Determination of Larval Instars of Dastarcus helophoroides (Coleoptera: Bothrideridae) Using Head Capsule Width Frequency Distribution
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Head Capsule Measurement
2.3. Analysis of Head Capsule Widths
3. Results
3.1. Instar-Wise Head Capsule Widths (Observed)
3.2. Instar-Wise Head Capsule Widths (Theoretical)
3.3. Regression Analysis of Head Capsule Widths
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rim, K.; Golec, J.R.; Duan, J.J. Host selection and potential non-target risk of Dastarcus helophoroides, a larval parasitoid of the Asian long-horned beetle, Anoplophora glabripennis. Biol. Control 2018, 123, 120–126. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Zhang, Y.; Zhang, Y. Research advances of Chinese major forest pests by integrated management based on biological control. Chin. J. Biol. Control 2018, 34, 163. [Google Scholar]
- Wang, Q. Cerambycid pests in agricultural and horticultural crops. In Cerambycidae of the World; CRC Press: Boca Raton, FL, USA, 2017; pp. 423–576. [Google Scholar]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus [Pinus densiflora, Pinus thunbergii, Pinus luchuensis, Japan]. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Robertson, L.; Cobacho Arcos, S.; Escuer, M.; Santiago Merino, R.; Esparrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 755–757. [Google Scholar]
- Meng, J. Sustainability: A framework of typology based on efficiency and effectiveness. J. Macromark. 2015, 35, 84–98. [Google Scholar] [CrossRef]
- Siviter, H.; Muth, F. Do novel insecticides pose a threat to beneficial insects? Proc. R. Soc. B 2020, 287, 20201265. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.; du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Deveci, H.A.; Nur, G.; Deveci, A.; Kaya, I.; Kaya, M.M.; Kükürt, A.; Gelen, V.; Başer, Ö.F.; Karapehlivan, M. An Overview of the Biochemical and Histopathological Effects of Insecticides; IntechOpen: London, UK, 2021. [Google Scholar]
