The Ontogeny and Dietary Differences in Queen and Worker Castes of Honey Bee (Apis cerana cerana)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bees
2.2. Developmental Stage of Workers
2.3. Developmental Stage of Queens
2.4. Measurement Methods
2.5. Statistical Analysis
3. Results
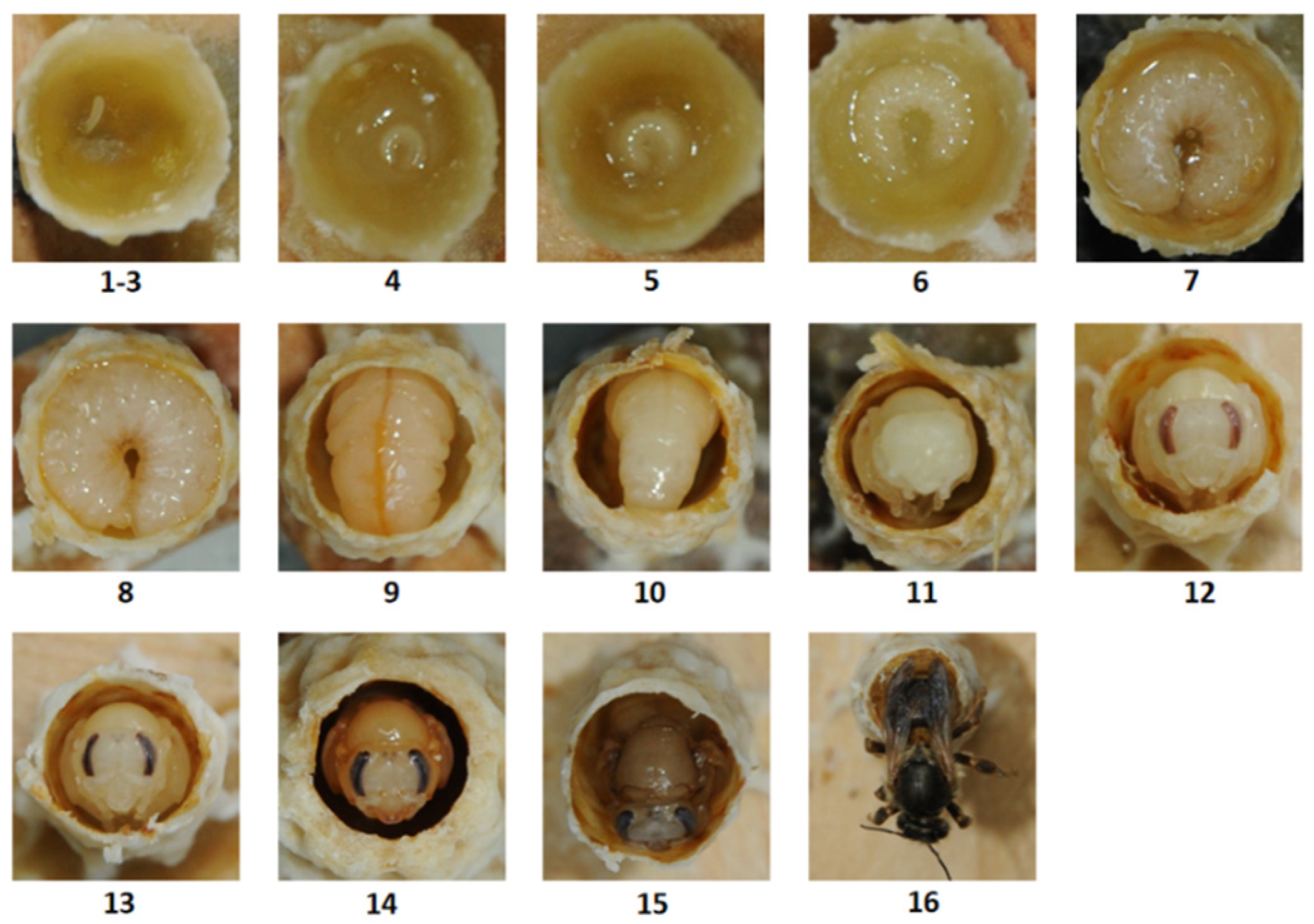
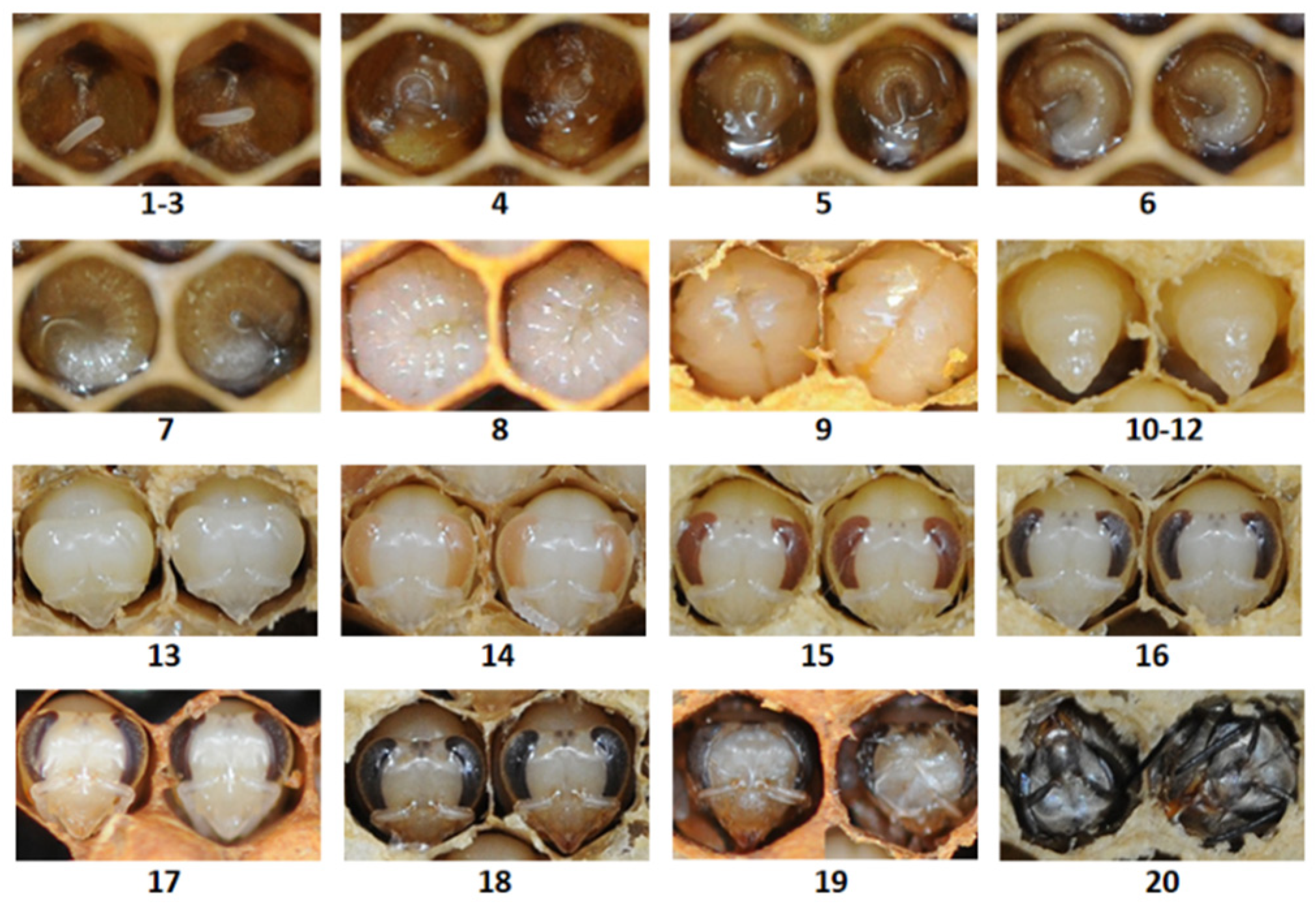
3.1. The Larval and Pupal Morphological Characteristics of A. c. cerana Queens and Workers
3.2. The Average Body Weight of the Queen and Workers During Ontogeny
3.3. Changes in Moisture and Crude Protein Contents in RJ and WJ at Different Ages
3.4. Changes in Amino Acid Content in RJ and WJ at Different Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, F.; Chen, X.; Lv, J.; Shi, X.; Feng, X.; Wang, Z.; Li, X. Population Structure and Genetic Diversity of Chinese Honeybee (Apis cerana cerana) in Central China. Genes 2022, 13, 1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.J.; Zhang, W.Q. Han RC. Differential gene expression of the honey bees Apis mellifera and A. cerana induced by Varroa destructor infection. J. Insect Physiol. 2010, 56, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Yang, S.; Wang, Z.W.; Radloff, S.E.; Oldroyd, B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie 2012, 43, 618–623. [Google Scholar] [CrossRef]
- Hou, C.; Li, B.; Luo, Y.; Deng, S.; Diao, Q. First detection of Apis mellifera filamentous virus in Apis cerana cerana in China. J Invertebr. Pathol. 2016, 138, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.R.; Kuttalam, S.; Ramaraju, K. Thai sacbrood virus (TSBV)—A potential threat to Indian honey bee. In Proceedings of the XXIII National Conference on “Recent Trends in Virology Research in the Omics Era”, Coimbatore, Tamil Nadu, 18–20 December 2014. [Google Scholar]
