A New SDM-Based Approach for Assessing Climate Change Effects on Plant–Pollinator Networks
Simple Summary
Abstract
1. Introduction
2. Method Description
2.1. SDMs
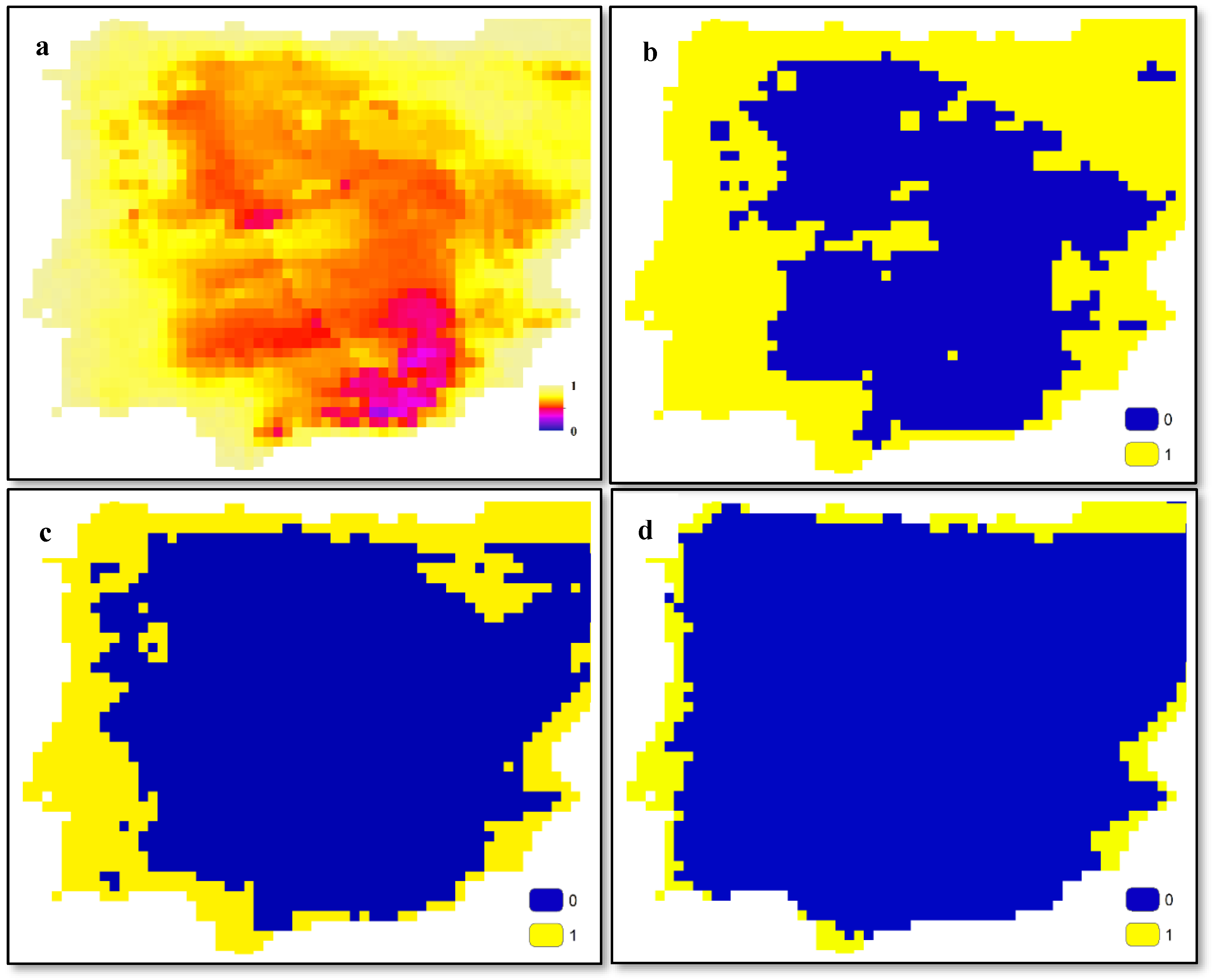
2.2. Outputs of SDMs
2.3. Overlapping of Outputs of SDMs
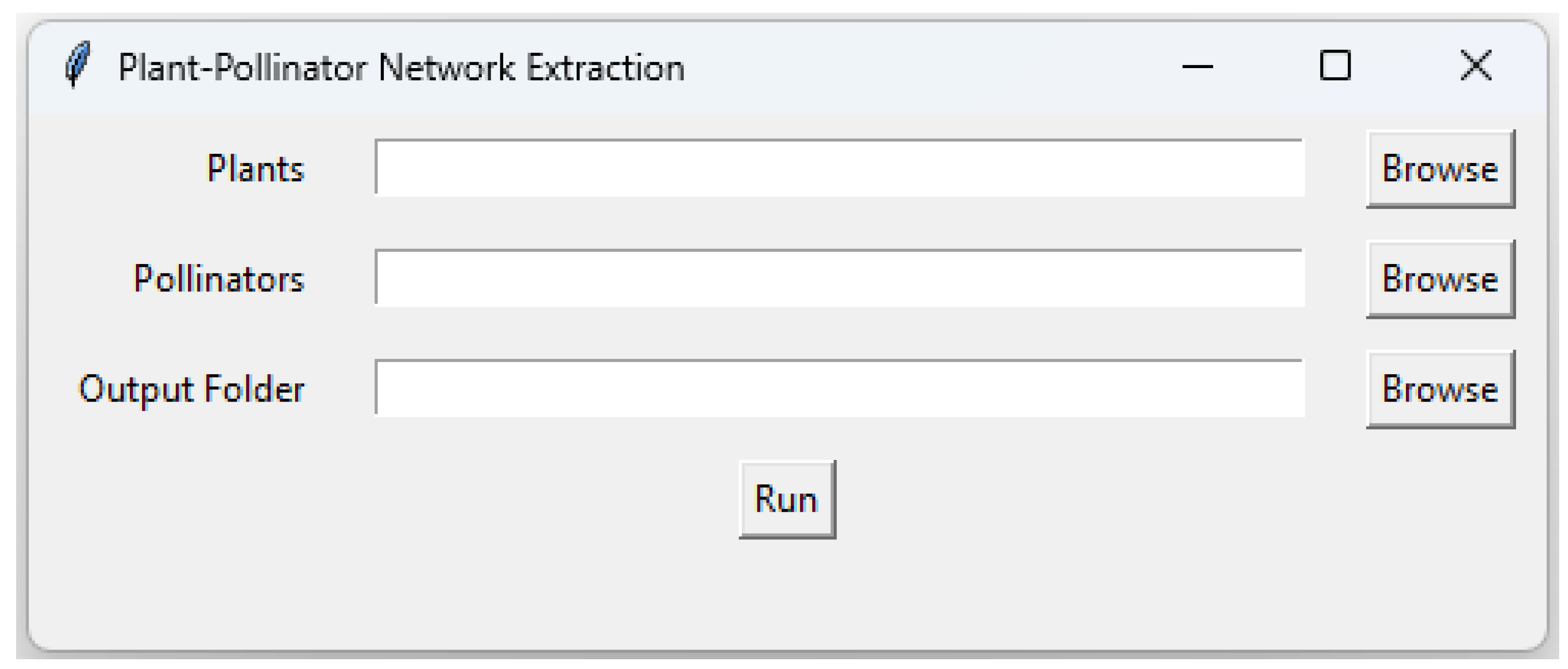
2.4. Interaction Network Extraction from Binary Maps
2.5. Estimating Climate Change Effects on Plant–Pollinator Interactions
2.6. Application to a Case Study
2.6.1. Plant–Pollinator Catalog for Chile
2.6.2. Occurrence Data
2.6.3. Environmental Variables
2.6.4. Model Fitting
2.6.5. Model Assessment Results
2.6.6. Bipartite Metrics Results
3. Results
3.1. Model Assessment
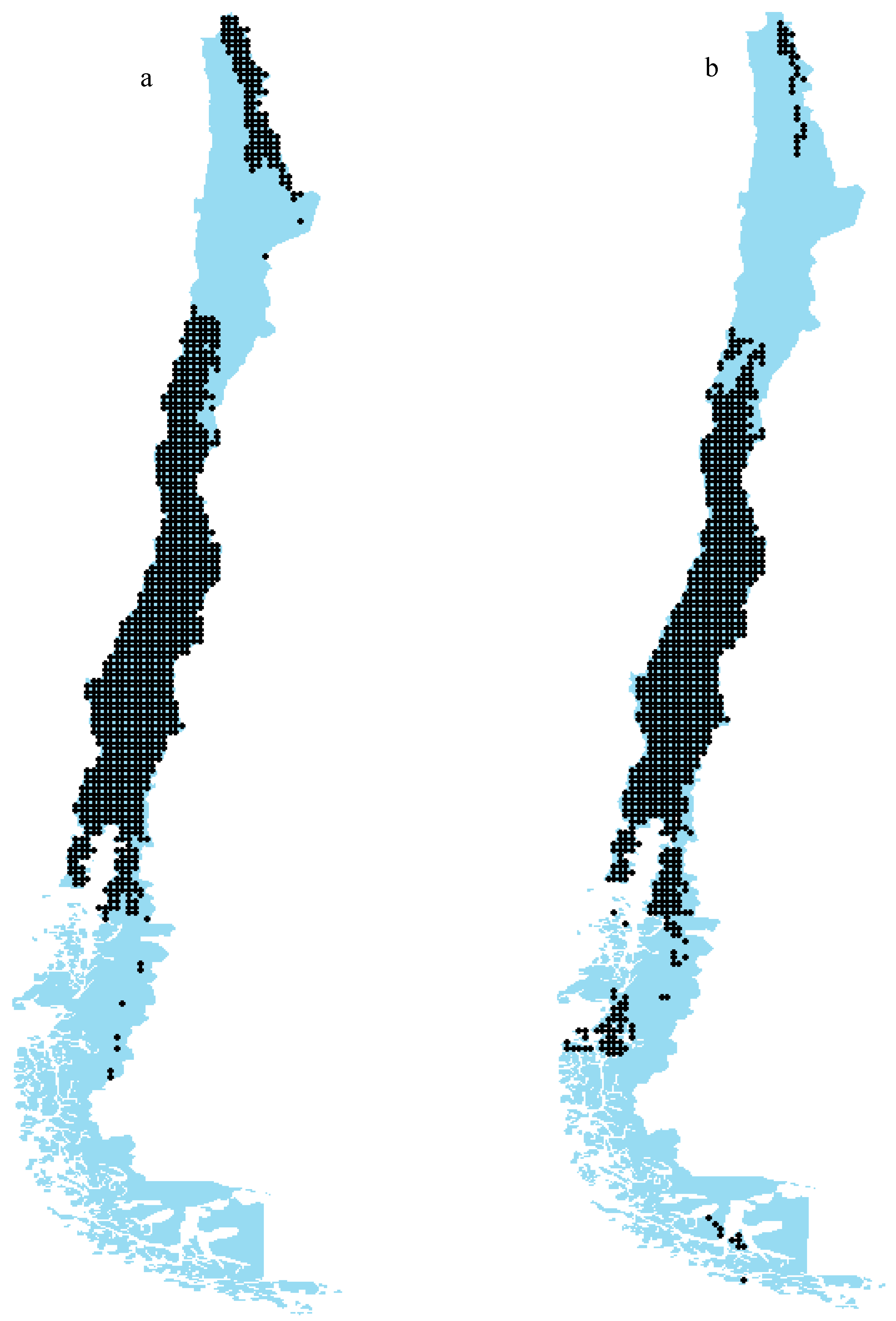
3.2. Potential Effects of Climate Change at the Individual Level
3.3. Potential Effects of Climate Change at the Network Level
3.4. Bipartite Metrics
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.; CaraDonna, P.J.; Rakosy, D.; Fründ, J.; Tudanca, M.P.P.; Dormann, C.F.; Burkle, L.A.; Kaiser-Bunbury, C.N.; Knight, T.M.; Resasco, J. Predicting plant–pollinator interactions: Concepts, methods, and challenges. Trends Ecol. Evol. 2024, 39, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.-Y.; Wu, L.-Y.; Feng, H.-H.; Zhang, M.; Armbruster, W.S.; Renner, S.S.; Huang, S.-Q. New calculations indicate that 90% of flowering plant species are animal-pollinated. Natl. Sci. Rev. 2023, 10, nwad219. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M. Seeds: The Ecology of Regeneration in Plant Communities; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Siopa, C.; Carvalheiro, L.G.; Castro, H.; Loureiro, J.; Castro, S. Animal-pollinated crops and cultivars—A quantitative assessment of pollinator dependence values and evaluation of methodological approaches. J. Appl. Ecol. 2024, 61, 1279–1288. [Google Scholar] [CrossRef]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef]
- Rahimi, E.; Jung, C. Plant–pollinator metanetworks in fragmented landscapes: A simulation study. Ecol. Process. 2023, 12, 29. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Biella, P.; Ollerton, J.; Barcella, M.; Assini, S. Network analysis of phenological units to detect important species in plant-pollinator assemblages: Can it inform conservation strategies? Community Ecol. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Bascompte, J.; Scheffer, M. The resilience of plant–pollinator networks. Annu. Rev. Entomol. 2023, 68, 363–380. [Google Scholar] [CrossRef]