- Wang, G.; Xu, X.; Cheng, Q.; Hu, J.; Xu, X.; Zhang, Y.; Guo, S.; Ji, Y.; Zhou, C.; Gao, F.; et al. Preparation of sustainable release mesoporous silica nano-pesticide for control of Monochamus alternatus. Sustain. Mater. Technol. 2023, 35, e00538. [Google Scholar] [CrossRef]
- Saddam, B.; Idrees, M.A.; Kumar, P.; Mahamood, M. Biopesticides: Uses and importance in insect pest control: A review. Int. J. Trop. Insect Sci. 2024, 44, 1013–1020. [Google Scholar] [CrossRef]
- Tadahisa, U. Preliminary release experiments in laboratory and outdoor cages of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) for biological control of Monochamus alternatus Hope (Coleoptera: Cerambycidae). Bull. For. For. Prod. Res. Inst. 2003, 2, 255–262. [Google Scholar]
- Li, M.; Li, Y.; Lei, Q.; Yang, Z. Biocontrol of Asian long-horned beetle larva by releasing eggs of Dastarcus helophoroides (Coleoptera: Bothrideridae). Sci. Silvae Sin. 2009, 45, 78–82. [Google Scholar]
- Tang, Y.; Gao, S.; Zhang, Y.; Yang, Z.; Lu, J.; Zhan, M.; Wang, J. Key Environmental Factors Affecting Parasitism of Monochamus alternatus Hope by Dastarcus helophoroides Fairmaire. Chin. J. Biol. Control 2015, 31, 830. [Google Scholar]
- Castoreña, M.M.V.; Valencia, E.A.C. Determinación de estadios larvales de Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) para la construcción de un modelo de predicción. Folia Entomol. Mex. 2004, 43, 307–312. [Google Scholar]
- Gullan, P.; Cranston, P. The Insects: An Outline of Entomology. Nature 1994, 370, 261. [Google Scholar]
- Yamany, A.S.; Abdel-Gaber, R. Identification of fourth-instar larvae of Aedes albopictus (Skuse) (Diptera: Culicidae) employing scanning electron microscopic tool. Microsc. Res. Tech. 2024, 87, 933–947. [Google Scholar] [CrossRef]
- Lambiase, S.; Corotti, S.; Sacchi, R. Morphometric analysis for determination of larval instars in Dermestes frischii Kugelann and Dermestes undulatus Brahm (Coleoptera: Dermestidae). J. Forensic Sci. 2024, 69, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Merville, A.; Vallier, A.; Venner, S.; Siberchicot, A.; Fouchet, D.; Heddi, A.; Bel-Venner, M.-C. Determining the instar of a weevil larva (Coleoptera: Curculionidae) using a parsimonious method. Eur. J. Entomol. 2014, 111, 567–573. [Google Scholar] [CrossRef]
