Numerical Modeling Reveals That Resistant Western Corn Rootworm Are Stronger Fliers than Their Susceptible Conspecifics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Wing Geometry and Aspect Ratio (AR)
2.3. Numerical Modeling: Finite Element Method (FEM)
3. Results
3.1. Wing Geometry: Aspect Ratio (AR)
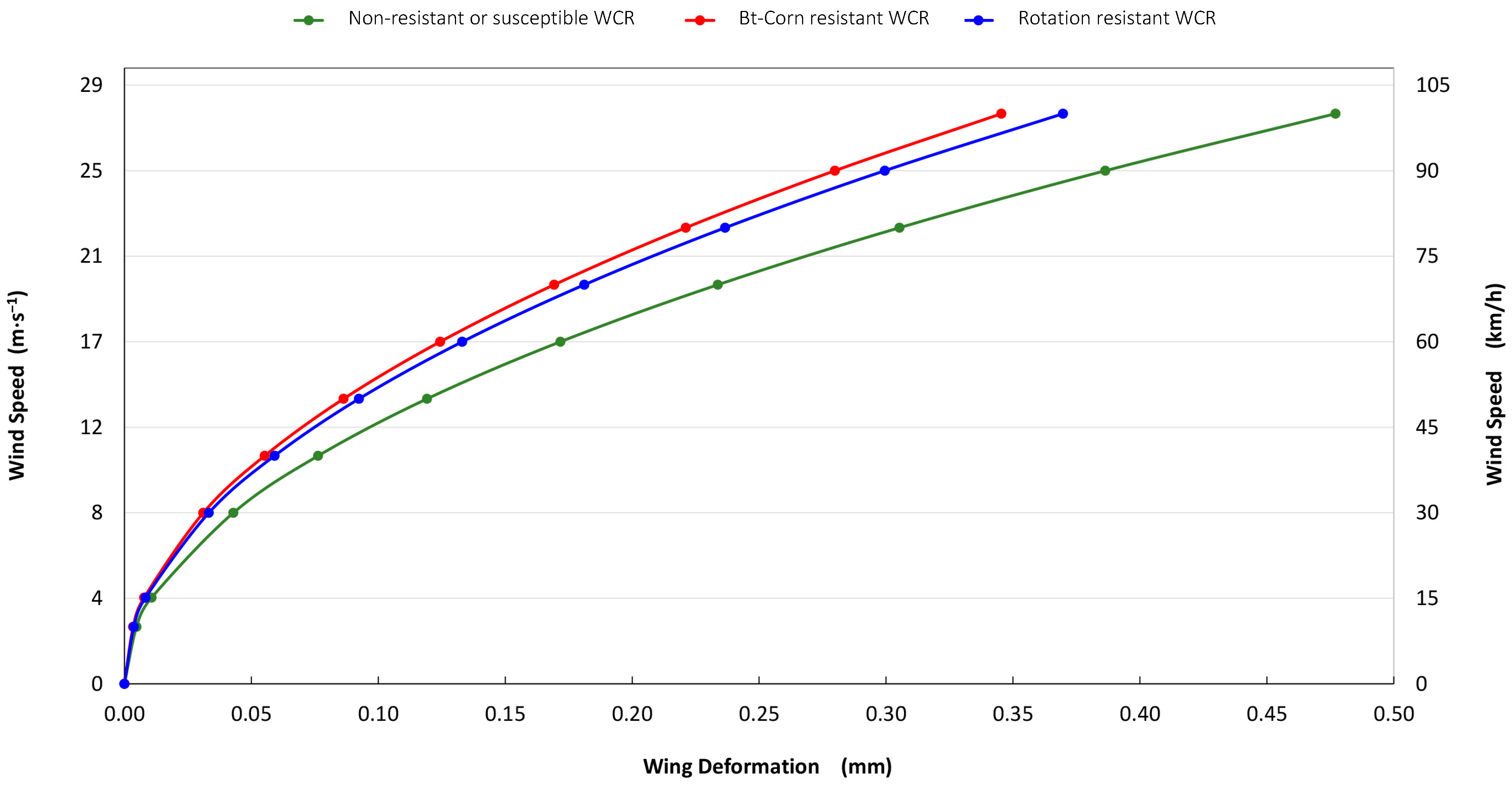
3.2. Finite Element Method (FEM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meinke, L.J.; Siegfried, B.D.; Wright, R.J.; Chandler, L.D. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J. Econ. Entomol. 1998, 91, 594–600. [Google Scholar] [CrossRef]
- Ball, H.J.; Weekman, G.T. Insecticide resistance in the adult western corn rootworm in Nebraska. J. Econ. Entomol. 1962, 55, 439–441. [Google Scholar] [CrossRef]
- Ball, H.J.; Weekman, G.T. Differential resistance of corn rootworms to insecticides in Nebraska and adjoining states. J. Econ. Entomol. 1963, 56, 553–555. [Google Scholar] [CrossRef]
- Wright, R.J.; Scharf, M.E.; Meinke, L.J.; Zhou, X.; Siegfried, B.D.; Chandler, L.D. Larval susceptibility of an insecticide-resistant western corn rootworm (Coleoptera: Chrysomelidae) population to soil insecticides: Laboratory bioassays, assays of detoxification enzymes, and field performance. J. Econ. Entomol. 2000, 93, 7–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, A.E.; Wang, H.; Zukoff, S.N.; Meinke, L.J.; French, B.W.; Siegfried, B.D. Evidence of field-evolved resistance to bifenthrin in western corn rootworm (Diabrotica virgifera virgifera LeConte) populations in Western Nebraska and Kansas. PLoS ONE 2015, 10, e0142299. [Google Scholar] [CrossRef]
- Pereira, A.E.; Souza, D.; Zukoff, S.N.; Meinke, L.J.; Siegfried, B.D. Cross-resistance and synergism bioassays suggest multiple mechanisms of pyrethroid resistance in western corn rootworm populations. PLoS ONE 2017, 12, e0179311. [Google Scholar] [CrossRef]
- Levine, E.; Oloumi-Sadeghi, H. Western corn rootworm (Coleoptera: Chrysomelidae) larval injury to corn grown for seed production following soybeans grown for seed production. J. Econ. Entomol. 1996, 89, 1010–1016. [Google Scholar] [CrossRef]
- Sammons, A.E.; Edwards, C.R.; Bledsoe, L.W.; Boeve, P.J.; Stuart, J.J. Behavioral and feeding assays reveal a western corn rootworm (Coleoptera: Chrysomelidae) variant that is attracted to soybean. Environ. Entomol. 1997, 26, 1336–1342. [Google Scholar] [CrossRef]
- Levine, E.; Spencer, J.L.; Isard, S.A.; Onstad, D.W.; Gray, M.E. Adaptation of the western corn rootworm to crop rotation: Evolution of a new strain in response to a management practice. Am. Entomol. 2002, 48, 94–107. [Google Scholar] [CrossRef]
- Gassmann, A.J. Resistance to Bt Maize by Western Corn Rootworm: Effects of Pest Biology, the Pest–Crop Interaction and the Agricultural Landscape on Resistance. Insects 2021, 12, 136. [Google Scholar] [CrossRef]