- Kellogg, C.R. Some Characteristics of the Oriental Honeybee, Apis indica F., in China. J. Econ. Entomol. 1941, 34, 717–719. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Zheng, H.; Cao, L.; Niu, D.; Yu, D.; Sun, Y.; Hu, S.; Hu, F. Transcriptome comparison between honey bee queen- and worker-destined larvae. Insect Biochem. Mol. Biol. 2012, 42, 665–673. [Google Scholar] [CrossRef]
- He, X.J.; Barron, A.B.; Yang, L.; Chen, H.; He, Y.Z.; Zhang, L.Z.; Huang, Q.; Wang, Z.L.; Wu, X.B.; Yan, W.Y.; et al. Extent and complexity of RNA processing in honey bee queen and worker caste development. iScience 2022, 25, 104301. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.-T.; Xu, B.-H. Diversity in life history of queen and worker honey bees, Apis mellifera L. J. Asia Pac. Entomol. 2015, 18, 145–149. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Patel, N.G.; Haydak, M.H.; Gochnauer, T.A. Electrophoretic Components of the Proteins in Honeybee Larval Food. Nature 1960, 186, 633–634. [Google Scholar] [CrossRef]
- Kevill, J.L.; Lee, K.; Goblirsch, M.; Mcdermott, E.; Tarpy, D.R.; Spivak, M.; Schroeder, D.C. The Pathogen Profile of a Honey Bee Queen Does Not Reflect That of Her Workers. Insects 2020, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Asencot, M.; Lensky, Y. The effect of soluble sugars in stored royal jelly on the differentiation of female honeybee (Apis mellifera L.) larvae to queens. Insect Biochem. 1988, 18, 127–133. [Google Scholar] [CrossRef]
- Wei, W.T.; Hu, Y.Q.; Zheng, H.Q.; Cao, L.F.; Hu, F.L.; Hepburn, H.R. Geographical influences on content of 10-hydroxy-trans-2-decenoic acid in royal jelly in China. J. Econ. Entomol. 2013, 106, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Hu, F.L.; Dietemann, V. Changes in composition of royal jelly harvested at different times: Consequences for quality standards. Apidologie 2011, 42, 39–47. [Google Scholar] [CrossRef]