- Panzavolta, T. Instar determination for Pissodes castaneus (Coleoptera: Curculionidae) using head capsule widths and lengths. Environ. Entomol. 2014, 36, 1054–1058. [Google Scholar] [CrossRef]
- Cazado, L.E.; Van Nieuwenhove, G.A.; O’brien, C.; Gastaminza, G.A.; Murúa, M.G. Determination of number of instars of Rhyssomatus subtilis (Coleoptera: Curculionidae) based on head capsule widths. Fla. Entomol. 2014, 97, 639–643. [Google Scholar] [CrossRef]
- Dyar, H.G. The number of molts of lepidopterous larvae. Psyche 1890, 5, 420–422. [Google Scholar] [CrossRef]
- Gaines, J.; Campbell, F. Dyar’s rule as related to the number of instars of the corn ear worm, Heliothis obsoleta (Fab.), collected in the field. Ann. Entomol. Soc. Am. 1935, 28, 445–461. [Google Scholar] [CrossRef]
- Chen, Y.; Seybold, S. Application of a frequency distribution method for determining instars of the beet armyworm (Lepidoptera: Noctuidae) from widths of cast head capsules. J. Econ. Entomol. 2013, 106, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Hao, C.F.; Li, M.L. Female reproductive system of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). J. Northwest AF Univ. 2016, 44, 100–108. [Google Scholar]
- Tang, H.; Yang, Z.; Zhang, Y.; Li, G. Technical researches on distinguishing female and male alive adults of the main parasite of longhorn beetles, Dastarcus helophoroides (Coleoptera: Bothrideridae) without injuring. Acta Zoot. Sin 2007, 32, 649–654. [Google Scholar]
- Han, X.; Duan, Y.L.; Li, X.F.; Wang, Z.Y.; Zhang, Y.N.; Qiao, L.Q. Sensilla Ultrastructure of Antennae and Mouthparts of the First Instar Larvae of Dastarcus helophoroides (Coleoptera: Bothrideridae). For. Sci. Res. 2021, 34, 180–184. [Google Scholar]
- Pei, P. Dastarcus helophoroides P450 Participates in the Molecular Mechanism of Cypermethrin Stress Response. Master’s Thesis, Northwest A&F University, Yangling, China, 2021. [Google Scholar]
