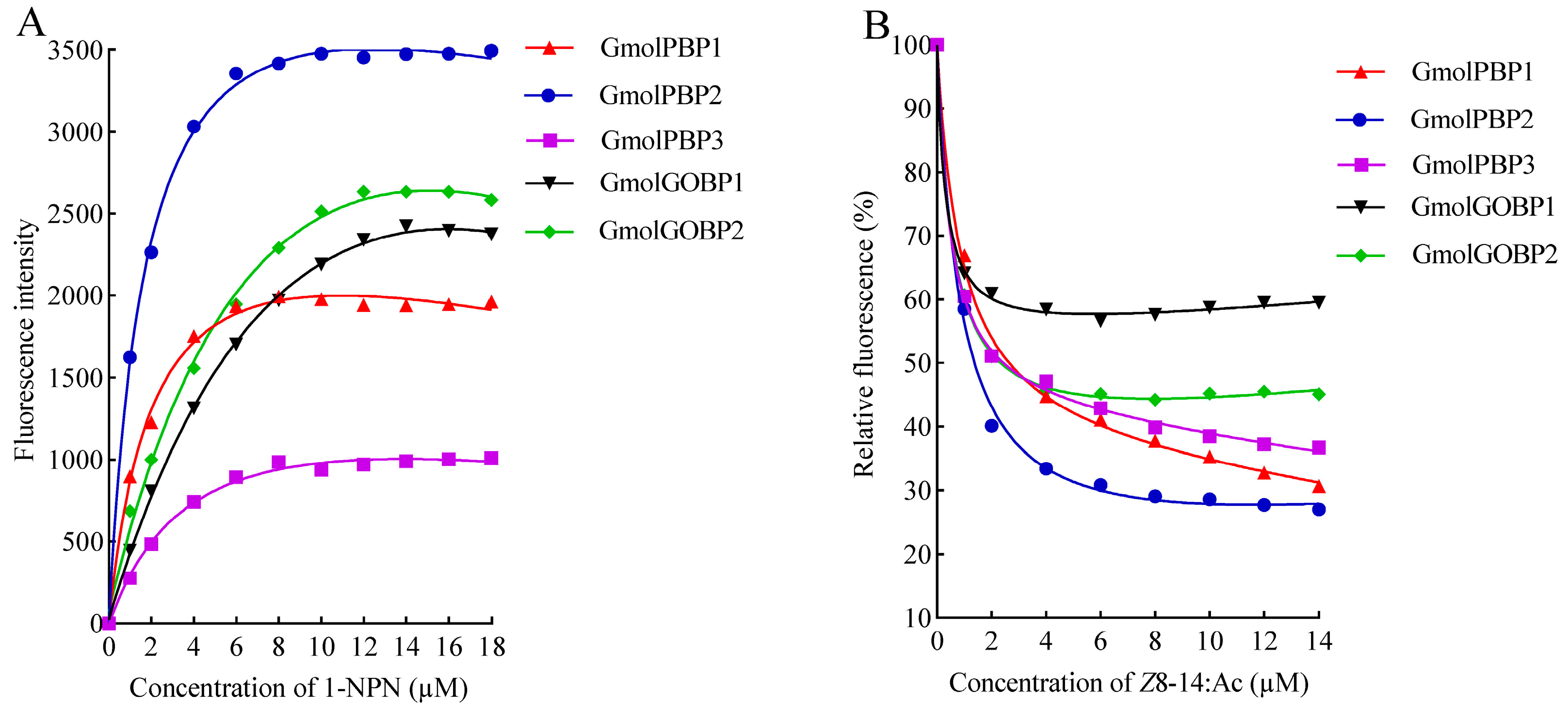
3.4. Binding Affinities of rGmolPBPs and rGmolGOBPs for Z8-14:Ac
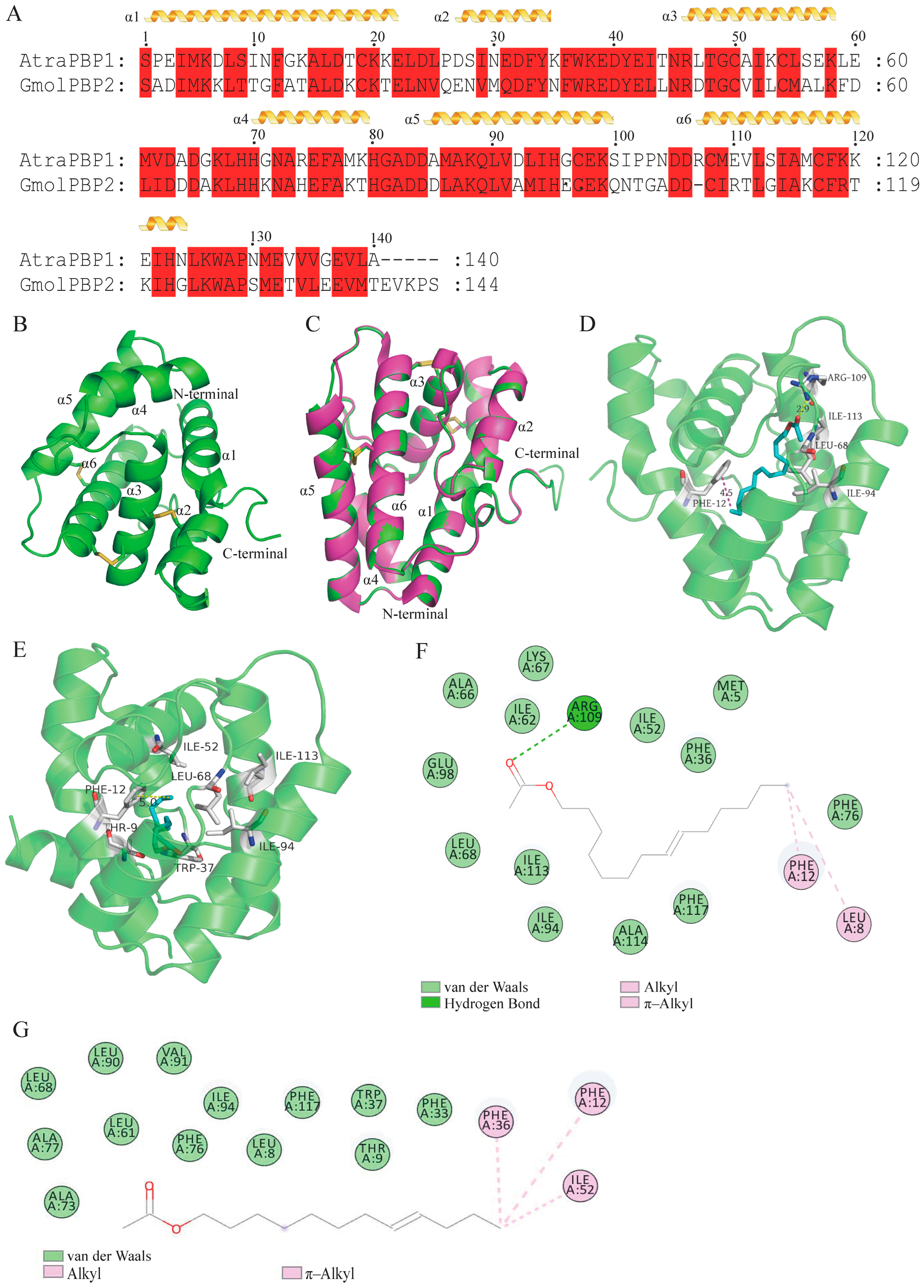
3.5. Homology Modeling and Molecular Dynamics Simulation of GmolPBP2
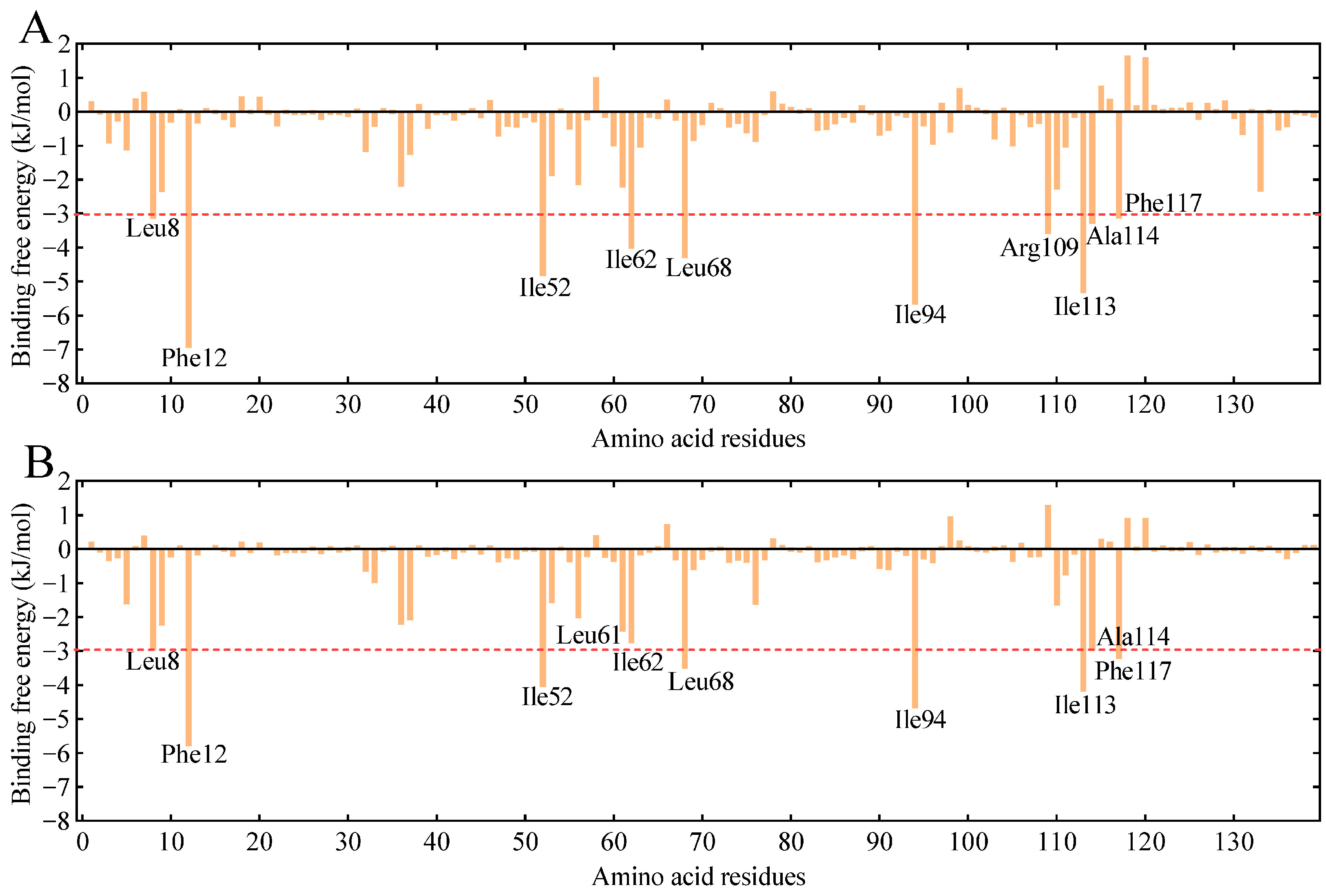
3.6. Validation of Key Amino Acid Residues for GmolPBP2 Binding to Z8-14:Ac
4. Discussion
4.1. Z8-14:Ac Exhibits an Inhibitory Effect on OFM Males
4.2. GmolPBPs and GmolGOBPs Predominantly Expressed in OFM Adult Antennae
4.3. GmolPBP2 Was the Most Likely Target for Binding Z8-14:Ac
4.4. Key Amino Acid Residues and Interaction Forces of GmolPBP2 Binding to Z8-14:Ac
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, C.T.; Hull, L.A.; Krawczyk, G. Effects of orchard host plants (apple and peach) on development of oriental fruit moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2007, 100, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Xu, B.Q.; Li, C.Q.; Li, B.L.; Luo, K.; Li, G.W.; Chen, X.L. Functional disparity of four pheromone-binding proteins from the plum fruit moth Grapholita funebrana Treitscheke in detection of sex pheromone components. Int. J. Biol. Macromol. 2023, 225, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Najar-Rodriguez, A.; Orschel, B.; Dorn, S. Season-long volatile emissions from peach and pear trees in situ, overlapping profiles, and olfactory attraction of an oligophagous fruit moth in the laboratory. J. Chem. Ecol. 2013, 39, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Fan, F.; Ma, C.S.; Wang, C.; Wei, G.S. Effect of host plants on the development, survivorship and reproduction of Grapholita molesta (Lepidoptera: Tortricidae) under laboratory conditions. Austral Entomol. 2016, 55, 433–438. [Google Scholar] [CrossRef]
- Sarker, S.; Lim, U.T. Olfactory responses of Grapholita molesta to different developmental stages of immature fruit of peach, plum, and apple. J. Asia-Pac. Entomol. 2023, 26, 102035. [Google Scholar] [CrossRef]
- Rizzo, R.; Farina, V.; Saiano, F.; Lombardo, A.; Ragusa, E.; Verde, G.L. Do Grapholita funebrana infestation rely on specific plum fruit features? Insects 2019, 10, 444. [Google Scholar] [CrossRef]
- Stefanova, D.; Vasilev, P.; Kutinkova, H.; Andreev, R.; Palagacheva, N.; Tityanov, M. Possibility for control of plum fruit moth Grapholita funebrana Tr. by pheromone dispensers. J. Biopestic. 2019, 12, 153–156. [Google Scholar]
- Zheng, Y.; Wu, R.X.; Dorn, S.; Chen, M.H. Diversity of Tortricid moths in apple orchards: Evidence for a cryptic species of Grapholita (Lepidoptera: Tortricidae) from China. Bull. Entomol. Res. 2017, 107, 268–280. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.M.; Lin, H.Z.; Chen, L.S.; Wang, S.S. Species and population dynamics of fruit borer in Shihezi reclamation zone. North Horticult. 2017, 12, 132–135. [Google Scholar]