- Li, S.; Tao, L.; Yu, X.; Zheng, H.; Wu, J.; Hu, F. Royal Jelly Proteins and Their Derived Peptides: Preparation, Properties, and Biological Activities. J. Agric. Food Chem. 2021, 69, 14415–14427. [Google Scholar] [CrossRef]
- Shan, L.; Liuhao, W.; Jun, G.; Yujie, T.; Yanping, C.; Jie, W.; Jilian, L. Chinese Sacbrood virus infection in Asian honey bees (Apis cerana cerana) and host immune responses to the virus infection. J. Invertebr. Pathol. 2017, 150, 63–69. [Google Scholar] [CrossRef]
- Hu, F.L.; Bíliková, K.; Casabianca, H.; Daniele, G.L.; Salmen Espindola, F.; Feng, M.; Guan, C.; Han, B.; Krištof Kraková, T.; Li, J.K. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2017, 58, 1–68. [Google Scholar] [CrossRef]
- Messia, M.C.; Caboni, M.F.; Marconi, E. Storage Stability Assessment of Freeze-Dried Royal Jelly by Furosine Determination. J. Agric. Food Chem. 2005, 53, 4440–4443. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Wang, L.; Zhang, L.; Wang, T.; Zhao, Y.; Xiao, S.; Qiao, N.; Xu, C.; Ding, L.; et al. Potential predictive value of serum targeted metabolites and concurrently mutated genes for EGFR-TKI therapeutic efficacy in lung adenocarcinoma patients with EGFR sensitizing mutations. Am. J. Cancer Res. 2020, 10, 4266–4286. [Google Scholar]
- Nunes-Silva, P.; Gonalves, L.S.; Francoy, T.M.; Jong, D.D. Rate of growth and development time of africanized honey bee (Apis mellifera) queens and workers during ontogenetic development. Silva 2006, 23, 325–332. [Google Scholar]
- Free, J.B. A social insect: The biology of the honey bee. Science 1987, 238, 1591–1592. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, H.R.; Radloff, S.E. Honeybees of Africa; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Zhao, H.; Li, G.; Guo, D.; Wang, Y.; Liu, Q.; Gao, Z.; Wang, H.; Liu, Z.; Guo, X.; Xu, B. Transcriptomic and metabolomic landscape of the molecular effects of glyphosate commercial formulation on Apis mellifera ligustica and Apis cerana cerana. Sci. Total Environ. 2020, 744, 140819. [Google Scholar] [CrossRef]
