The Cap and the Spermatostyle Protecting the Sperm Bundle Have a Similar Origin—Ultrastructural Study of the Spermatogenesis from the Ground Beetle Carabus (Chaetocarabus) lefebvrei Dejean, 1826 (Adephaga Carabidae)
Simple Summary
Abstract
1. Introduction
- (1)
- Individual sperm attached with their heads and the initial part of their flagella to a rod of extracellular material (spermatostyle). The sperm can be attached only along one side of the rod as it occurs in the Gyrinidae Gyretes sp. [17] or on whatever side as in Harpalus sp. [18] and other species of Carabids [12,19,20,21,22,23,24,25,26], in several species of Dineutus and in the whirligig Orectochilus villosus [27];
- (2)
- (3)
- (4)
- (5)
- Sperm rouleaux when the tip of a sperm head slips into another sperm head to form a stack. This is made possible by apposition of one sperm cone-shaped side with the concave side of the successive sperm in the stack. This peculiar type of sperm aggregation has been described in some Dytiscidae Hydroporinae such as Stictonectes optatus [31] and Hydroporus sp. [16].
2. Materials and Methods
2.1. Fluorescence Microscopy
2.2. Scanning Electron Microscopy (SEM)
2.3. Transmission Electron Microscopy (TEM)
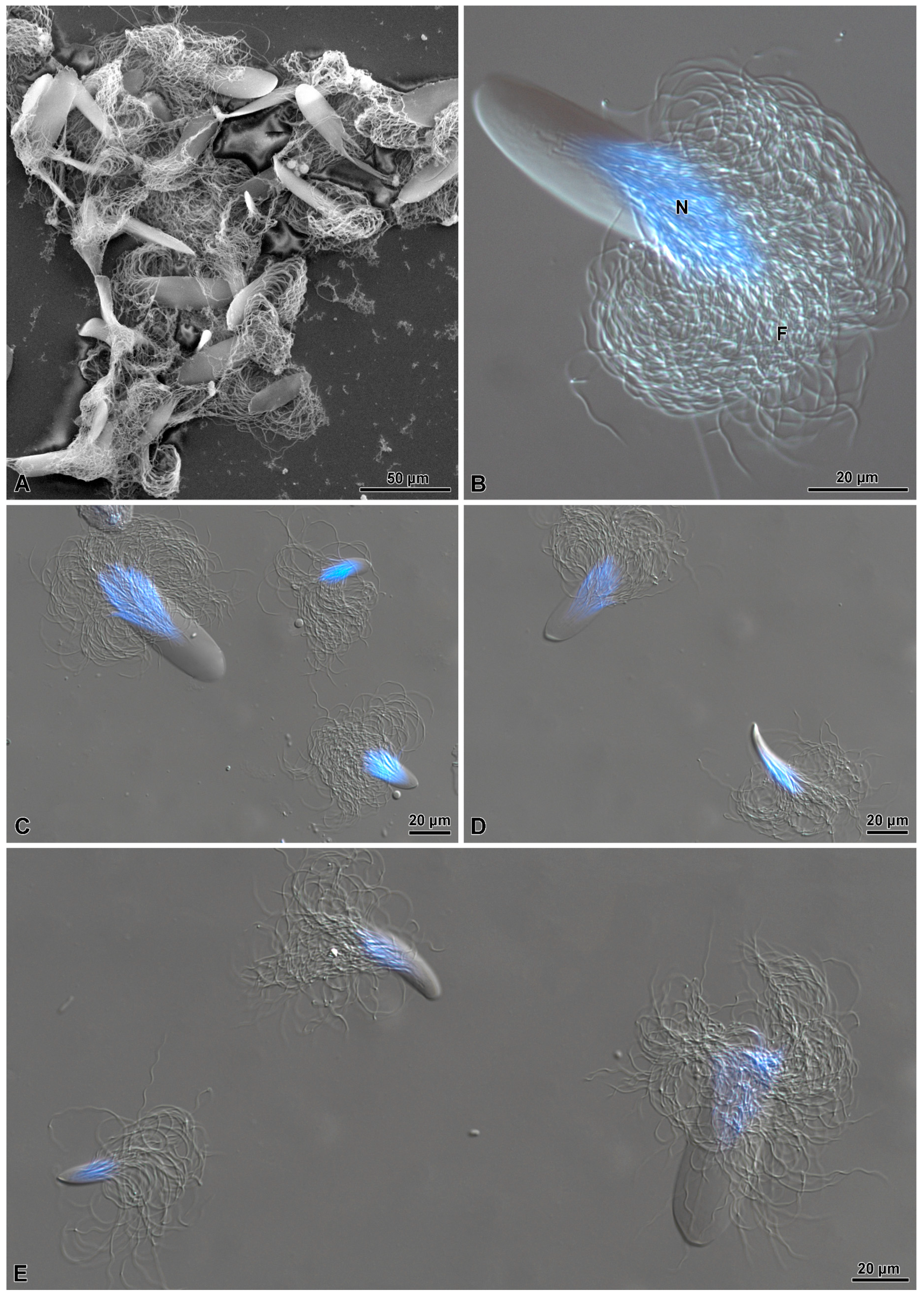