- Chen, X.L.; Chen, Y.X.; Bao, L.J.; Kang, L.; Sun, Y.; Li, G.W. Species and population dynamic of fruit-borer in apple orchard of Yan’an area. Plant Protect. 2021, 47, 219–225. [Google Scholar]
- Zheng, Y.; Qiao, X.F.; Wang, K.; Dorn, S.; Chen, M.H. Population genetics affected by pest management using fruitbagging: A case study with Grapholita molesta (Lepidoptera: Tortricidae) in China. Entomol. Exp. Appl. 2015, 156, 117–127. [Google Scholar] [CrossRef]
- Sziràki, G. Specificity of sexual attractant traps for signalization of oriental fruit moth, Grapholitha molesta Busck. Acta Phytopathol. Acad. Sci. Hung. 1978, 13, 205–213. [Google Scholar]
- Tòth, M.; Sziràki, G.; Szöcs, G.; Sàringer, E. A pheromone inhibitor for male Grapholitha funebrana Tr., and its use for increasing the specifcity of the lure for G. molesta Busck (Lepidoptera: Tortricidae). Agric. Ecosyst. Environ. 1991, 35, 65–72. [Google Scholar]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Deng, J.Y.; Shen, Z.J.; Wang, F.M.; Liu, T.; Hong, W.Y.; Fang, M.H.; Wo, L.F.; Chu, S.J. Enhancement of attraction to sex pheromone of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) by structurally unrelated sex pheromone compounds of Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae). J. Asia-Pac. Entomol. 2022, 25, 101859. [Google Scholar] [CrossRef]
- Meier, L.; Zou, Y.F.; Millar, J.; Mongold-Diers, J.; Hanks, L. Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of Cerambycid beetles. J. Chem. Ecol. 2016, 42, 1181–1192. [Google Scholar] [CrossRef]
- Silva, W.D.; Millar, J.G.; Hanks, L.M.; Costa, C.M.; Leite, M.O.G.; Tonelli, M.; Bento, J.M.S. Interspecific cross-attraction between the South American Cerambycid beetles Cotyclytus curvatus and Megacyllene acuta is averted by minor pheromone components. J. Chem. Ecol. 2018, 44, 268–275. [Google Scholar] [CrossRef]
- Guerin, P.M.; Arn, H.; Buser, H.R.; Charmillot, P.; Tth, M.; Szirki, G. Sex pheromone of Grapholita funebrana. Occurrence of Z-8- and Z-10-tetradecenyl acetate as secondary components. J. Chem. Ecol. 1986, 12, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Lacey, M.J.; Sanders, C.J. Chemical composition of sex pheromone of oriental fruit moth and rates of release by individual female moths. J. Chem. Ecol. 1992, 18, 1421–1435. [Google Scholar] [CrossRef]
- Cardé, A.M.; Baker, T.C.; Cardé, R.T. Identification of a four component sex pheromone of the female oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae). J. Chem. Ecol. 1979, 5, 423–427. [Google Scholar] [CrossRef]
- Han, K.S.; Jung, J.K.; Choi, K.H.; Lee, S.W.; Boo, K.S. Sex pheromone composition and male trapping of oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae) in Korea. J. Asia-Pac. Entomol. 2001, 4, 31–35. [Google Scholar] [CrossRef]
