Influence of Temperature and Host Plant on the Digestion of Frankliniella intonsa (Trybom) Revealed by Molecular Detection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Feeding Experiment
2.3. DNA Extraction
2.4. Plant DNA Detection
2.5. Plant Nutrient Measurement
2.6. Statistics
3. Results
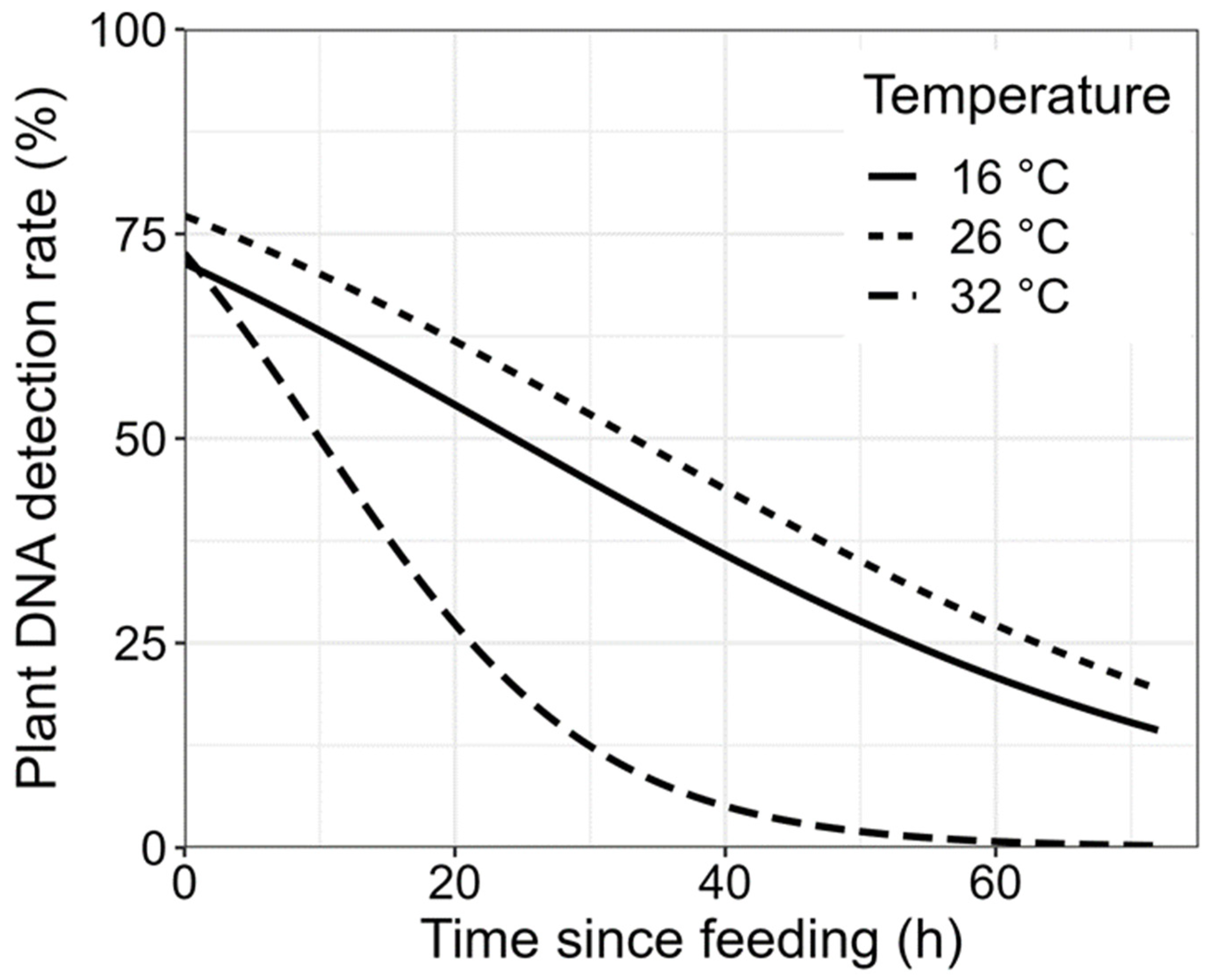
3.1. Detectability Half-Life
3.2. The Effects of Plant Species and Temperature on Plant DNA Detection
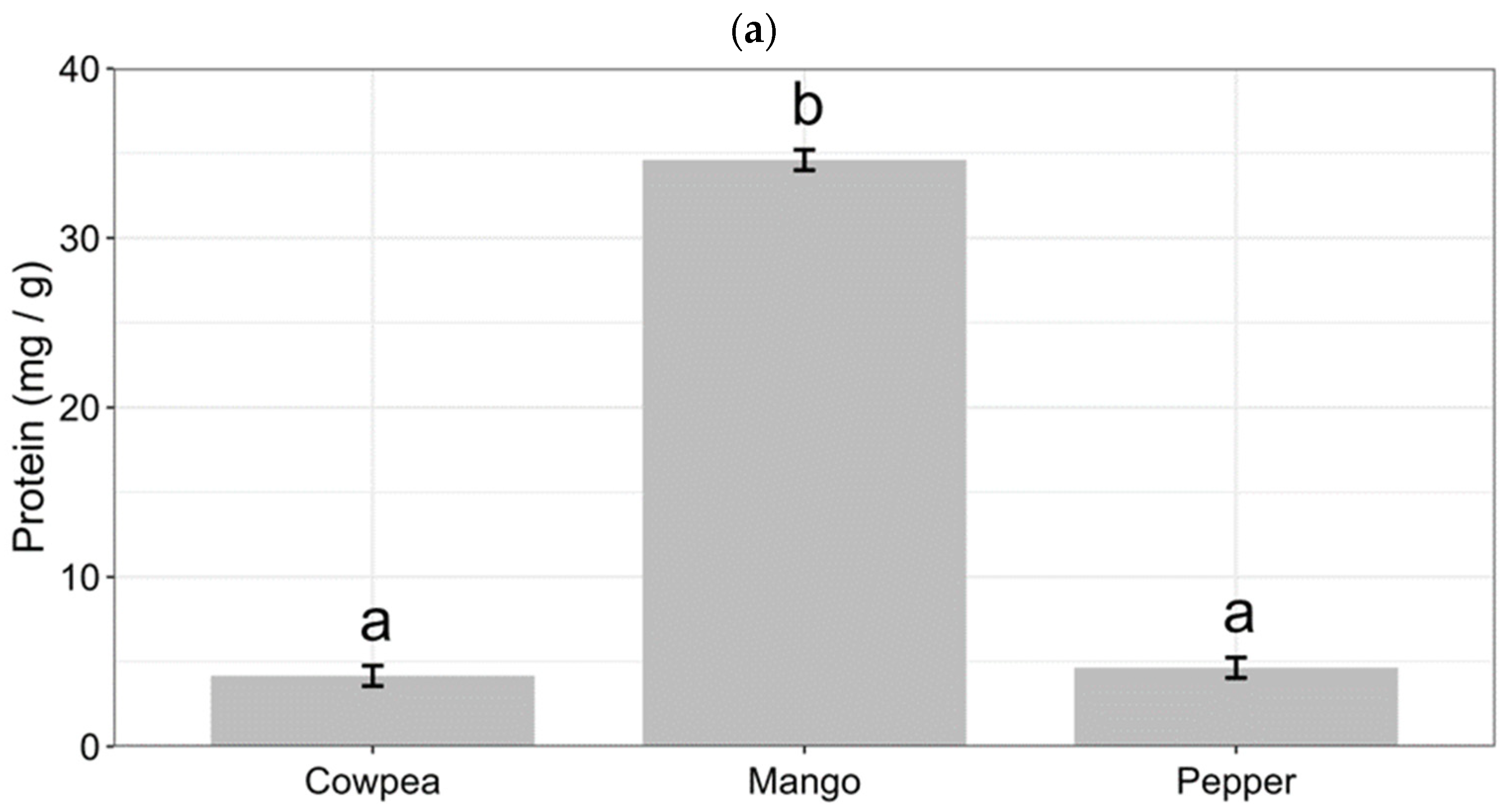
3.3. Content of Plant Nutrients
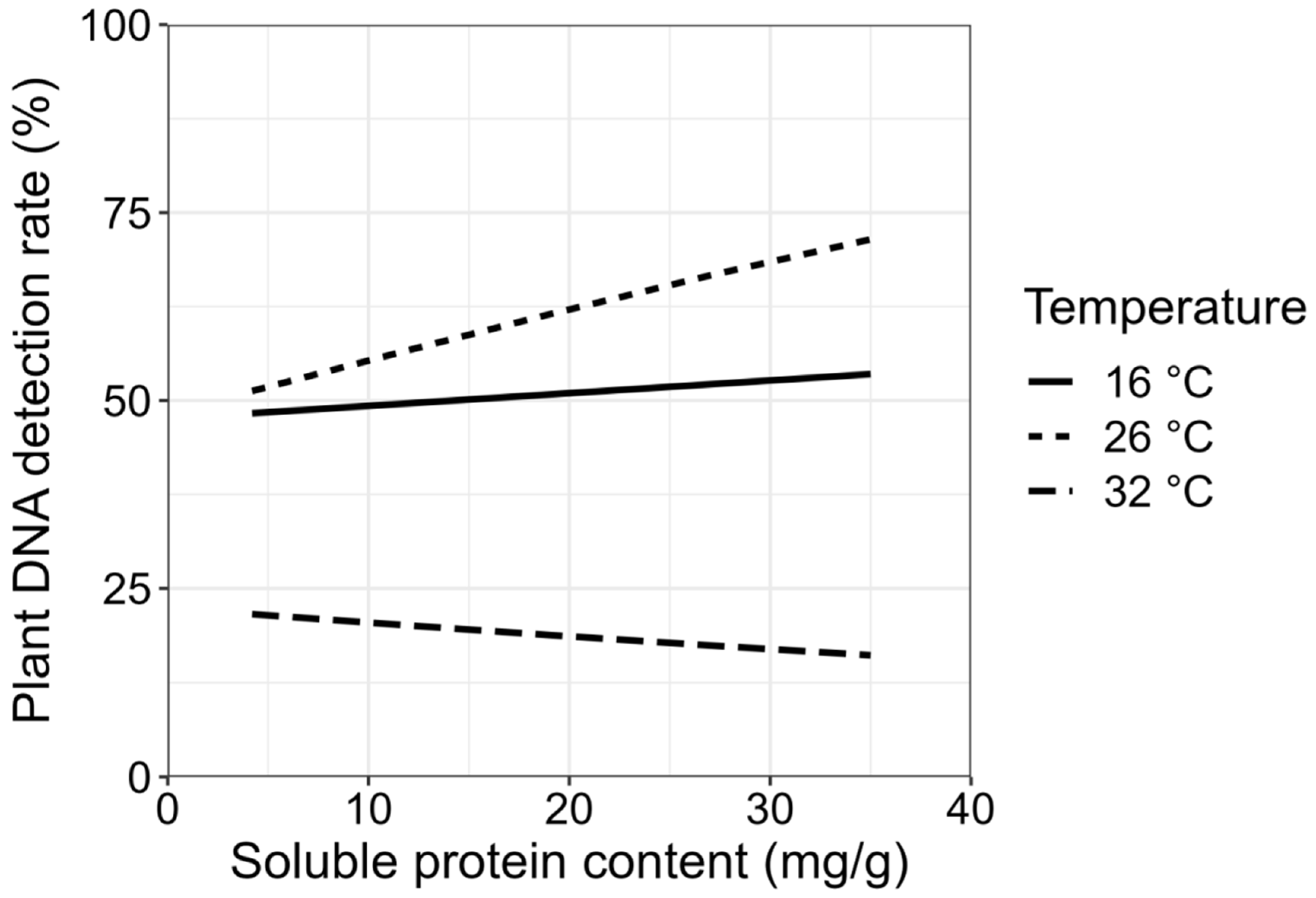
3.4. Effects of Plant Nutrients and Temperature on Plant DNA Detection
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.-H.; Qiao, F.-J.; Lu, Z.; Li, C.-Y.; Liu, T.-X.; Gao, Y.-L.; Zhang, B. Interspecific Competitions between Frankliniella intonsa and Frankliniella occidentalis on Fresh Lentil Bean Pods and Pepper Plants. Insects 2022, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Frantz, G.; Mellinger, H.C. Shifts in Western Flower Thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), Population Abundance and Crop Damage. Fla. Entomol. 2009, 92, 29–34. [Google Scholar] [CrossRef]
- Sierra-Baquero, P.; Varon, E.; Gomes Dias, L.; Monje, B. Economic Injury Level for the Flower Thrips Frankliniella Cf. Gardeniae Moulton (Thysanoptera: Thripidae) in Mango. Rev. Fac. Nac. Agron. Medellín 2020, 73, 9213–9220. [Google Scholar] [CrossRef]
- Tang, L.-D.; Guo, L.-H.; Wu, J.-H.; Zang, L.-S. Thrips in Genus Megalurothrips (Thysanoptera: Thripidae): Biodiversity, Bioecology, and IPM. J. Integr. Pest Manag. 2023, 14, 8. [Google Scholar] [CrossRef]