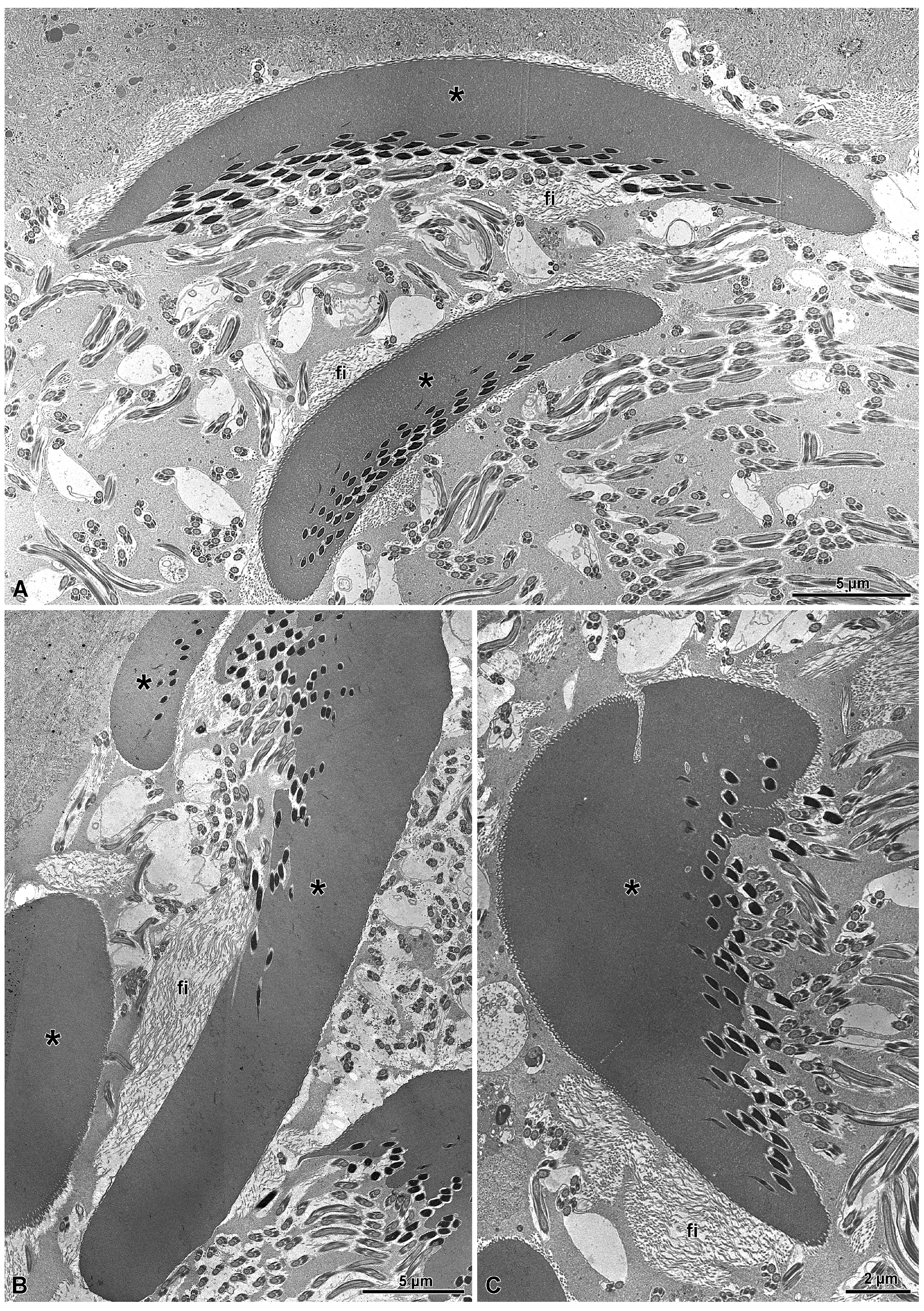
3. Results
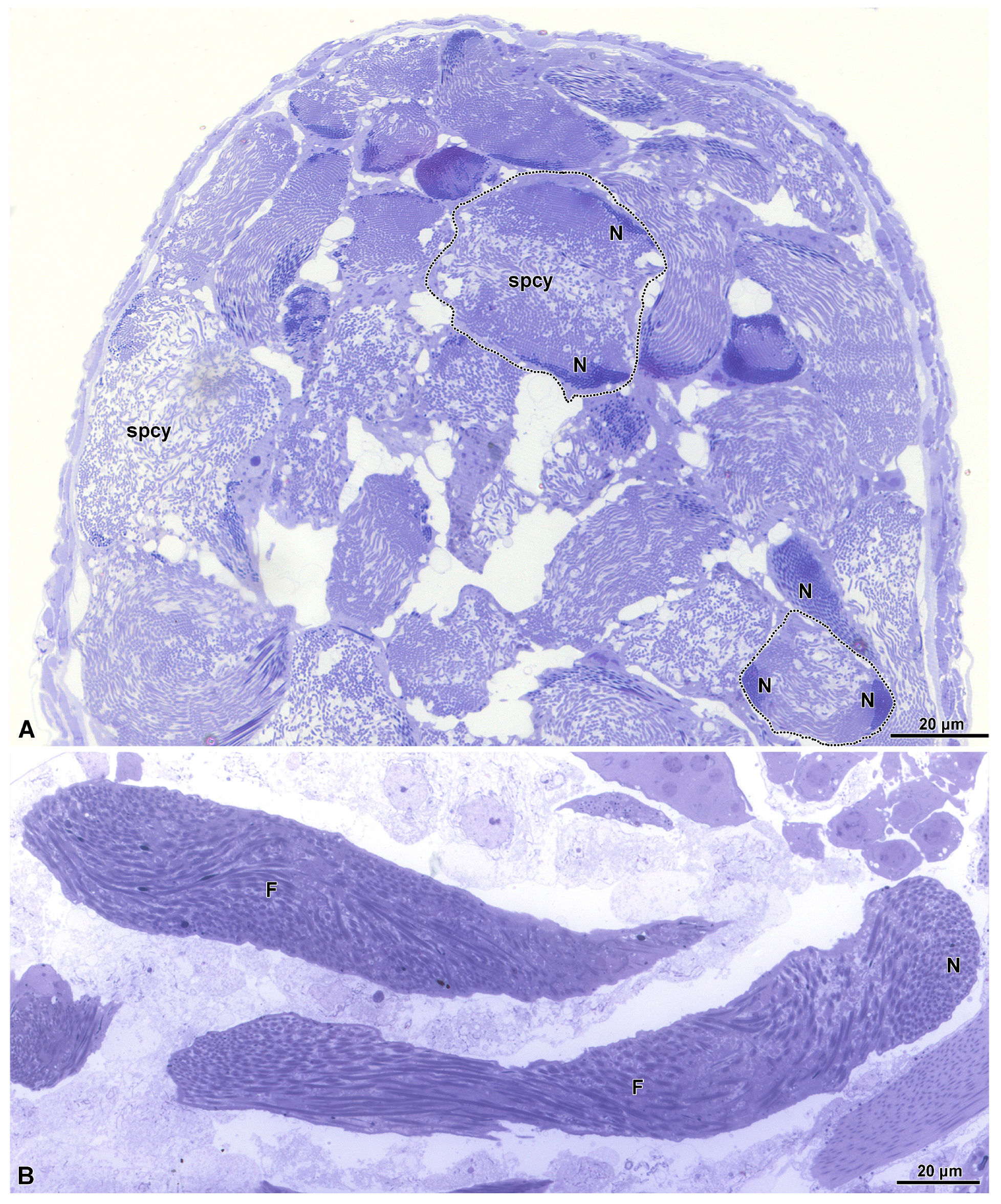
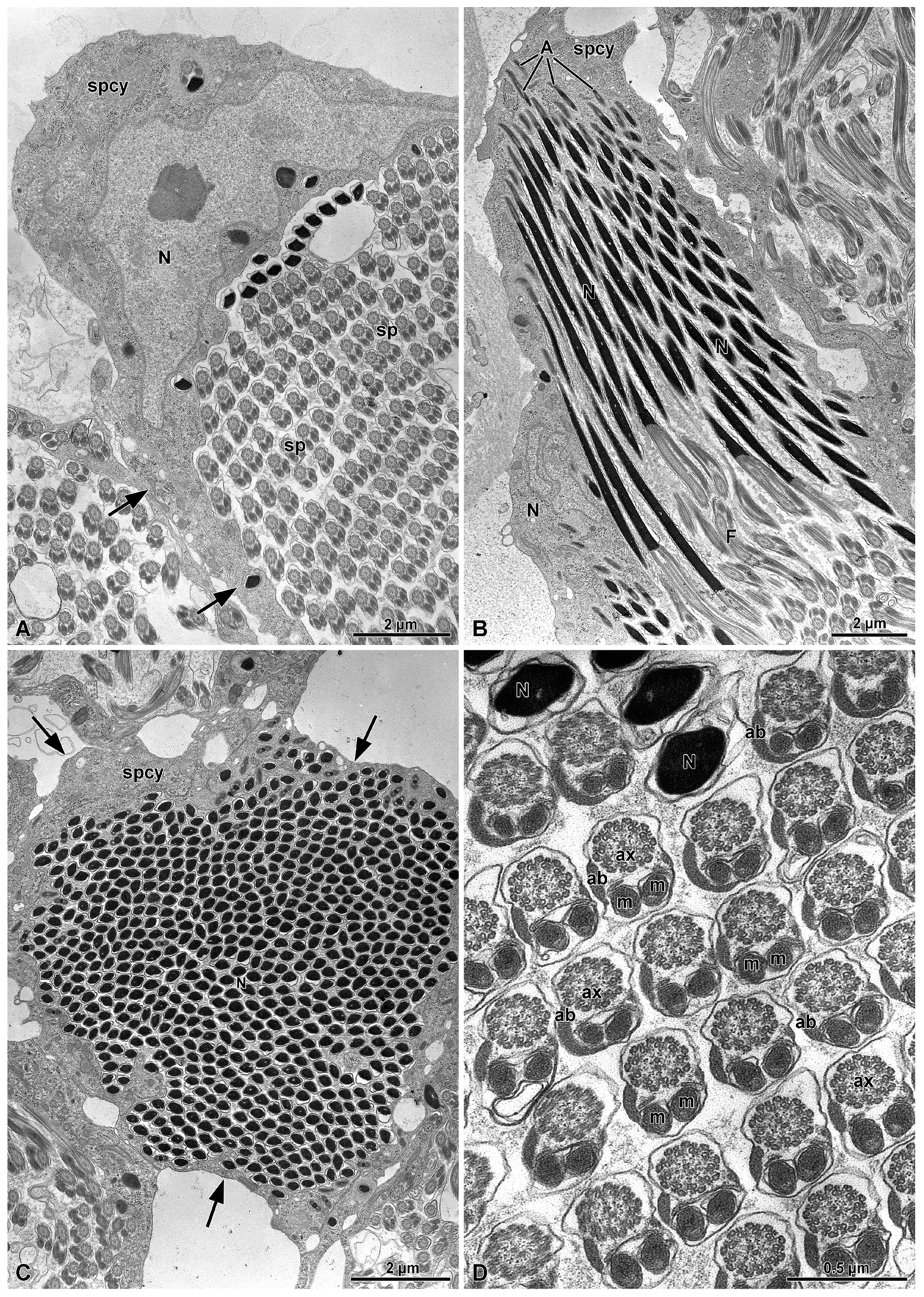
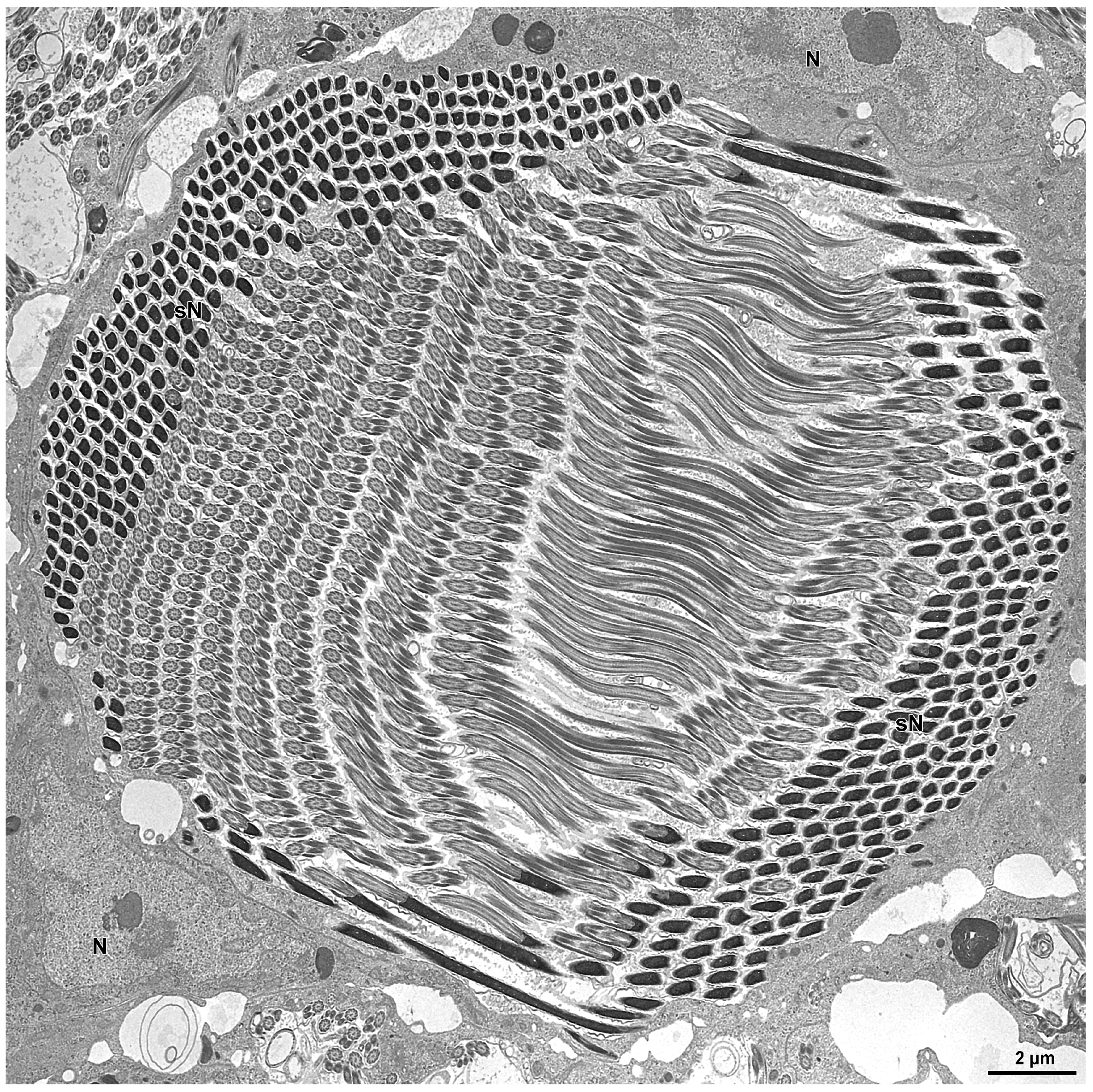
3.1. Spermatogenesis and Spermiogenesis
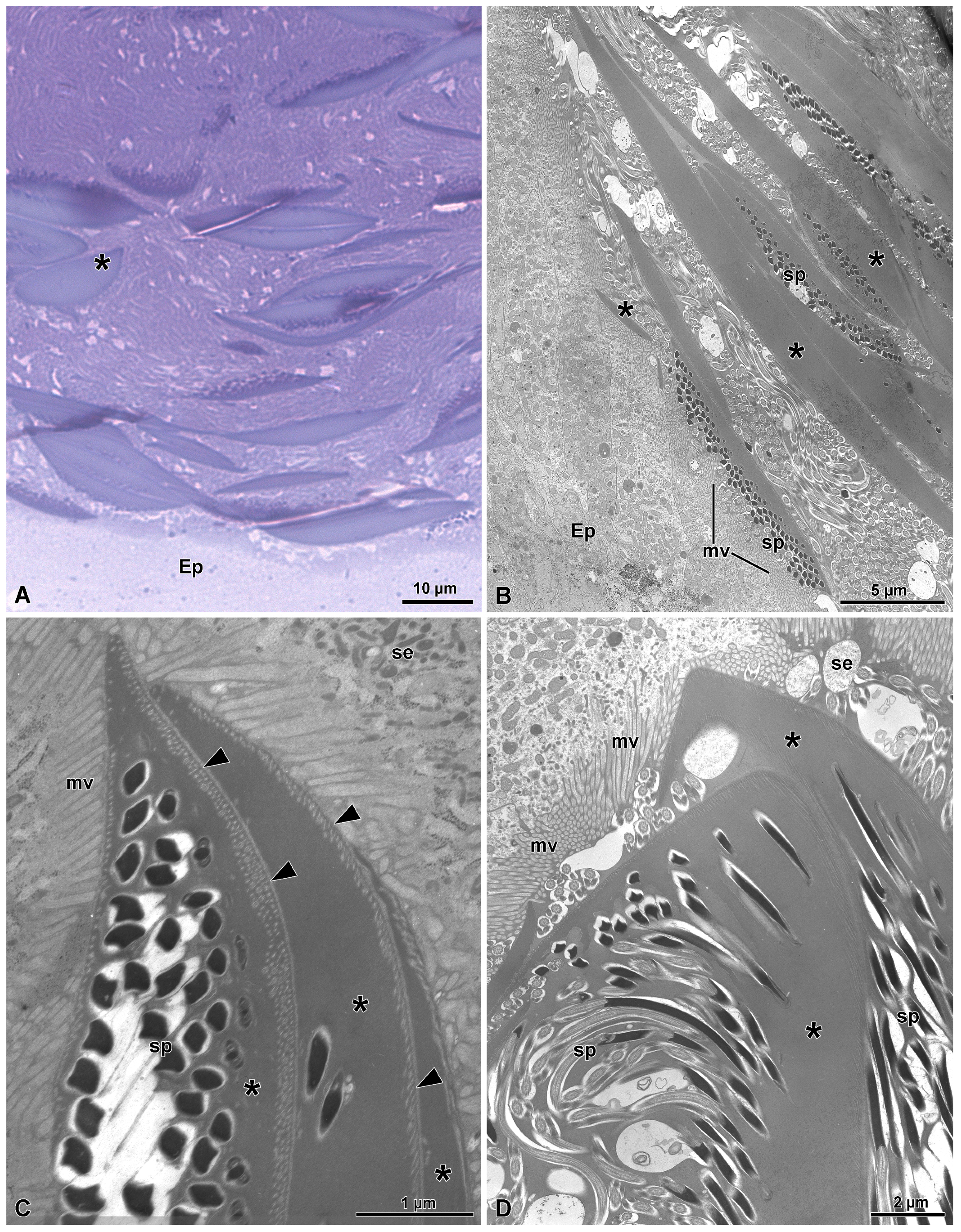
3.2. The Distal Deferent Duct (Vas Deferens I, Sensu [22])
3.3. The Most Proximal Deferent Duct (Vas Deferens II, Sensu [22])
3.4. The Seminal Vesicles (Sensu [22])
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alcock, J.; Thornhill, R. The Evolution of Insect mating systems. In The Evolution of Insect Mating Systems; Shuker, D., Simmons, L., Eds.; Oxford Academic: Oxford, UK, 2014; pp. 275–278. [Google Scholar] [CrossRef]
- Kokko, H.; Klug, H.; Jennions, M.D. Mating systems. In The Evolution of Insect Mating Systems; Shuker, D., Simmons, L., Eds.; Oxford Academic: Oxford, UK, 2014; pp. 42–58. [Google Scholar] [CrossRef]
- Simmons, L.W. Sexual selection and genital evolution. Aust. Entomol. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Simmons, L.W. Inter-male competition and mating success in the field cricket Gryllus bimaculatus (De Geer). Anim. Behav. 1986, 34, 567–579. [Google Scholar] [CrossRef]