- Gray, M.E.; Sappington, T.W.; Miller, N.J.; Moeser, J.; Bohn, M.O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 2009, 54, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Hibbard, B.E.; Moeser, J.; Onstad, D.W. Behaviour and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agric. For. Entomol. 2009, 11, 9–27. [Google Scholar] [CrossRef]
- Mabry, T.R.; Spencer, J.L.; Levine, E.; Isard, S.A. Western corn rootworm (Coleoptera: Chrysomelidae) behavior is affected by alternating diets of aorn and soybean. Environ. Entomol. 2004, 33, 860–871. [Google Scholar] [CrossRef]
- Li, H.; Toepfer, S.; Kuhlmann, U. Relationship between phenotypic traits and selected fitness components of Diabrotica virgifera virgifera. Entomol. Exp. Appl. 2009, 131, 254–263. [Google Scholar] [CrossRef]
- Li, H.; Toepfer, S.; Kuhlmann, U. Flight and crawling activities of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) in relation to morphometric traits. J. Appl. Entomol. 2010, 131, 254–263. [Google Scholar] [CrossRef]
- Benítez, H.A.; Lemic, D.; Bažok, R.; Gallardo-Araya, C.M.; Mikac, K.M. Evolutionary directional asymmetry and shape variation in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae): An example using hind wings. Biol. J. Linn. Soc. 2013, 111, 110–118. [Google Scholar] [CrossRef]
- Mikac, K.M.; Lemic, D.; Bažok, R.; Benítez, H.A. Wing shape changes: A morphological view of the Diabrotica virgifera virgifera European invasion. Biol. Invasions 2016, 18, 3401–3407. [Google Scholar] [CrossRef]
- Mikac, K.M.; Lemic, D.; Benítez, H.A.; Bažok, R. Changes in corn rootworm wing morphology are related to resistance development. J. Pest Sci. 2019, 92, 443–451. [Google Scholar] [CrossRef]
- Mrganić, M.; Bažok, R.; Mikac, K.M.; Benítez, H.A.; Lemic, D. Two decades of invasive western corn rootworm population monitoring in Croatia. Insects 2018, 9, 160. [Google Scholar] [CrossRef]
- Kadoić Balaško, M.; Mikac, K.M.; Benitez, H.A.; Bažok, R.; Lemic, D. Genetic and Morphological Approach for Western Corn Rootworm Resistance Management. Agriculture 2021, 11, 585. [Google Scholar] [CrossRef]
- Mikac, K.M.; Douglas, J.; Spencer, J.L. Wing shape and size of the western corn rootworm (Coleoptera: Chrysomelidae) is related to sex and resistance to soybean-maize crop rotation. J. Econ. Entomol. 2013, 106, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.A.; Tollefson, J.J.; Mutchmor, J.A. Study of migratory flight in the western corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 1986, 5, 620–625. [Google Scholar] [CrossRef]
- Naranjo, S.E. Comparative flight behavior of Diabrotica virgifera virgifera and Diabrotica barberi in the laboratory. Entomol. Exp. Appl. 1990, 15, 620–625. [Google Scholar] [CrossRef]
- Isard, S.A.; Spencer, J.L.; Mabry, T.R.; Levine, E. Influence of atmospheric conditions on high-elevation flight of western corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 2004, 33, 650–656. [Google Scholar] [CrossRef]
- Isard, S.A.; Spencer, J.L.; Nasser, M.A.; Levine, E. Aerial Movement of Western Corn Rootworm (Coleoptera: Chrysomelidae): Diel Periodicity of Flight Activity in Soybean Fields. Environ. Entomol. 2000, 29, 226–234. [Google Scholar] [CrossRef]