- Wang, X.; Ni, X.; Duan, C.; Li, R.; Jiang, X.; Xu, M.; Yu, R.J. The effect of ultrasound treatment on the structural and functional properties of Tenebrio molitor Myofibrillar protein. Foods 2024, 13, 2817. [Google Scholar] [CrossRef]
- Sun, C.W. Flying Ability and It’s Influencing Factors of Dastarcus helophoroides Adults. Master’s Thesis, Shandong Agricultural University, Jinan, China, 2019. [Google Scholar]
- Zhang, X. Effects of Dastarcus helophoroides Larval Density on the Degeneration of Subsitute Host Nutrition and Metabolism. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2018. [Google Scholar]
- Delbac, L.; Lecharpentier, P.; Thiery, D. Larval instars determination for the European Grapevine Moth (Lepidoptera: Tortricidae) based on the frequency distribution of head-capsule widths. Crop Prot. 2010, 29, 623–630. [Google Scholar] [CrossRef]
- Sukovata, L. A comparison of three approaches for larval instar separation in insects—A case study of Dendrolimus pini. Insects 2019, 10, 384. [Google Scholar] [CrossRef]
- Li, N.; Wu, L.; Geng, Y.; Wei, D.; Chen, M. Determination of larval instars of Semanotus bifasciatus (Coleoptera: Cerambycidae) based on frequency distributions of morphological variables. J. Entomol. Sci. 2020, 55, 405–415. [Google Scholar] [CrossRef]
- Ramasubramanian, T.; Rajan, T.S.; Sudhanan, E.M. Instar determination for sugarcane internode borer Chilo sacchariphagus indicus (Kapur) (Lepidoptera: Crambidae). J. Asia-Pac. Entomol. 2021, 24, 461–469. [Google Scholar] [CrossRef]
- Brockelsby, W.D.; Miskelly, C.M.; Glare, T.R.; Minor, M.A. The number of larval instars in the flax weevil (Anagotus fairburni) (Coleoptera: Curculionidae). N. Z. J. Zool. 2023, 52, 1–11. [Google Scholar] [CrossRef]
- McClellan, Q.; Logan, J. Instar determination for the gypsy moth (Lepidoptera: Lymantriidae) based on the frequency distribution of head capsule widths. Environ. Entomol. 1994, 23, 248–253. [Google Scholar] [CrossRef]
- Logan, J.; Bentz, B.; Vandygriff, J.; Turner, D. General program for determining instar distributions from headcapsule widths: Example analysis of mountain pine beetle (Coleoptera: Scolytide) data. Environ. Entomol. 1998, 27, 555–563. [Google Scholar] [CrossRef]