- Simmons, L.W.; Beveridge, M.; Krauss, S. Genetic analysis of parentage within experimental populations of a male dimorphic beetle, Onthophagus taurus, using amplified fragment length polymorphism. Behav. Ecol. Sociobiol. 2004, 57, 164–173. [Google Scholar] [CrossRef]
- Parker, G.A. Why are there so many tiny sperm? Sperm competition and the maintenance of two sex. J. Theor. Biol. 1982, 96, 281–294. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm competition games: Sperm size and sperm number under adult control. Proc. R. Soc. Lond. B Biol. Sci. 1993, 253, 245–254. [Google Scholar] [CrossRef]
- Gage, M.J.G. Association between body size, mating pattern, testis size, and sperm length across butterflies. Proc. R. Soc. Lond. B Biol. Sci. 1994, 258, 247–254. [Google Scholar] [CrossRef]
- Pitnick, S.; Markow, T.A.; Spicer, G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 1999, 53, 1804–1822. [Google Scholar] [CrossRef]
- Takami, Y. Mating behavior, insemination and sperm transfer in the ground beetle Carabus insulicola. Zool. Sci. 2002, 19, 1067–1073. [Google Scholar] [CrossRef][Green Version]
- Sasakawa, K. Morphological association between the spermatophore and male genitalia in carabid beetles of the tribe Pterostichini (Coleoptera: Carabidae). Zool. Sci. 2006, 23, 587–591. [Google Scholar] [CrossRef]