- Grant, R.H.; Seevers, K.P. Local and long-range movement of adult western corn rootworm (Coleoptera: Chrysomelidae) as evidenced by washup along southern Lake Michigan shores. Environ. Entomol. 1989, 18, 266–272. [Google Scholar] [CrossRef]
- Grant, R.H.; Seevers, K.P. The vertical movement of adult western corn rootworms (Diabrotica virgifera virgifera) relative to the transport of momentum and heat. Agric. For. Meteorol. 1990, 49, 191–203. [Google Scholar] [CrossRef]
- Kesel, A.B.; Philippi, U.; Nachtigall, W. Biomechanical aspects of the insect wing: An analysis using the finite element method. Comp. Biol. Med. 1998, 28, 423–437. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Into thin air: Contributions of aerodynamic and inertial-elastic forces to wing bending in the hawkmoth Manduca sexta. J. Exp. Biol. 2003, 206, 2999–3006. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural stiffness in insect wings I. Scaling and the influence of wing venation. J. Exp. Biol. 2003, 206, 2979–2987. [Google Scholar] [CrossRef]
- Wootton, R.J.; Herbert, R.C.; Young, P.G.; Evans, K.E. Approaches to the structural modelling of insect wings. Philos. Trans. R. Soc. B 2003, 358, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, S.R.; Lentink, D. Structural analysis of a dragonfly wing. Exp. Mech. 2010, 50, 1323–1334. [Google Scholar] [CrossRef]
- Li, X.; Guo, C. Structural characteristics analysis of the hind wings in a bamboo weevil (Cyrtotrachelus buqueti). IET Nanobiotechnol. 2019, 13, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Song, W.P.; Song, B.F.; Li, Y.B. The Effects of force on the structure deformation of wing for flapping-wing. Adv. Nat. Sci. 2010, 3, 285–290. [Google Scholar]
- Ha, N.S.; Truong, Q.T.; Goo, N.S.; Park, H.C. Biomechanical properties of insect wings: The stress stiffening effects on the asymmetric bending of the Allomyrina dichotoma beetle’s hind wing. PLoS ONE 2013, 8, e80689. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Barić, B.; Drmić, Z.; Kadoić Balaško, M.; Bažok, R.; Lemic, D.; Benitez, H.A.; Dominguez Davila, J.H.; Mikac, K.M. Codling moth wing morphology changes due to insecticide resistance. Insects 2019, 10, 310. [Google Scholar] [CrossRef]
- Dirks, J.H.; Taylor, D. Veins improve fracture toughness of insect wings. PLoS ONE 2012, 7, e43411. [Google Scholar] [CrossRef]
- Rajabi, H.; Darvizeh, A.; Shafiei, A.; Taylor, D.; Dirks, J.H. Numerical investigation of insect wing fracture behaviour. J. Biomech. 2015, 48, 89–94. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghoroubi, N.; Darvizeh, A.; Dirks, J.H.; Appel, E.; Gorb, S.N. A comparative study of the effects of vein-joints on the mechanical behaviour of insect wings: I. Single joints. Bioinspir. Biomim. 2015, 10, 056003. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghoroubi, N.; Malaki, M.; Darvizeh, A.; Gorb, S.N. Basal complex and basal venation of Odonata wings: Structural diversity and potential role in the wing deformation. PLoS ONE 2016, 1, e0160610. [Google Scholar] [CrossRef]