- Vizentin-Bugoni, J.; Debastiani, V.J.; Bastazini, V.A.; Maruyama, P.K.; Sperry, J.H. Including rewiring in the estimation of the robustness of mutualistic networks. Methods Ecol. Evol. 2020, 11, 106–116. [Google Scholar] [CrossRef]
- Yurk, B.P.; Powell, J.A. Modeling the evolution of insect phenology. Bull. Math. Biol. 2009, 71, 952–979. [Google Scholar] [CrossRef] [PubMed]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant–pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar]
- Haq, I.U.; Ali, S.; Ali, A.; Ali, H. Effect of Climate Change on Insect Pollinator. In Climate Change and Insect Biodiversity; CRC Press: Boca Raton, FL, USA, 2024; pp. 179–195. [Google Scholar]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Jaworski, T.; Hilszczański, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change. Lesn. Pr. Badaw. 2013, 74, 345. [Google Scholar]
- Rafferty, N.E. Effects of global change on insect pollinators: Multiple drivers lead to novel communities. Curr. Opin. Insect Sci. 2017, 23, 22–27. [Google Scholar] [CrossRef]
- Schweiger, O.; Biesmeijer, J.C.; Bommarco, R.; Hickler, T.; Hulme, P.E.; Klotz, S.; Kühn, I.; Moora, M.; Nielsen, A.; Ohlemüller, R. Multiple stressors on biotic interactions: How climate change and alien species interact to affect pollination. Biol. Rev. 2010, 85, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Jha, S.; Burkle, L.; Kremen, C. Vulnerability of Pollination Ecosystem Services; UC Berkeley: Berkeley, CA, USA, 2013. [Google Scholar]
- Rahimi, E.; Jung, C. Global Trends in Climate Suitability of Bees: Ups and Downs in a Warming World. Insects 2024, 15, 127. [Google Scholar] [CrossRef]
- Midgley, G.F.; Thuiller, W.; Higgins, S.I. Plant species migration as a key uncertainty in predicting future impacts of climate change on ecosystems: Progress and challenges. In Terrestrial Ecosystems in a Changing World; Springer: Berlin/Heidelberg, Switzerland, 2007; pp. 129–137. [Google Scholar] [CrossRef]