- Sasakawa, K. Sperm bundle and reproductive organs of carabid beetles tribe Pterostichini (Coleoptera, Carabidae). Naturwissenschaften 2007, 94, 384–391. [Google Scholar] [CrossRef]
- Takami, Y.; Sota, T. Sperm competition promotes diversity of sperm bundles in Ohomopterus ground beetles. Naturwissenschaften 2007, 94, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F. Sperm-cooperation in the fish-fly, Parachaulioides japonicus. Funct. Ecol. 1998, 12, 347–350. [Google Scholar] [CrossRef]
- Phillips, D.M. Insect sperm: Their structure and morphogenesis. J. Cell Biol. 1970, 44, 243–277. [Google Scholar] [CrossRef]
- Higginson, D.M.; Pitnick, S. Evolution of intra-ejaculate sperm interactions: Do sperm cooperate? Biol. Rev. Camb. Philos. Soc. 2011, 86, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Salazar, K.; Novais, A.; Lino-Neto, J.L.; Serrão, J.E. The sperm aggregation in a whirligig beetle (Coleoptera, Gyrinidae): Structure, functions, and comparison with related taxa. Org. Divers. Evol. 2022, 22, 355–375. [Google Scholar] [CrossRef]
- Breland, O.P.; Simmons, E. Preliminary studies of the spermatozoa and the male reproductive system of some whirligig beetle (Coleoptera: Gyrinidae). Entomol. News 1970, 81, 101–110. [Google Scholar]