- Yang, C.Y.; Jung, J.K.; Han, K.S.; Boo, K.S.; Yiem, M.S. Sex pheromone composition and monitoring of the oriental fruit moth Grapholitha molesta (Lepidoptera: Tortricidae) in Naju pear orchards. J. Asia-Pac. Entomol. 2002, 5, 201–207. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Miller, J.R.; Ledebuhr, R.; Siegert, P.; Gut, L.J. Season-long mating disruption of Grapholita molesta (Lepidoptera: Tortricidae) by one machine application of pheromone in wax drops. J. Pest Sci. 2007, 80, 109–117. [Google Scholar] [CrossRef]
- Rosu-Mares, S.D.; Feneșan, M.P.; Ciotlaus, I.; Balea, A.; Andreica, A. Pheromone monitoring of harmful Lepidoptera present in Bistrita area in apple and plum orchards. Rom. J. Hortic. 2021, 2, 99–106. [Google Scholar]
- Puskás, J.; Nowinszky, L.; Barczikay, G.; Kúti, Z.S. The pheromone trap catch of harmful moths in connection with solar activity featured by q-index. Appl. Ecol. Env. Res. 2010, 8, 261–266. [Google Scholar]
- Pelosi, P.; Maida, R. Odorant-binding proteins in insects. Comp. Biochem. Phys. B 1995, 111, 503–514. [Google Scholar] [CrossRef]
- Brito, N.F.; Moreira, M.F.; Melo, A.C.A. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef]
- Zhou, J.J.; Robertson, G.; He, X.L.; Dufour, S.; Hooper, A.M.; Pickett, J.A.; Keep, N.H.; Field, L.M. Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J. Mol. Biol. 2009, 389, 529–545. [Google Scholar] [CrossRef]
- Forstner, M.; Gohl, T.; Breer, H.; Krieger, J. Candidate pheromone binding proteins of the silk moth Bombyx mori. Invertebr. Neurosci. 2006, 6, 177–187. [Google Scholar]
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272. [Google Scholar]
- Cao, D.P.; Liu, Y.; Wei, J.J.; Liao, X.Y.; Walker, W.B.; Li, J.H.; Wang, G.R. Identifcation of candidate olfactory genes in Chilo suppressalis by antennal transcriptome analysis. Int. J. Biol. Sci. 2014, 10, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Yang, L.; Xu, Z.F.; He, L. Transcriptomics and identifcation of candidate chemosensory genes in antennae of Conogethes punctiferalis (Lepidoptera: Crambidae). J. Asia-Pac. Entomol. 2016, 19, 911–920. [Google Scholar] [CrossRef]
- Jing, D.P.; Zhang, T.T.; Prabu, S.; Bai, S.X.; He, K.L.; Wang, Z.Y. Molecular characterization and volatile binding properties of pheromone binding proteins and general odorant binding proteins in Conogethes pinicolalis (Lepidoptera: Crambidae). Int. J. Biol. Macromol. 2020, 146, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Z.; Liu, J.T.; Zhou, J.J.; Wang, Q.; Dong, J.Z.; Zhang, Y.J.; Li, X.C.; Li, J.; Gu, S.H. Expressional and functional comparisons of two general odorant binding proteins in Agrotis ipsilon. Insect Biochem. Mol. Biol. 2018, 98, 34–47. [Google Scholar] [CrossRef]
- Yang, H.H.; Li, S.P.; Yi, M.Z.; Zhu, X.Y.; Li, J.B.; Zhang, Y.N.; Li, X.M. Functional differentiation of two general odorant-binding proteins to sex pheromones in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 191, 105348. [Google Scholar] [CrossRef]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar]