- Imbach, P.; Fung, E.; Hannah, L.; Navarro-Racines, C.E.; Roubik, D.W.; Ricketts, T.H.; Harvey, C.A.; Donatti, C.I.; Läderach, P.; Locatelli, B. Coupling of pollination services and coffee suitability under climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 10438–10442. [Google Scholar] [CrossRef]
- Morton, E.M.; Rafferty, N.E. Plant–pollinator interactions under climate change: The use of spatial and temporal transplants. Appl. Plant Sci. 2017, 5, 1600133. [Google Scholar] [CrossRef]
- Schleuning, M.; Fründ, J.; Schweiger, O.; Welk, E.; Albrecht, J.; Albrecht, M.; Beil, M.; Benadi, G.; Blüthgen, N.; Bruelheide, H. Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Commun. 2016, 7, 13965. [Google Scholar] [CrossRef]
- Encinas-Viso, F.; Goodwin, E.; Saunders, M.E.; Florez, J.; Lumbers, J.; Rader, R. The missing links: Bee and non-bee alpine visitor observation networks differ to pollen transport networks. Ecol. Entomol. 2024, 49, 377–385. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Elberling, H.; Rasmussen, C.; Jordano, P. Missing and forbidden links in mutualistic networks. Proc. R. Soc. B Biol. Sci. 2011, 278, 725–732. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chatterjee, S.; Smith, B.M.; Basu, P. Seasonal dynamics of plant pollinator networks in agricultural landscapes: How important is connector species identity in the network? Oecologia 2021, 196, 825–837. [Google Scholar] [CrossRef]
- Dalsgaard, B. Land-use and climate impacts on plant–pollinator interactions and pollination services. Diversity 2020, 12, 168. [Google Scholar] [CrossRef]
- Freimuth, J.; Bossdorf, O.; Scheepens, J.; Willems, F.M. Climate warming changes synchrony of plants and pollinators. Proc. R. Soc. B 2022, 289, 20212142. [Google Scholar] [CrossRef]
- Vidal, M.C.; Anneberg, T.J.; Curé, A.E.; Althoff, D.M.; Segraves, K.A. The variable effects of global change on insect mutualisms. Curr. Opin. Insect Sci. 2021, 47, 46–52. [Google Scholar] [CrossRef]
- Ávila-Thieme, M.I.; Kusch, E.; Corcoran, D.; Castillo, S.P.; Valdovinos, F.S.; Navarrete, S.A.; Marquet, P.A. NetworkExtinction: An R package to simulate extinction propagation and rewiring potential in ecological networks. Methods Ecol. Evol. 2023, 14, 1952–1966. [Google Scholar] [CrossRef]