- Carcupino, M.; Stocchino, A.G.; Corso, G.; Manca, I.; Casale, A. Morphology of the male reproductive apparatus and spermatodesms formation in Percus strictus strictus (Coleoptera, Carabidae). In Proceedings of the 9th International Symposium on Spermatology, Cape Town, South Africa, 6–11 October 2002; van der Horst, G., Franken, D., Bornman, R., Bornman, T., Dyer, S., Eds.; Monduzzi Editore: Bologna, Italy, 2002; pp. 31–34. [Google Scholar]
- Sasakawa, K. Marked sperm dimorphism in the ground beetle Scarites terricola: A novel type of insect sperm polymorphism. Physiol. Entomol. 2009, 34, 387–390. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Ferenz, H.J.; Schneider, S. Formation of sperm bundles in Pterostichus nigrita (Coleoptera: Carabidae). Invertebr. Reprod. Dev. 2013, 57, 120–131. [Google Scholar] [CrossRef]
- Schubert, L.F.; Krüger, S.; Moritz, G.B.; Schubert, V. Male reproductive system and spermatogenesis of Limodromus assimilis (Paykull 1790). PLoS ONE 2017, 12, e0180492. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Giglio, A.; Lupetti, P. Sperm ultrastructure in several species of Carabidae beetles (Insecta, Adephaga) and their organization in spermatozeugmata. Arthropod Struct. Dev. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Fanciulli, P.P.; Petrioli, A.; Lupetti, P. New findings on the sperm ultrastructure of Carabidae (Insecta, Coleoptera). Arthropod Struct. Dev. 2020, 54, 100912. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.A.; Maddison, D.R. Novelty and emergent patterns in sperm: Morphological diversity and evolution of spermatozoa and sperm conjugation in ground beetles (Coleoptera: Carabidae). J. Morphol. 2020, 8, 862–892. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.; Mercati, D.; Lupetti, P.; Brandmayr, P.; Dallai, R. The sperm structure of Clinidium canaliculatum (Costa): A contribution to the systematic position of Rhysodidae (Coleoptera: Carabidae). Arthropod Struct. Dev. 2024, 78, 101330. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.A.; Dallai, R.; Sims-West, D.J.; Mercati, D.; Sinka, R.; Ahmed-Braimah, Y.; Pitnick, S.; Dorus, S. Proteomic diversification of spermatostyles among six species of whirligig beetles. Mol. Reprod. Dev. 2024, 91, e23745. [Google Scholar] [CrossRef] [PubMed]
- Dallai, R.; Afzelius, B.A. Membrane specializations in the paired spermatozoa of dytiscid water beetle. Tissue Cell 1985, 17, 561–572. [Google Scholar] [CrossRef]