- Zhan, S.; Merlin, C.; Boore, J.L.; Reppert, S.M. The monarch butterfly genome yields insights into long-distance migration. Cell 2011, 147, 1171–1185. [Google Scholar] [CrossRef]
- Dasmahapatra, K.K.; Walters, J.R.; Briscoe, A.D.; Davey, J.W.; Whibley, A.; Nadeau, N.J.; Zimin, A.V.; Hughes, D.S.T.; Ferguson, L.C.; Martin, S.H.; et al. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 2012, 487, 94–98. [Google Scholar]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Gu, F.M.; Chen, H.C.; Liu, Z.X.; Song, W.M.; Wu, F.A.; Sheng, S.; Wang, J. Binding characteristics of pheromone-binding protein 1 in Glyphodes pyloalis to organophosphorus insecticides: Insights from computational and experimental approaches. Int. J. Biol. Macromol. 2024, 260, 129339. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yan, Q.; Li, L.L.; Xu, J.W.; Mang, D.; Wang, X.L.; Hoh, H.H.; Ye, J.; Ju, Q.; Ma, Y.; et al. Different binding properties of two general odorant binding proteins in Athetis lepigone with sex pheromones, host plant volatiles and insecticides. Pestic. Biochem. Physiol. 2020, 164, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Du, J.; Li, Y.P.; Wu, J.X. Identification of putative olfactory genes from the oriental fruit moth Grapholita molesta via an antennal transcriptome analysis. PLoS ONE 2015, 10, e0142193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Y.Q.; Dong, J.F.; Qiao, H.L.; Wu, J.X. Molecular characterization, expression patterns and binding properties of two pheromone-binding proteins from the oriental fruit moth, Grapholita molesta (Busck). J. Integr. Agr. 2014, 13, 2709–2720. [Google Scholar] [CrossRef]
- Li, G.W.; Chen, X.L.; Li, B.L.; Zhang, G.H.; Li, Y.P.; Wu, J.X. Binding properties of general odorant binding proteins from the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae). PLoS ONE 2016, 11, e0155096. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chen, J.; Yu, H.L.; Tian, X.L.; Wu, J.X. Molecular and functional characterization of pheromone binding protein 1 from the oriental fruit moth, Grapholita molesta (Busck). Sci. Rep. 2018, 8, 2276. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.R.; Wu, J.X. Effect of four different artificial diets on development and reproduction of Grapholitha molesta (Lepidoptera: Tortricidae). J. Shanxi Agric. Univ. Nat. Sci. Ed. 2010, 30, 228–231. [Google Scholar]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by genometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Kelmansky, D.M.; Martínez, E.J.; Leiva, V. A new variance stabilizing transformation for gene expression data analysis. Stat. Appl. Genet. Mol. Biol. 2013, 12, 653–666. [Google Scholar] [CrossRef]