- Devoto, M.; Zimmermann, M.; Medan, D. Robustness of plant-flower visitor webs to simulated climate change. Ecol. Austral 2007, 17, 37–50. [Google Scholar]
- Lázaro, A.; Gómez-Martínez, C. Habitat loss increases seasonal interaction rewiring in plant–pollinator networks. Funct. Ecol. 2022, 36, 2673–2684. [Google Scholar] [CrossRef]
- Magrach, A.; Artamendi, M.; Lapido, P.D.; Parejo, C.; Rubio, E. Indirect interactions between pollinators drive interaction rewiring through space. Ecosphere 2023, 14, e4521. [Google Scholar] [CrossRef]
- Vizentin-Bugoni, J.; Maruyama, P.K. To rewire or not to rewire: To what extent rewiring to surviving partners can avoid extinction? J. Anim. Ecol. 2023, 92, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Filazzola, A.; Matter, S.F.; MacIvor, J.S. The direct and indirect effects of extreme climate events on insects. Sci. Total Environ. 2021, 769, 145161. [Google Scholar] [CrossRef] [PubMed]
- Pollock, L.J.; Tingley, R.; Morris, W.K.; Golding, N.; O’Hara, R.B.; Parris, K.M.; Vesk, P.A.; McCarthy, M.A. Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM). Methods Ecol. Evol. 2014, 5, 397–406. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Edwards, T.C., Jr.; Guisan, A.; McMahon, S.M.; Normand, S.; Thuiller, W.; Wüest, R.O.; Zimmermann, N.E.; Elith, J. What do we gain from simplicity versus complexity in species distribution models? Ecography 2014, 37, 1267–1281. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Colwell, R.K.; Rangel, T.F. Hutchinson’s duality: The once and future niche. Proc. Natl. Acad. Sci. USA 2009, 106, 19651–19658. [Google Scholar] [CrossRef] [PubMed]
- Filazzola, A.; Matter, S.F.; Roland, J. Inclusion of trophic interactions increases the vulnerability of an alpine butterfly species to climate change. Glob. Chang. Biol. 2020, 26, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Miličić, M.S.; Janković, M.A.; Milić, D.M.; Radenković, S.R.; Vujić, A.A. Strictly protected species of hoverflies (Diptera: Syrphidae) in Serbia in the face of climate change. Zb. Matice Srp. Za Prir. Nauk. 2018, 135, 53–62. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performance depends on evaluation criteria–Dutch hoverflies as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef]