- Brooks, W.K. Report on the Stomatopoda collected by HMS Challenger during the years 1873–76. In Report on the Scientific Results of the Voyage of HMS; Challenger Reports; 1886; Volume 16, pp. 1–16. [Google Scholar] [CrossRef]
- Crosby, T. Dyar’s rule predated by Brooks’ rule. N. Z. Entomol. 1973, 5, 175–176. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Li, S.; Zhu, H.; Fan, B.; Wang, Y.; Su, P.; Han, Y.; Hao, D. Biological traits and life history of Pagiophloeus tsushimanus (Coleoptera: Curculionidae), a weevil pest on camphor trees in China. J. For. Res. 2021, 32, 1979–1988. [Google Scholar] [CrossRef]
- Yu, Y.; Cen, G.; Wei, D.; Zeng, X.; Zeng, T. Division of larval instars of Dorysthenes granulosus based on Crosby growth rule. J. South. Agric. 2012, 43, 1485–1489. [Google Scholar]
- Castañeda-Vildózola, Á.; González-Hernández, H.; Equihua-Martínez, A.; Valdez-Carrasco, J.; Peña, J.E.; Cazado, L.E.; Franco-Mora, O. Head capsule width is useful for determining larval instar in Heilipus lauri (Coleoptera: Curculionidae). Fla. Entomol. 2016, 99, 822–825. [Google Scholar] [CrossRef]
- Luo, W.; Ji, Y.-C.; Wen, J.-B. Application of a frequency distribution method for determining instars of Eucryptorrhynchus brandti (Coleoptera: Curculionidae) from several morphological variables. Biocontrol Sci. Technol. 2016, 26, 1329–1336. [Google Scholar] [CrossRef]
- Velásquez, Y.; Viloria, Á.L. Instar determination of the neotropical beetle Oxelytrum discicolle (Coleoptera: Silphidae). J. Med. Entomol. 2010, 47, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Fauteux, A.; Gonzalez, N.; Soares, A.O.; Lucas, É. Morphological determination of the larval instars of Eupeodes americanus (Diptera: Syrphidae). Phytoprotection 2022, 102, 30–34. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Dou, W.; Jiang, H.-B.; Wei, D.-D.; Wei, D.; Niu, J.-Z.; Wang, J.-J. Determination of instars of Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2017, 100, 270–275. [Google Scholar] [CrossRef]