- Gombač, P.; Trdan, S. The Efficacy of Intercropping with Birdsfoot Trefoil and Summer Savoury in Reducing Damage Inflicted by Onion Thrips (Thrips tabaci, Thysanoptera, Thripidae) on Four Leek Cultivars. J. Plant Dis. Prot. 2014, 121, 117–124. [Google Scholar] [CrossRef]
- Riefler, J.; Koschier, E.H. Comparing Behavioural Patterns of Thrips tabaci Lindeman on Leek and Cucumber. J. Insect Behav. 2009, 22, 111–120. [Google Scholar] [CrossRef]
- Yue, W.B.; Zhou, D.; Li, D.Y.; Zhi, J.R.; Fang, X.L.; Qiu, X.Y. Multigenerational Variation in the Nutrients and Digestion of Western Flower Thrips (Frankliniella occidentalis) Depends on the Nutritive Quality of Different Foods. J. Insect Sci. 2023, 23, 12. [Google Scholar] [CrossRef]
- Zhi, J.; Liu, L.; Hou, X.; Xie, W.; Yue, W.; Zeng, G. Role of Digestive Enzymes in the Adaptation of Frankliniella occidentalis to Preferred and Less-preferred Host Plants. Entomol. Exp. Appl. 2021, 169, 688–700. [Google Scholar] [CrossRef]
- Scott Brown, A.S.; Simmonds, M.S.J.; Blaney, W.M. Relationship between Nutritional Composition of Plant Species and Infestation Levels of Thrips. J. Chem. Ecol. 2002, 28, 2399–2409. [Google Scholar] [CrossRef]
- Hosseini, R.; Schmidt, O.; Keller, M.A. Factors Affecting Detectability of Prey DNA in the Gut Contents of Invertebrate Predators: A Polymerase Chain Reaction-based Method. Entomol. Exp. Appl. 2008, 126, 194–202. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Burkepile, D.E. Temperature-induced Mismatches between Consumption and Metabolism Reduce Consumer Fitness. Ecology 2012, 93, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Woods, H.A. Thermal Sensitivity of Growth and Feeding in Manduca sexta Caterpillars. Physiol. Zool. 1997, 70, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.P.; Drews, W.A.; Burkepile, D.E.; Parker, J.D. Increased Temperature Alters Feeding Behavior of a Generalist Herbivore. Oikos 2013, 122, 1669–1678. [Google Scholar] [CrossRef]
- Grčić, A.; Ilijin, L.; Filipović, A.; Matić, D.; Mrdaković, M.; Todorović, D.; Vlahović, M.; Perić-Mataruga, V. Digestive Enzyme Activity and Macromolecule Content in the Hemolymph of Differentially Adapted Lymantria dispar L. Populations after Short-Term Increases in Ambient Temperature. Environ. Res. 2023, 236, 116461. [Google Scholar] [CrossRef]
- Hereward, J.P.; Walter, G.H. Molecular Interrogation of the Feeding Behaviour of Field Captured Individual Insects for Interpretation of Multiple Host Plant Use. PLoS ONE 2012, 7, e44435. [Google Scholar] [CrossRef][Green Version]
- Otte, D.; Joern, A. On Feeding Patterns in Desert Grasshoppers and the Evolution of Specialized Diets. Proc. Acad. Nat. Sci. USA 1976, 128, 89–126. [Google Scholar]
- Sheppard, S.K.; Bell, J.; Sunderland, K.D.; Fenlon, J.; Skervin, D.; Symondson, W.O.C. Detection of Secondary Predation by PCR Analyses of the Gut Contents of Invertebrate Generalist Predators. Mol. Ecol. 2005, 14, 4461–4468. [Google Scholar] [CrossRef]