- Sbaraglia, C. Climate Change Effects on Habitat Suitability of a Butterfly in the Past, Present, and Future: Biotic Interaction Between Parnassius Apollo and Its Host Plants. Master’s Thesis, University of Pisa, Pisa, Italy, 2022. [Google Scholar]
- Tabor, J.A.; Koch, J.B. Ensemble models predict invasive bee habitat suitability will expand under future climate scenarios in Hawai’i. Insects 2021, 12, 443. [Google Scholar] [CrossRef]
- Martínez-López, O.; Koch, J.B.; Martínez-Morales, M.A.; Navarrete-Gutiérrez, D.; Enríquez, E.; Vandame, R. Reduction in the potential distribution of bumble bees (Apidae: Bombus) in Mesoamerica under different climate change scenarios: Conservation implications. Glob. Chang. Biol. 2021, 27, 1772–1787. [Google Scholar] [CrossRef]
- Abrol, D.P. Defensive behaviour of Apis cerana F. against predatory wasps. J. Apic Sci. 2006, 50, 39. [Google Scholar]
- Zhang, L.; Huettmann, F.; Liu, S.; Sun, P.; Yu, Z.; Zhang, X.; Mi, C. Classification and regression with random forests as a standard method for presence-only data SDMs: A future conservation example using China tree species. Ecol. Inform. 2019, 52, 46–56. [Google Scholar] [CrossRef]
- Scherrer, D.; D’Amen, M.; Fernandes, R.F.; Mateo, R.G.; Guisan, A. How to best threshold and validate stacked species assemblages? Community optimisation might hold the answer. Methods Ecol. Evol. 2018, 9, 2155–2166. [Google Scholar] [CrossRef]
- Lawson, C.R.; Hodgson, J.A.; Wilson, R.J.; Richards, S.A. Prevalence, thresholds and the performance of presence–absence models. Methods Ecol. Evol. 2014, 5, 54–64. [Google Scholar] [CrossRef]
- Gábor, L.; Šímová, P.; Keil, P.; Zarzo-Arias, A.; Marsh, C.J.; Rocchini, D.; Malavasi, M.; Barták, V.; Moudrý, V. Habitats as predictors in species distribution models: Shall we use continuous or binary data? Ecography 2022, 2022, e06022. [Google Scholar] [CrossRef]
- Poisot, T. Guidelines for the prediction of species interactions through binary classification. Methods Ecol. Evol. 2023, 14, 1333–1345. [Google Scholar] [CrossRef]