- Peterson, M.K.; Appel, A.G.; Hu, X.P. Instar determination of Blattella asahinai (Blattodea: Ectobiidae) from digital measurements of the pronotum using Gaussian mixture modeling and the number of cercal annuli. J. Insect Sci. 2019, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Haeussler, G. Some observations on the number of larval instars of the oriental peach moth, Laspeyresia molesta Busck. J. Econ. Entomol. 1928, 21, 843–852. [Google Scholar] [CrossRef]
- Chen, Y.; Dallara, P.L.; Nelson, L.J.; Coleman, T.W.; Hishinuma, S.M.; Carrillo, D.; Seybold, S.J. Comparative morphometric and chemical analyses of phenotypes of two invasive ambrosia beetles (Euwallacea spp.) in the United States. Insect Sci. 2017, 24, 647–662. [Google Scholar] [CrossRef]
- Preto, C.R.; Bellamy, D.E.; Walse, S.S.; Zalom, F.G. Predicting larval stage distribution of Lobesia botrana (Lepidoptera: Tortricidae) at three constant temperatures. J. Econ. Entomol. 2019, 112, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Molina, J.M. Head capsule width and instar determination for larvae of Streblote panda (Lepidoptera: Lasiocampidae). Ann. Entomol. Soc. Am. 2008, 101, 881–886. [Google Scholar] [CrossRef]
- Hansen, J.D.; Owens, J.C.; Huddleston, E.W. Relation of head capsule width to instar development in larvae of the range caterpillar, Hemileuca oliviae Cockerell (Lepidoptera: Saturniidae). J. Kans. Entomol. Soc. 1981, 54, 1–7. [Google Scholar]
- Cave, G.; Smith, C. Number of instars of the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1983, 76, 293–294. [Google Scholar] [CrossRef]
- Zawadneak, M.A.; Goncalves, R.B.; Poltronieri, A.S.; Santos, B.; Bischoff, A.M.; Borba, A.M.; Pimentel, I.C. Biological parameters of Duponchelia fovealis (Lepidoptera: Crambidae) reared in the laboratory on two diets. Eur. J. Entomol. 2017, 114, 291–294. [Google Scholar] [CrossRef]
- Gousul, N.; Buhroo, A.A. Bionomics of slender burnished brass (Thysanoplusia orichalcea [Fabricius, 1775], Lepidoptera: Noctuidae) on potato (Solanum tuberosum L.) in Kashmir. Acta Agric. Slov. 2021, 117, 1–8. [Google Scholar]