- Wang, D.I. Growth Rates of Young Queen and Worker Honeybee Larvae. J. Apic. Res. 1965, 4, 3–5. [Google Scholar] [CrossRef]
- Li, H.; Deng, S.; Meng, Q.; Yang, S.; Jiang, W.; Zhao, W.; Tian, Y.; Dong, K.; Gong, X. Impact of Apis cerana cerana (Hymenoptera: Apidae) body size on newly built cell size. J. Asia-Pac. Entomol. 2024, 27, 102277. [Google Scholar] [CrossRef]
- Jie, H.; Li, P.M.; Zhao, G.J.; Feng, X.L.; Chen, Q. Amino acid composition of royal jelly harvested at different times after larval transfer. Genet. Mol. Res. 2016, 15, gmr.5038306. [Google Scholar] [CrossRef]
- Li, Z. Amino Acids Composition and Nutritional Functions Analysis of Royal Jelly. Food Res. Dev. 2014, 35, 94–96. [Google Scholar]
- Martin, N.; Hulbert, A.J.; Brenner, G.C.; Brown, S.H.J.; Mitchell, T.W. Honey bee caste lipidomics in relation to life-history stage and the long life of the queen. J. Exp. Biol. 2019, 222, jeb.207043. [Google Scholar]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef]
- Miller, R.A.; Buehner, G.; Chang, Y.; Harper, J.M.; Sigler, R.; Smith-Wheelock, M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005, 4, 119–125. [Google Scholar] [CrossRef]
- Lee, B.C.; Kaya, A.; Ma, S.; Kim, G.; Gerashchenko, M.V.; Yim, S.H.; Hu, Z.; Harshman, L.G.; Gladyshev, V.N. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014, 5, 3592. [Google Scholar] [CrossRef] [PubMed]
- Obata, F.; Tsuda-Sakurai, K.; Yamazaki, T.; Nishio, R.; Nishimura, K.; Kimura, M.; Funakoshi, M.; Miura, M. Nutritional Control of Stem Cell Division through S-Adenosylmethionine in Drosophila Intestine. Dev. Cell 2018, 44, 741–751.e3. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.D.; Haydak, M.H. Seasonal Variation in Weight and Ovarian Development in the Worker Honeybee. J. Econ. Entomol. 1951, 44, 54–57. [Google Scholar] [CrossRef]