- Semmler, T.; Danilov, S.; Rackow, T.; Sidorenko, D.; Barbi, D.; Hegewald, J.; Pradhan, H.K.; Sein, D.; Wang, Q.; Jung, T. AWI-CM-1.1-MR model output prepared for CMIP6 ScenarioMIP: Links to SSP126, SSP245, SSP370, and SSP585 scenarios. Earth Syst. Grid Fed. 2019. [Google Scholar] [CrossRef]
- Nazarenko, L.S.; Tausnev, N.; Russell, G.L.; Rind, D.; Miller, R.L.; Schmidt, G.A.; Bauer, S.E.; Kelley, M.; Ruedy, R.; Ackerman, A.S. Future climate change under SSP emission scenarios with GISS-E2. 1. J. Adv. Model. Earth Syst. 2022, 14, e2021MS002871. [Google Scholar] [CrossRef]
- Muschett, G.; Fontúrbel, F.E. A comprehensive catalogue of plant-pollinator interactions for Chile. Sci. Data 2022, 9, 78. [Google Scholar] [CrossRef]
- Rahimi, E.; Barghjelveh, S.; Dong, P. Estimating potential range shift of some wild bees in response to climate change scenarios in northwestern regions of Iran. J. Ecol. Environ. 2021, 45, 130–142. [Google Scholar] [CrossRef]
- Stockwell, D.R.; Peterson, A.T. Effects of sample size on accuracy of species distribution models. Ecol. Model. 2002, 148, 1–13. [Google Scholar] [CrossRef]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef]
- Singh, A. Niche Divergence. In Encyclopedia of Animal Cognition and Behavior; Springer: Berlin/Heidelberg Germany, 2022; pp. 4664–4666. [Google Scholar]
- Buckley, L.B.; Davies, T.J.; Ackerly, D.D.; Kraft, N.J.; Harrison, S.P.; Anacker, B.L.; Cornell, H.V.; Damschen, E.I.; Grytnes, J.-A.; Hawkins, B.A. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B Biol. Sci. 2010, 277, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Olalla-Tárraga, M.Á.; McInnes, L.; Bini, L.M.; Diniz-Filho, J.A.; Fritz, S.A.; Hawkins, B.A.; Hortal, J.; Orme, C.D.L.; Rahbek, C.; Rodríguez, M.Á. Climatic niche conservatism and the evolutionary dynamics in species range boundaries: Global congruence across mammals and amphibians. J. Biogeogr. 2011, 38, 2237–2247. [Google Scholar] [CrossRef]
- Castro-Insua, A.; Gómez-Rodríguez, C.; Wiens, J.J.; Baselga, A. Climatic niche divergence drives patterns of diversification and richness among mammal families. Sci. Rep. 2018, 8, 8781. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Freckleton, R.P.; Jetz, W. Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B: Biol. Sci. 2011, 278, 2384–2391. [Google Scholar] [CrossRef]