- Cen, G.; Yu, Y.; Zeng, X.; Long, X.; Wei, D.; Gao, X.; Zeng, T. An adaptive kernel smoothing method for classifying Austrosimulium tillyardianum (Diptera: Simuliidae) larval instars. J. Insect Sci. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, J.; Wang, W.; Zheng, Y. Larval Instars and Adult Flight Period of Monochamus saltuarius (Coleoptera: Cerambycidae). Forests 2022, 13, 910. [Google Scholar] [CrossRef]
- Yang, R.; Qiu, P.; Gu, Y.; Ni, M.; Xue, Z.; Han, J.; Jiang, Y.; Jin, Y.; Wang, Y.; Zhou, X. Biology of Rhynchaenus maculosus provides insights and implications for integrated management of this emerging pest. Sci. Rep. 2022, 12, 14650. [Google Scholar] [CrossRef]
- Esperk, T.; Tammaru, T.; Nylin, S. Intraspecific variability in number of larval instars in insects. J. Econ. Entomol. 2007, 100, 627–645. [Google Scholar] [CrossRef]
- Langor, D.W.; Spence, J.R.; Pohl, G.R. Host effects on fertility and reproductive success of Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Evolution 1990, 44, 609–618. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric analysis of instar variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Guo, W.; Gao, P.; Wang, F.; Hu, Z. Occurrence and control techniques of Anoplophora glabripennis and Anoplophora chinensis. Mod. Gard. 2016, 8, 53. [Google Scholar]
- Yang, C.; Wei, Y.; Li, C.; Huang, S.; Li, L.; Ma, Z. Division of larval instars of the coconut black-headed caterpillar, Opisina arenosella. Plant Prot. 2015, 41, 70–74. [Google Scholar]
- Xu, J.H.; Huang, X.F.; Xu, H.C.; Cheng, J.M.; Jiang, Y.; Fang, W.J. Rearing and biological properties of Monochamus alternatus. J. Zhejiang For. Sci. 2009, 29, 86–88. [Google Scholar]
- Huang, D.; Wang, R.J.; Tang, P.; Li, G.Z.; Peng, J.Y.; Wang, S.F.; Zhu, L.X. Study on the age division of Monochamus alternatus in Panzhihua City and the regularity of each age. Sichuan Agric. Sci. Tech. 2018, 373, 31–33. [Google Scholar]
- Bleiker, K.; Régnière, J. Determining the instar of mountain pine beetle (Coleoptera: Curculionidae) larvae by the width of their head capsules. Can. Entomol. 2015, 147, 635–640. [Google Scholar] [CrossRef]