- Harbo, J.R.; Bolten, A.B.; Rinderer, T.E.; Collins, A. Development Periods for Eggs of Africanized and European Honeybees. J. Apic. Res. 2015, 20, 156–159. [Google Scholar] [CrossRef]


| Time (min) | Mobile Phase | |
|---|---|---|
| A (v%) | B (v%) | |
| 0 | 96 | 4 |
| 0.5 | 96 | 4 |
| 2.5 | 90 | 10 |
| 5 | 72 | 28 |
| 6 | 5 | 95 |
| 7 | 5 | 95 |
| 7.1 | 96 | 4 |
| 9 | 96 | 4 |
| Day of Development | Body Weight (mg) | |
|---|---|---|
| Queen | Worker | |
| 1–3 | 0.18 ± 0.01 | 0.18 ± 0.01 |
| 4 | 0.54 ± 0.02 | 0.54 ± 0.02 |
| 5 | 2.28 ± 0.06 | 2.15 ± 0.05 |
| 6 | 15.64 ± 0.36 | 6.54 ± 0.22 |
| 7 | 69.58 ± 0.64 | 35.12 ± 0.71 |
| 8 | 180.66 ± 3.33 | 85.67 ± 1.18 |
| 9 | 230.69 ± 1.95 | 126.80 ± 0.70 |
| 10 | 216.03 ± 2.24 | 112.70 ± 0.73 |
| 11 | 208.30 ± 1.87 | 109.39 ± 0.82 |
| 12 | 202.50 ± 1.97 | 105.85 ± 1.09 |
| 13 | 195.26 ± 2.07 | 100.77 ± 0.69 |
| 14 | 181.69 ± 1.65 | 99.92 ± 0.67 |
| 15 | 172.03 ± 1.86 | 97.28 ± 0.78 |
| 16 | 154.75 ± 2.65 | 96.10 ± 0.83 |
| 17 | — | 95.85 ± 0.73 |
| 18 | — | 92.28 ± 0.42 |
| 19 | — | 89.98 ± 0.52 |
| 20 | — | 84.50 ± 0.63 |
| Amino Acid Content % | 1 d | 2 d | 3 d | 4 d | 5 d | |
|---|---|---|---|---|---|---|
| Histidine (His) | WJ | 0.35 ± 0.04 a | 0.34 ± 0.03 a | 0.35 ± 0.01 a | 0.31 ± 0.01 ab | 0.24 ± 0.01 b |
| RJ | 0.35 ± 0.04 | 0.42 ± 0.04 | 0.49 ± 0.05 | 0.46 ± 0.03 * | 0.36 ± 0.04 | |
| Arginine (Arg) | WJ | 0.77 ± 0.08 a | 0.63 ± 0.02 ab | 0.65 ± 0.03 ab | 0.49 ± 0.02 c | 0.55 ± 0.01 bc |
| RJ | 0.77 ± 0.08 bc | 0.94 ± 0.07 *ab | 0.97 ± 0.03 *a | 0.78 ± 0.04 *bc | 0.76 ± 0.02 *c | |
| Serine (Ser) | WJ | 0.45 ± 0.04 a | 0.39 ± 0.01 ab | 0.43 ± 0.02 a | 0.38 ± 0.02 ab | 0.35 ± 0.01 b |
| RJ | 0.45 ± 0.04 b | 0.57 ± 0.03 *a | 0.59 ± 0.01 *a | 0.56 ± 0.01 *a | 0.53 ± 0.02 *ab | |
| Glycine (Gly) | WJ | 0.49 ± 0.05 a | 0.42 ± 0.01 ab | 0.46 ± 0.02 a | 0.41 ± 0.03 ab | 0.37 ± 0.01 b |