- Tong, J.; Chang, Z.; Yang, X.; Zhang, J.; Liu, X.; Chetwynd, D.G.; Chen, D.; Sun, J. Nanoindentation mechanical properties and structural biomimetic models of three species of insects wings. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 831–839. [Google Scholar] [CrossRef]
- Perrard, A.; Baylac, M.; Carpenter, J.M.; Villemant, C. Evolution of wing shape in hornets: Why is the wing venation efficient for species identification? J. Evol. Biol. 2014, 27, 2665–2675. [Google Scholar] [CrossRef]
- Upton, M.F.S.; Mantel, B.L. Methods for Collecting, Preserving and Studying Insects and Other Terrestrial Arthropods; The Australian Entomological Society Miscellaneous Pub: Sydney, Australia, 2010. [Google Scholar]
- Altizer, S.; Davis, A.K. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 2010, 64, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Lua, K.B.; Lai, K.C.; Lim, T.T.; Yeo, K.S. On the aerodynamic characteristics of hovering rigid and flexible hawkmoth-like wings. Exp. Fluids 2010, 49, 1263–1291. [Google Scholar] [CrossRef]
- Rohlf, F.J. TPSdig, v. 2.17; State University of Stony Brook: Stony Brook, NY, USA, 2016. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural stiffness in insect wings. II. Spatial distribution and dynamic wing bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef]
- Dingle, H. Geographic variation and behavioral flexibility in milkweed bug life histories. In Insect Life History Patterns; Springer: New York, NY, USA, 1981; pp. 57–73. [Google Scholar]
- Dudley, R.; Srygley, R. Flight physiology of neotropical butterflies: Allometry of airspeeds during natural free flight. J. Exp. Biol. 1994, 191, 125–139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikac, K.M.; Lemic, D.; Pajač Živković, I.; Dominguez Davila, J.H. Numerical Modeling Reveals That Resistant Western Corn Rootworm Are Stronger Fliers than Their Susceptible Conspecifics. Insects 2024, 15, 834. https://doi.org/10.3390/insects15110834
Mikac KM, Lemic D, Pajač Živković I, Dominguez Davila JH. Numerical Modeling Reveals That Resistant Western Corn Rootworm Are Stronger Fliers than Their Susceptible Conspecifics. Insects. 2024; 15(11):834. https://doi.org/10.3390/insects15110834
Chicago/Turabian StyleMikac, Katarina M., Darija Lemic, Ivana Pajač Živković, and Jose H. Dominguez Davila. 2024. "Numerical Modeling Reveals That Resistant Western Corn Rootworm Are Stronger Fliers than Their Susceptible Conspecifics" Insects 15, no. 11: 834. https://doi.org/10.3390/insects15110834
APA StyleMikac, K. M., Lemic, D., Pajač Živković, I., & Dominguez Davila, J. H. (2024). Numerical Modeling Reveals That Resistant Western Corn Rootworm Are Stronger Fliers than Their Susceptible Conspecifics. Insects, 15(11), 834. https://doi.org/10.3390/insects15110834