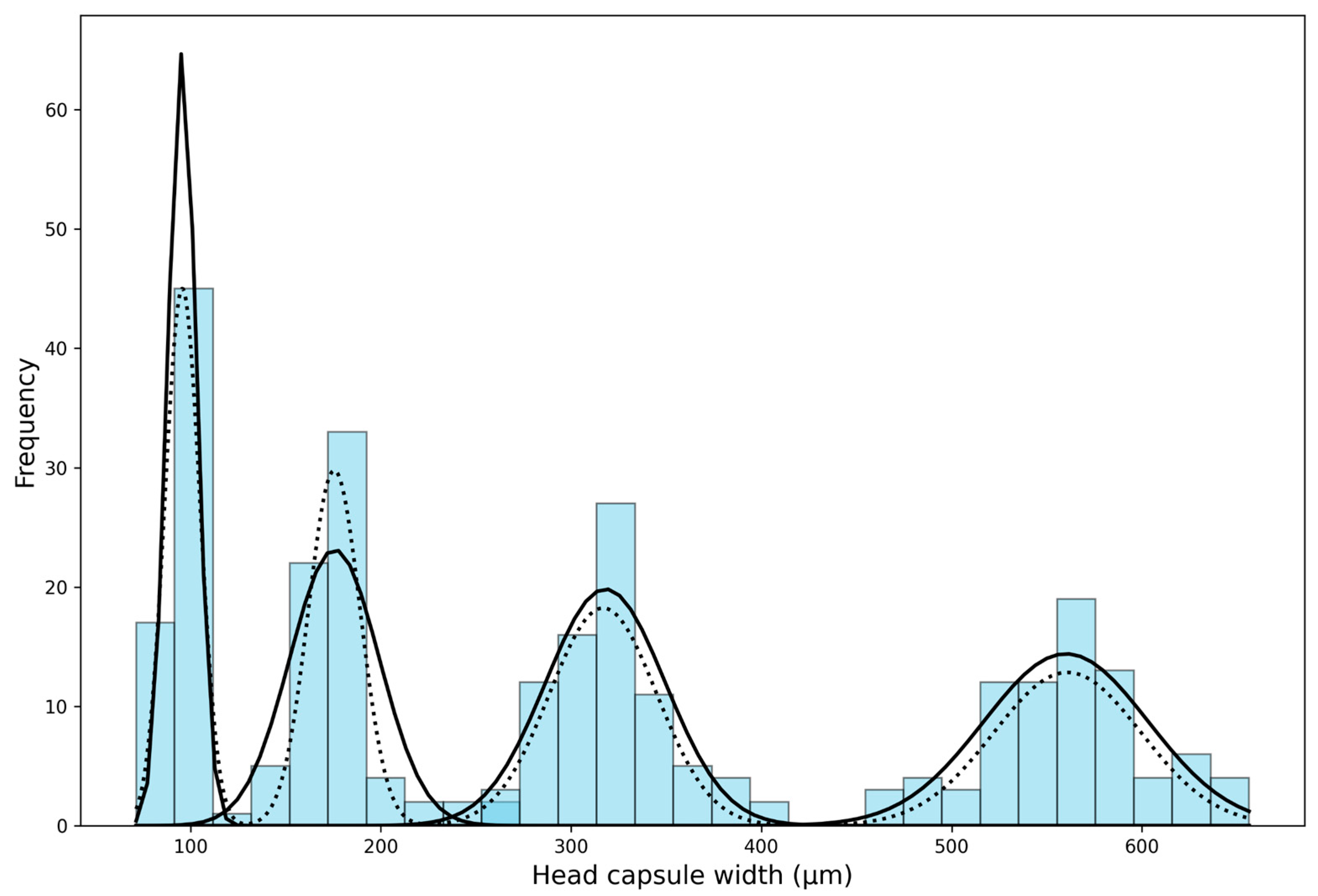
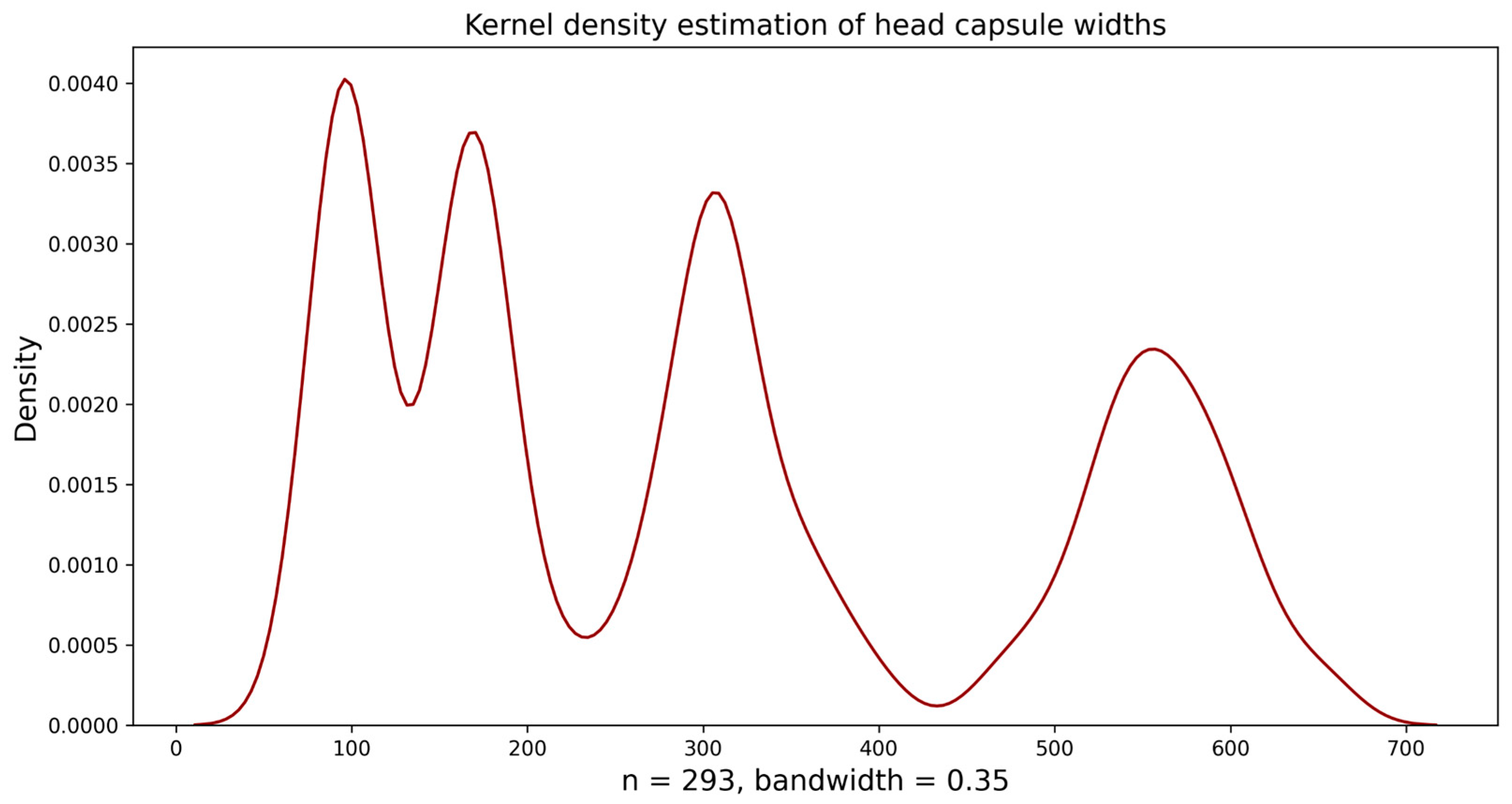
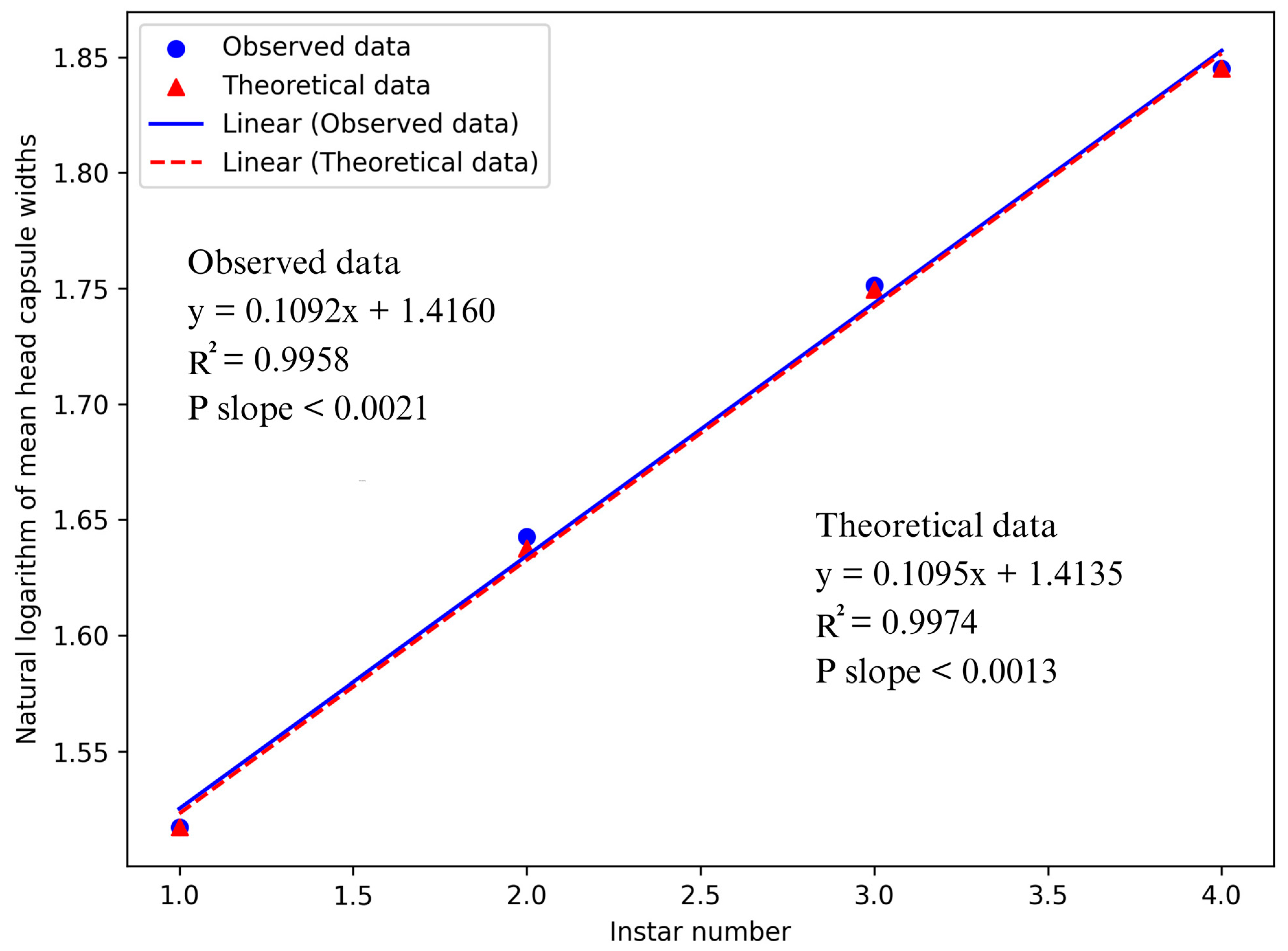
| Instar | n | Head Capsule Width | CV (%) | Brooks–Dyar’s Ratio | Crosby’s Growth Ratio | |
|---|---|---|---|---|---|---|
| Mean ± SD (µm) | Range | |||||
| 1 | 63 | 95.52 ± 7.62 | 71.41–112.13 | 4.60 | ||
| 2 | 70 | 175.64 ± 23.69 | 142.83–255.15 | 7.78 | 1.83 | |
| 3 | 80 | 318.01 ± 31.57 | 270.18–406 | 5.73 | 1.81 | −0.01 |
| 4 | 80 | 560.06 ± 43.46 | 466.6–649.93 | 4.48 | 1.76 | −0.02 |
| Mean growth rate | 1.80 |
| Instar | n | Head Capsule Width | CV (%) | Brooks–Dyar’s Ratio | |
|---|---|---|---|---|---|
| Mean ± SD (µm) | Range | ||||
| 1 | 63 | 95.68 ± 9.21 | ˂116.14 | 4.60 | |
| 2 | 66 | 175.52 ± 13.64 | 116.14–235.42 | 5.37 | 1.83 |
| 3 | 84 | 316.80 ± 28.17 | 235.42–429 | 6.30 | 1.80 |
| 4 | 80 | 560.83 ± 38.78 | >429 | 4.48 | 1.77 |
| Mean growth rate | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, T.; Guo, J.; Wang, Y.; Zhou, J.; Tang, G.; Zhang, Z. Determination of Larval Instars of Dastarcus helophoroides (Coleoptera: Bothrideridae) Using Head Capsule Width Frequency Distribution. Insects 2024, 15, 1013. https://doi.org/10.3390/insects15121013
Shaheen T, Guo J, Wang Y, Zhou J, Tang G, Zhang Z. Determination of Larval Instars of Dastarcus helophoroides (Coleoptera: Bothrideridae) Using Head Capsule Width Frequency Distribution. Insects. 2024; 15(12):1013. https://doi.org/10.3390/insects15121013
Chicago/Turabian StyleShaheen, Tayyab, Jiali Guo, Yun Wang, Jiaojiao Zhou, Guanghui Tang, and Zhengqing Zhang. 2024. "Determination of Larval Instars of Dastarcus helophoroides (Coleoptera: Bothrideridae) Using Head Capsule Width Frequency Distribution" Insects 15, no. 12: 1013. https://doi.org/10.3390/insects15121013
APA StyleShaheen, T., Guo, J., Wang, Y., Zhou, J., Tang, G., & Zhang, Z. (2024). Determination of Larval Instars of Dastarcus helophoroides (Coleoptera: Bothrideridae) Using Head Capsule Width Frequency Distribution. Insects, 15(12), 1013. https://doi.org/10.3390/insects15121013