- Zhang, T.T.; Mei, X.D.; Feng, J.N.; Berg, B.G.; Zhang, Y.J.; Guo, Y.Y. Characterization of three pheromone-binding proteins (PBPs) of Helicoverpa armigera (Hübner) and their binding properties. J. Insect Physiol. 2012, 58, 941–948. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.J.; O’Connell, R.J.; Hammond, A.M. A comparative study of pheromone perception in two species of Noctuid moths. J. Insect Behav. 1988, 1, 75–96. [Google Scholar] [CrossRef]
- Mozūraitis, R.; Liblikas, I.; Noreika, R. Sex pheromone communication of tentiform leaf-miners Phyllonorycter insignitella and Phyllonorycter nigrescentella from two related species groups. Chemoecology 2009, 18, 171–176. [Google Scholar] [CrossRef]
- Greenberg, L.; Johnson, C.A.; Trager, J.C.; McElfresh, J.S.; Rodstein, J.; Millar, J.G. Sex attractant pheromones of virgin queens of sympatric slave-making ant species in the genus Polyergus, and their possible roles in reproductive isolation. J. Chem. Ecol. 2018, 44, 547–555. [Google Scholar] [CrossRef]
- Xu, T.; Hansen, L.; Cha, D.H.; Hao, D.; Zhang, L.; Teale, S.A. Identification of a female-produced pheromone in a destructive invasive species: Asian longhorn beetle, Anoplophora glabripennis. J. Pest Sci. 2020, 93, 1321–1332. [Google Scholar] [CrossRef]
- Klun, J.A.; Bierl-Leonhardt, B.A.; Plimmer, J.R.; Sparks, A.N.; Primiani, M.; Chapman, O.L.; Lepone, G.; Lee, G.H. Sex pheromone chemistry of the female tobacco budworm moth, Heliothis virescens. J. Chem. Ecol. 1980, 6, 177–183. [Google Scholar] [CrossRef]
- Dunkelblum, E.; Kehat, M. Female sex pheromone components of Heliothis peltigera (Lepidoptera: Noctuidae). Chemical identification from gland extracts and male response. J. Chem. Ecol. 1989, 15, 2233–2245. [Google Scholar] [CrossRef]
- Chang, X.Q.; Nie, X.P.; Zhang, Z.; Zeng, F.F.; Lv, L. De novo analysis of the oriental armyworm Mythimna separata antennal transcriptome and expression patterns of odorant-binding proteins. Comp. Biochem. Phys. D 2017, 22, 120–130. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Sun, L.; Li, Y.Y.; Quan, L.F.; Zhang, H.J.; Yan, W.T.; Yue, Q.; Qiu, G.S. Antennal transcriptome analysis of the chemosensory gene families in Carposina sasakii (Lepidoptera: Carposinidae). BMC Genom. 2018, 19, 544. [Google Scholar] [CrossRef]
- Li, L.L.; Xu, B.Q.; Li, C.Q.; Li, B.L.; Chen, X.L.; Li, G.W. Different binding affinities of three general odorant-binding proteins in Grapholita funebrana (Treitscheke) (Lepidoptera: Tortricidae) to sex pheromones, host plant volatiles, and insecticides. J. Econ. Entomol. 2022, 115, 1129–1145. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Sun, Y.L.; Tian, C.H.; Wang, S.L. Antennal transcriptome analysis of odorant-binding proteins and characterization of GOBP2 in the variegated cutworm Peridroma saucia. Front. Physiol. 2023, 14, 1241324. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Liu, Y.; Yang, T.; Pelosi, P.; Dong, S.; Wang, G. Pheromone binding proteins enhance the sensitivity of olfactory receptors to sex pheromones in Chilo suppressalis. Sci. Rep. 2015, 5, 13093. [Google Scholar] [CrossRef]