| RJ | 0.49 ± 0.05 b | 0.59 ± 0.03 *ab | 0.62 ± 0.01 *a | 0.57 ± 0.02 *ab | 0.54 ± 0.03 *ab | |
| Aspartic acid (Asp) | WJ | 1.72 ± 0.18 a | 1.55 ± 0.01 ab | 1.67 ± 0.02 ab | 1.37 ± 0.08 bc | 1.24 ± 0.04 c |
| RJ | 1.72 ± 0.18 b | 2.32 ± 0.16 *a | 2.42 ± 0.04 *a | 2.21 ± 0.11 *a | 2.08 ± 0.13 *ab | |
| Glutamic acid (Glu) | WJ | 1.29 ± 0.14 a | 1.15 ± 0.01 ab | 1.27 ± 0.01 a | 1.07 ± 0.05 ab | 0.98 ± 0.02 b |
| RJ | 1.29 ± 0.14 b | 1.69 ± 0.11 *a | 1.79 ± 0.05 *a | 1.60 ± 0.04 *ab | 1.49 ± 0.10 *ab | |
| Threonine (Thr) | WJ | 0.61 ± 0.06 a | 0.52 ± 0.01 ab | 0.58 ± 0.02 a | 0.51 ± 0.03 ab | 0.46 ± 0.01 b |
| RJ | 0.61 ± 0.06 b | 0.76 ± 0.04 *a | 0.80 ± 0.02 *a | 0.74 ± 0.01 *a | 0.70 ± 0.03 *ab | |
| Alanine (Ala) | WJ | 0.52 ± 0.05 a | 0.45 ± 0.01 abc | 0.48 ± 0.02 ab | 0.42 ± 0.02 bc | 0.38 ± 0.01 c |
| RJ | 0.52 ± 0.05 b | 0.63 ± 0.04 *ab | 0.67 ± 0.02 *a | 0.62 ± 0.01 *ab | 0.59 ± 0.03 *ab | |
| Proline (Pro) | WJ | 0.46 ± 0.04 a | 0.39 ± 0.01 ab | 0.44 ± 0.02 ab | 0.39 ± 0.02 ab | 0.38 ± 0.01 b |
| RJ | 0.46 ± 0.04 b | 0.56 ± 0.03 *a | 0.59 ± 0.02 *a | 0.54 ± 0.01 *ab | 0.52 ± 0.03 *ab | |
| Lysine (Lys) | WJ | 0.99 ± 0.09 a | 0.85 ± 0.01 ab | 0.93 ± 0.04 ab | 0.81 ± 0.04 bc | 0.68 ± 0.01 c |
| RJ | 0.99 ± 0.09 c | 1.31 ± 0.07 *ab | 1.37 ± 0.03 *a | 1.25 ± 0.05 *ab | 1.12 ± 0.05 *bc | |
| Cysteine (Cys) | WJ | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 |
| RJ | 0.07 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.08 ± 0.01 | |
| Tyrosine (Tyr) | WJ | 0.55 ± 0.07 a | 0.46 ± 0.01 ab | 0.47 ± 0.02 a | 0.36 ± 0.01 bc | 0.34 ± 0.01 c |
| RJ | 0.55 ± 0.07 b | 0.65 ± 0.04 *ab | 0.70 ± 0.01 *a | 0.59 ± 0.03 *ab | 0.54 ± 0.02 *b | |
| Methionine (Met) | WJ | 0.29 ± 0.04 a | 0.21 ± 0.02 ab | 0.15 ± 0.04 b | 0.11 ± 0.03 b | 0.10 ± 0.03 b |
| RJ | 0.29 ± 0.04 a | 0.12 ± 0.04 b | 0.16 ± 0.03 ab | 0.11 ± 0.03 b | 0.15 ± 0.04 b | |
| Valine (Val) | WJ | 0.87 ± 0.07 a | 0.73 ± 0.01 b | 0.77 ± 0.02 ab | 0.64 ± 0.04 bc | 0.58 ± 0.01 c |