- Olalla-Tárraga, M.Á.; González-Suárez, M.; Bernardo-Madrid, R.; Revilla, E.; Villalobos, F. Contrasting evidence of phylogenetic trophic niche conservatism in mammals worldwide. J. Biogeogr. 2017, 44, 99–110. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Winter, M.; Herrmann, D. Evolution of climate niches in European mammals? Biol. Lett. 2010, 6, 229–232. [Google Scholar] [CrossRef]
- Rubidge, E.M.; Monahan, W.B.; Parra, J.L.; Cameron, S.E.; Brashares, J.S. The role of climate, habitat, and species co-occurrence as drivers of change in small mammal distributions over the past century. Glob. Chang. Biol. 2011, 17, 696–708. [Google Scholar] [CrossRef]
- Santos, A.M.; Cianciaruso, M.V.; Barbosa, A.M.; Bini, L.M.; Diniz-Filho, J.A.F.; Faleiro, F.V.; Gouveia, S.F.; Loyola, R.; Medina, N.G.; Rangel, T.F. Current climate, but also long-term climate changes and human impacts, determine the geographic distribution of European mammal diversity. Glob. Ecol. Biogeogr. 2020, 29, 1758–1769. [Google Scholar] [CrossRef]
- Billman, P.D.; Beever, E.A.; McWethy, D.B.; Thurman, L.L.; Wilson, K.C. Factors influencing distributional shifts and abundance at the range core of a climate-sensitive mammal. Glob. Chang. Biol. 2021, 27, 4498–4515. [Google Scholar] [CrossRef]
- Naimi, B. Package ‘usdm’. Uncertainty Analysis for Species Distribution Models. 2017. Available online: www.cran.r-project.org (accessed on 1 January 2024).
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Rose, M.B.; de Andrade, A.F.A.; Minoli, I.; Franklin, J. flexsdm: An R package for supporting a comprehensive and flexible species distribution modelling workflow. Methods Ecol. Evol. 2022, 13, 1661–1669. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. Interaction 2008, 1, 8–11. [Google Scholar]
- Dormann, C.F.; Fruend, J.; Gruber, B.; Dormann, M.C.F.; LazyData, T. Package ‘bipartite’. Visualizing Bipartite Networks and Calculating Some (Ecological) Indices (Version 2.04). (R Foundation for Statistical Computing). 2014. Available online: https://cran.r-project.org/web/packages/bipartite/index.html (accessed on 28 July 2015).