- Khuhro, S.A.; Liao, H.; Dong, X.T.; Yu, Q.; Yan, Q.; Dong, S.L. Two general odorant binding proteins display high bindings to both host plant volatiles and sex pheromones in a pyralid moth Chilo suppressalis (Lepidoptera: Pyralidae). J. Asia-Pac. Entomol. 2017, 20, 521–528. [Google Scholar] [CrossRef]
- Guo, X.; Xuan, N.; Liu, G.; Xie, H.; Lou, Q.; Arnaud, P.; Offmann, B.; Picimbon, J.-F. An expanded survey of the moth PBP/GOBP clade in Bombyx mori: New insight into expression and functional roles. Front. Physiol. 2021, 12, 712593. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Brockmann, A. Sex-specific molecular specialization and activity rhythm-dependent gene expression in honey bee antennae. J. Exp. Biol. 2020, 223, jeb217406. [Google Scholar] [CrossRef]
- Tonini, C.; Cassani, G.; Piccardi, P.; Maini, S.; Castellari, P.L.; Pasqualini, E. Sex pheromone components of the leafroller moth Pandemis cerasana. J. Insect Physiol. 1982, 28, 443–446. [Google Scholar] [CrossRef]
- Priesner, E.; Buser, H.R.; Bengtsson, M.; Chambon, P.J.; Wildbolz, T.; Arn, H.; Witzgall, P. Sex pheromones of Spilonota ocellana and Spilonota laricana. Entomol. Exp. Appl. 1991, 60, 219–223. [Google Scholar]
- Alessandro, G.; Thrimawithana, A.H.; Bernd, S.; Newcomb, R.D. Differential gene expression in the evolution of sex pheromone communication in New Zealand’s endemic leafroller moths of the genera Ctenopseustis and Planotortrix. BMC Genom. 2018, 19, 94. [Google Scholar]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.R.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Moural, T.W.; Wei, H.S.; Zhu, F. Roles of insect odorant binding proteins in communication and xenobiotic adaptation. Front. Insect Sci. 2023, 3, 1274197. [Google Scholar] [CrossRef]
- Sandler, B.H.; Nikonova, L.; Leal, W.S.; Clardy, J. Sexual attraction in the silkworm moth: Structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 2000, 7, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, J.Y.; Zhang, Y.L. Key residues involved in the interaction between Cydia pomonella pheromone binding protein 1 (CpomPBP1) and codlemone. J. Agric. Food Chem. 2016, 64, 7994–8001. [Google Scholar] [CrossRef]
- Falchetto, M.; Ciossani, G.; Scolari, F.; Di Cosimo, A.; Nenci, S.; Field, L.M.; Mattevi, A.; Zhou, J.J.; Gasperi, G.; Forneris, F. Structural and biochemical evaluation of Ceratitis capitata odorant-binding protein 22 affinity for odorants involved in intersex communication. Insect Mol. Biol. 2019, 28, 431–443. [Google Scholar] [CrossRef]
- Lescop, E.; Briand, L.; Pernollet, J.C.; Guittet, E. Structural basis of the broad specificity of a general odorant-binding protein from Honeybee. Biochemistry 2009, 48, 2431–2441. [Google Scholar] [CrossRef]