- Afzelius, B.A.; Dallai, R. Conjugated spermatozoa. In New Horizons in Sperm Cell Research; Mohri, H., Ed.; Japan Science Society Press and Gordon & Breach Scientific Publ.: Tokyo, Japan, 1987; pp. 349–355. [Google Scholar]
- Mackie, J.B.; Walker, M.H. A study of the conjugate sperm of the dytiscid water beetles Dytiscus marginalis and Colymbetes fuscus. Cell Tissue Res. 1974, 148, 505–519. [Google Scholar] [CrossRef]
- Mercati, D.; Fanciulli, P.P.; Lupetti, P.; Dallai, R. The sperm structure of the diving beetles Stictonectes optatus (Seidlitz, 1887) and Scarodytes halensis (Fabricius, 1787) (Dytiscidae, Hydroporinae) with evidence of a spermatostyle in the sperm conjugation. Micron 2023, 166, 103412. [Google Scholar] [CrossRef]
- Gomez, R.A.; Mercati, D.; Lupetti, P.; Fanciulli, P.P.; Dallai, R. Morphology of male and female reproductive systems in the ground beetle Apotomus and the peculiar sperm ultrastructure of A. rufus (P. Rossi, 1790) (Coleoptera, Carabidae). Arthropod Struct Dev. 2023, 72, 101217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupetti, P.; Mercati, D.; Giglio, A.; Brandmayr, P.; Dallai, R. The Cap and the Spermatostyle Protecting the Sperm Bundle Have a Similar Origin—Ultrastructural Study of the Spermatogenesis from the Ground Beetle Carabus (Chaetocarabus) lefebvrei Dejean, 1826 (Adephaga Carabidae). Insects 2024, 15, 864. https://doi.org/10.3390/insects15110864