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 2605–2611. [Google Scholar] [CrossRef]
- Gao, J.; Barzel, B.; Barabási, A.-L. Universal resilience patterns in complex networks. Nature 2016, 530, 307–312. [Google Scholar] [CrossRef]
- Bastolla, U.; Fortuna, M.A.; Pascual-García, A.; Ferrera, A.; Luque, B.; Bascompte, J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 2009, 458, 1018–1020. [Google Scholar] [CrossRef]
- Grilli, J.; Rogers, T.; Allesina, S. Modularity and stability in ecological communities. Nat. Commun. 2016, 7, 12031. [Google Scholar] [CrossRef]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef]
- New, M.; Hulme, M. Representing uncertainty in climate change scenarios: A Monte-Carlo approach. Integr. Assess. 2000, 1, 203–213. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Araújo, M.; Anderson, R.; Márcia Barbosa, A.; Beale, C.; Dormann, C.; Early, R.; Garcia, R.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]


| Metric | Definition |
|---|---|
| Connectance | The realized proportion of possible links |
| Web asymmetry | The balance between numbers in the two levels: positive values indicate higher trophic level species, negative values indicate more lower-trophic-level species |
| Links per species | The mean number of links per species (qualitative): sum of links divided by number of species |
| Modularity Q | Compartments are sub-sets of the web that are not connected (through either higher or lower trophic levels) to another compartment |
| Nestedness | The nestedness temperature of the matrix (0 means cold, i.e., high nestedness, 100 means hot, i.e., chaos) |
| NODF | Another index for nestedness: high values indicate nestedness. |
| Weighted nestedness | A nestedness version that considers interaction frequencies (and is hence weighted) |
| Linkage density | Marginal total-weighted diversity of interactions per species (quantitative) |
| Number of species HL | Number of pollinators |
| Number of species LL | Number of plants |
| Robustness HL | The area below the “secondary extinction” curve for pollinators |
| Robustness LL | The area below the “secondary extinction” curve for plants |
| Contingent | AUC | BOYCE | IMAE |
|---|---|---|---|
| Plants | 0.99 (0.00) | 0.93 (0.04) | 0.98 (0.01) |
| Pollinators | 0.98 (0.01) | 0.92 (0.05) | 0.97 (0.01) |
| No. Species (Increase) | Increase% | No. Species (Decrease) | Decrease% | |
|---|---|---|---|---|
| Plants | 40 | 18.3 (14) | 147 | −33.4 (20) |
| Pollinators | 47 | 24.3 (32) | 124 | −25.7 (18) |
| Bipartite Metrics | Current | Future |
|---|---|---|
| Connectance | 0.25 | 0.31 |
| Web asymmetry | −0.18 | −0.19 |
| Links per species | 1.13 | 1.05 |
| Modularity Q | 0.53 | 0.55 |
| Nestedness | 13.48 | 12.99 |
| NODF | 23.77 | 24.09 |
| Weighted nestedness | 0.64 | 0.67 |
| Linkage density | 5.91 | 5.29 |
| Number of species HL | 25.55 | 19.29 |
| Number of species LL | 33.22 | 27.35 |
| Robustness HL | 0.49 | 0.49 |
| Robustness LL | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi, E.; Jung, C. A New SDM-Based Approach for Assessing Climate Change Effects on Plant–Pollinator Networks. Insects 2024, 15, 842. https://doi.org/10.3390/insects15110842
Rahimi E, Jung C. A New SDM-Based Approach for Assessing Climate Change Effects on Plant–Pollinator Networks. Insects. 2024; 15(11):842. https://doi.org/10.3390/insects15110842
Chicago/Turabian StyleRahimi, Ehsan, and Chuleui Jung. 2024. "A New SDM-Based Approach for Assessing Climate Change Effects on Plant–Pollinator Networks" Insects 15, no. 11: 842. https://doi.org/10.3390/insects15110842
APA StyleRahimi, E., & Jung, C. (2024). A New SDM-Based Approach for Assessing Climate Change Effects on Plant–Pollinator Networks. Insects, 15(11), 842. https://doi.org/10.3390/insects15110842