- Tsitsanou, K.E.; Thireou, T.; Drakou, C.E.; Koussis, K.; Keramioti, M.V.; Leonidas, D.D.; Eliopoulos, E.; Iatrou, K.; Zographos, S.E. Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: Implications for structure-based design of novel mosquito repellents. Cell. Mol. Life Sci. 2012, 69, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Zhang, X.Q.; Zhang, X.C.; Xu, J.W.; Li, L.L.; Zhu, X.Y.; Wang, J.J.; Wei, J.Y.; Mang, D.Z.; Zhang, F.; et al. Key amino acid residues influencing binding affinities of pheromone-binding protein from Athetis lepigone to two sex pheromones. J. Agric. Food Chem. 2020, 68, 6092–6103. [Google Scholar] [CrossRef] [PubMed]
- Hamiaux, C.; Carraher, C.; Löfstedt, C.; Corcoran, J.A. Crystal structure of Epiphyas postvittana pheromone binding protein 3. Sci. Rep. 2020, 10, 16366. [Google Scholar] [CrossRef]
- Pesenti, M.E.; Spinelli, S.; Bezirard, V.; Briand, L.; Pernollet, J.C.; Campanacci, V.; Tegoni, M.; Cambillau, C. Queen bee pheromone binding protein pH-induced domain swapping favors pheromone release. J. Mol. Biol. 2009, 390, 981–990. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Xing, Y.J.; Li, R.C.; Liu, J.Y. Structural insights into two representative conformations of the complex formed by Grapholita molesta (Busck) pheromone binding protein 2 and Z-8-dodecenyl acetate. J. Agric. Food Chem. 2019, 67, 4425–4434. [Google Scholar]

| Pheromone Loadings (μg) | Mass Ratio of Z8-12:Ac, E8-12:Ac, and Z8-14:Ac | Total PFM Males/Trap | Total OFM Males/Trap | Inhibition Rate of OFM Males (%) | ||
|---|---|---|---|---|---|---|
| Z8-12:Ac | E8-12:Ac | Z8-14:Ac | ||||
| 500 | 20 | 0 | 100:4:0 | 302.20 ± 51.88 a | 111.87±6.24 a | 0.00 |
| 500 | 20 | 5 | 100:4:1 | 387.73 ± 18.31 a | 46.08±3.25 b | 58.81 |
| 500 | 20 | 10 | 100:4:2 | 287.22 ± 7.96 a | 40.89±2.35 b | 63.45 |
| 500 | 20 | 25 | 100:4:5 | 314.53 ± 26.14 a | 14.82±1.47 c | 86.75 |
| 500 | 20 | 50 | 100:4:10 | 331.58 ± 17.46 a | 9.04±1.08 c | 91.92 |
| 500 | 20 | 75 | 100:4:15 | 297.81 ± 4.75 a | 9.55±1.09 c | 91.46 |
| 500 | 20 | 100 | 100:4:20 | 285.62 ± 6.55 a | 9.67±1.52 c | 91.36 |
| 500 | 20 | 125 | 100:4:25 | 352.89 ± 32.42 a | 6.86±0.24 c | 93.87 |
| 500 | 20 | 150 | 100:4:30 | 348.30 ± 22.77 a | 7.96±1.19 c | 92.88 |
| Pheromone Loadings (μg) | Mass Ratio of Z8-12:Ac, E8-12:Ac, and Z8-14:Ac | Total PFM Males/Trap | Total OFM Males/Trap | Inhibition Rate of OFM Males (%) | ||
|---|---|---|---|---|---|---|
| Z8-12:Ac | E8-12:Ac | Z8-14:Ac | ||||
| 500 | 20 | 0 | 100:4:0 | 56.49 ± 3.80 a | 169.47 ± 9.83 a | 0.00 |
| 500 | 20 | 5 | 100:4:1 | 64.07 ± 6.89 a | 59.24 ± 10.14 b | 65.04 |
| 500 | 20 | 10 | 100:4:2 | 45.47 ± 6.64 a | 61.31 ± 7.21 b | 63.82 |
| 500 | 20 | 25 | 100:4:5 | 43.06 ± 0.34 a | 21.36 ± 6.22 c | 87.40 |
| 500 | 20 | 50 | 100:4:10 | 53.73 ± 7.04 a | 12.05 ± 0.91 c | 92.89 |