| RJ | 0.87 ± 0.07 b | 1.02 ± 0.06 *ab | 1.10 ± 0.03 *a | 1.00 ± 0.03 *ab | 0.93 ± 0.05 *ab | |
| Isoleucine (Ile) | WJ | 0.75 ± 0.07 a | 0.64 ± 0.01 ab | 0.67 ± 0.02 ab | 0.57 ± 0.03 bc | 0.50 ± 0.01 c |
| RJ | 0.75 ± 0.07 b | 0.89 ± 0.06 *ab | 0.95 ± 0.03 *a | 0.87 ± 0.02 *ab | 0.81 ± 0.04 *ab | |
| Leucine (Leu) | WJ | 1.14 ± 0.11 a | 0.98 ± 0.01 ab | 1.05 ± 0.04 ab | 0.87 ± 0.05 bc | 0.77 ± 0.01 c |
| RJ | 1.14 ± 0.11 b | 1.40 ± 0.08 *a | 1.52 ± 0.04 *a | 1.38 ± 0.04 *a | 1.27 ± 0.07 *ab | |
| Phenylalanine (Phe) | WJ | 0.64 ± 0.06 a | 0.53 ± 0.01 bc | 0.57 ± 0.02 ab | 0.48 ± 0.02 bc | 0.43 ± 0.01 c |
| RJ | 0.64 ± 0.06 c | 0.78 ± 0.05 *ab | 0.84 ± 0.02 *a | 0.76 ± 0.02 *abc | 0.70 ± 0.03 *bc | |
| Total Amino Acid (TAA) | WJ | 11.95 ± 1.19 a | 10.30 ± 0.12 ab | 11.03 ± 0.34 ab | 9.26 ± 0.47 ab | 8.83 ± 0.14 b |
| RJ | 11.95 ± 1.19 b | 14.76 ± 0.94 *ab | 15.70 ± 0.32 *a | 14.19 ± 0.51 *ab | 13.17 ± 0.66 *ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Lei, L.; Wang, Y.; Xu, B.; Liu, Z. The Ontogeny and Dietary Differences in Queen and Worker Castes of Honey Bee (Apis cerana cerana). Insects 2024, 15, 855. https://doi.org/10.3390/insects15110855
Yang C, Lei L, Wang Y, Xu B, Liu Z. The Ontogeny and Dietary Differences in Queen and Worker Castes of Honey Bee (Apis cerana cerana). Insects. 2024; 15(11):855. https://doi.org/10.3390/insects15110855
Chicago/Turabian StyleYang, Chunyu, Li Lei, Ying Wang, Baohua Xu, and Zhenguo Liu. 2024. "The Ontogeny and Dietary Differences in Queen and Worker Castes of Honey Bee (Apis cerana cerana)" Insects 15, no. 11: 855. https://doi.org/10.3390/insects15110855
APA StyleYang, C., Lei, L., Wang, Y., Xu, B., & Liu, Z. (2024). The Ontogeny and Dietary Differences in Queen and Worker Castes of Honey Bee (Apis cerana cerana). Insects, 15(11), 855. https://doi.org/10.3390/insects15110855