Lupetti P, Mercati D, Giglio A, Brandmayr P, Dallai R. The Cap and the Spermatostyle Protecting the Sperm Bundle Have a Similar Origin—Ultrastructural Study of the Spermatogenesis from the Ground Beetle Carabus (Chaetocarabus) lefebvrei Dejean, 1826 (Adephaga Carabidae). Insects. 2024; 15(11):864. https://doi.org/10.3390/insects15110864
Chicago/Turabian StyleLupetti, Pietro, David Mercati, Anita Giglio, Pietro Brandmayr, and Romano Dallai. 2024. "The Cap and the Spermatostyle Protecting the Sperm Bundle Have a Similar Origin—Ultrastructural Study of the Spermatogenesis from the Ground Beetle Carabus (Chaetocarabus) lefebvrei Dejean, 1826 (Adephaga Carabidae)" Insects 15, no. 11: 864. https://doi.org/10.3390/insects15110864
APA StyleLupetti, P., Mercati, D., Giglio, A., Brandmayr, P., & Dallai, R. (2024). The Cap and the Spermatostyle Protecting the Sperm Bundle Have a Similar Origin—Ultrastructural Study of the Spermatogenesis from the Ground Beetle Carabus (Chaetocarabus) lefebvrei Dejean, 1826 (Adephaga Carabidae). Insects, 15(11), 864. https://doi.org/10.3390/insects15110864