| 500 | 20 | 75 | 100:4:15 | 48.57 ± 6.31 a | 8.95 ± 2.69 c | 94.72 |
| 500 | 20 | 100 | 100:4:20 | 42.36 ± 8.46 a | 12.74 ± 6.57 c | 92.48 |
| 500 | 20 | 125 | 100:4:25 | 57.52 ± 21.06 a | 5.86 ± 1.50 c | 96.54 |
| 500 | 20 | 150 | 100:4:30 | 72.67 ± 12.44 a | 10.33 ± 2.98 c | 93.90 |
| Pheromone Loadings (μg) | Mass Ratio of Z8-12:Ac, E8-12:Ac, and Z10-14:Ac | Total PFM Males/Trap | Total OFM Males/Trap | Inhibition Rate of OFM Males (%) | ||
|---|---|---|---|---|---|---|
| Z8-12:Ac | E8-12:Ac | Z10-14:Ac | ||||
| 500 | 20 | 0 | 100:4:0 | 142.55 ± 11.16 a | 193.28 ± 60.19 a | 0.00 |
| 500 | 20 | 25 | 100:4:5 | 121.32 ± 12.10 ab | 125.55 ± 14.47 ab | 35.04 |
| 500 | 20 | 50 | 100:4:10 | 101.39 ± 7.72 b | 97.92 ± 10.31 bc | 49.34 |
| 500 | 20 | 100 | 100:4:20 | 102.73 ± 10.21 b | 83.33 ± 9.82 cd | 56.89 |
| 500 | 20 | 150 | 100:4:30 | 66.08 ± 8.27 c | 33.56 ± 2.24 e | 82.64 |
| 500 | 20 | 250 | 100:4:50 | 62.46 ± 5.63 c | 31.28 ± 3.22 e | 83.82 |
| 500 | 20 | 350 | 100:4:70 | 51.35 ± 7.21cd | 37.49 ± 3.45 e | 80.70 |
| 500 | 20 | 450 | 100:4:90 | 41.24 ± 5.10 d | 23.64 ± 1.23 e | 87.77 |
| 500 | 20 | 550 | 100:4:110 | 42.33 ± 3.95 d | 29.69 ± 2.18 e | 84.64 |
| Protein Name | Kd (μM) | IC50 (μM) | Ki (μM) |
|---|---|---|---|
| GmolPBP1 | 1.29 ± 0.02 | 2.93 ± 0.07 | 1.66 ± 0.04 c |
| GmolPBP2 | 1.09 ± 0.02 | 1.26 ± 0.04 | 0.66 ± 0.02 d |
| GmolPBP3 | 11.24 ± 0.08 | 2.78 ± 0.32 | 2.56 ± 0.27 b |
| GmolGOBP1 | 5.76 ± 0.01 | >14 | >14 a |
| GmolGOBP2 | 4.32 ± 0.02 | 3.53 ± 0.22 | 2.86 ± 0.18 b |
| Protein Name | IC50 (μM) | Ki (μM) |
|---|---|---|
| wild-type | 1.260 ± 0.036 | 0.660 ± 0.019 |
| F12A | >14 | >14 |
| L68A | 1.129 ± 0.026 | 0.589 ± 0.007 |
| I94A | 1.082 ± 0.008 | 0.564 ± 0.004 |
| R109A | 1.257 ± 0.029 | 0.655 ± 0.015 |
| I113A | 0.980 ± 0.038 | 0.520 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Chen, X.; Xu, S.; Li, B.; Luo, K.; Li, G. Functional Role of Odorant-Binding Proteins in Response to Sex Pheromone Component Z8-14:Ac in Grapholita molesta (Busck). Insects 2024, 15, 918. https://doi.org/10.3390/insects15120918
Luo Y, Chen X, Xu S, Li B, Luo K, Li G. Functional Role of Odorant-Binding Proteins in Response to Sex Pheromone Component Z8-14:Ac in Grapholita molesta (Busck). Insects. 2024; 15(12):918. https://doi.org/10.3390/insects15120918
Chicago/Turabian StyleLuo, Yuqing, Xiulin Chen, Shiyan Xu, Boliao Li, Kun Luo, and Guangwei Li. 2024. "Functional Role of Odorant-Binding Proteins in Response to Sex Pheromone Component Z8-14:Ac in Grapholita molesta (Busck)" Insects 15, no. 12: 918. https://doi.org/10.3390/insects15120918
APA StyleLuo, Y., Chen, X., Xu, S., Li, B., Luo, K., & Li, G. (2024). Functional Role of Odorant-Binding Proteins in Response to Sex Pheromone Component Z8-14:Ac in Grapholita molesta (Busck). Insects, 15(12), 918. https://doi.org/10.3390/insects15120918