- Briem, F.; Zeisler, C.; Guenay, Y.; Staudacher, K.; Vogt, H.; Traugott, M. Identifying Plant DNA in the Sponging–Feeding Insect Pest Drosophila suzukii. J. Pest Sci. 2018, 91, 985–994. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Rowley, D.L.; Weber, D.C.; Payton, M.E.; Hawthorne, D.J. Feeding Mode and Prey Detectability Half-Lives in Molecular Gut-Content Analysis: An Example with Two Predators of the Colorado Potato Beetle. Bull. Entomol. Res. 2007, 97, 201–209. [Google Scholar] [CrossRef]
- Staudacher, K.; Schallhart, N.; Thalinger, B.; Wallinger, C.; Juen, A.; Traugott, M. Plant Diversity Affects Behavior of Generalist Root Herbivores, Reduces Crop Damage, and Enhances Crop Yield. Ecol. Appl. A Publ. Ecol. Soc. Am. 2013, 23, 1135–1145. [Google Scholar] [CrossRef]
- Guenay, Y.; Trager, H.; Glarcher, I.; Traugott, M.; Wallinger, C. Limited Detection of Secondarily Consumed Plant Food by DNA-based Diet Analysis of Omnivorous Carabid Beetles. Environ. DNA 2021, 3, 426–434. [Google Scholar] [CrossRef]
- Ye, Z.; Vollhardt, I.M.G.; Girtler, S.; Wallinger, C.; Tomanovic, Z.; Traugott, M. An Effective Molecular Approach for Assessing Cereal Aphid-Parasitoid-Endosymbiont Networks. Sci. Rep. 2017, 7, 3138. [Google Scholar] [CrossRef] [PubMed]
- Greenstone, M.H.; Hunt, J.H. Determination of Prey Antigen Half-life in Polistes metricus Using a Monoclonal Antibody-based Immunodot Assay. Entomol. Exp. Appl. 1993, 68, 1–7. [Google Scholar] [CrossRef]
- Guenay-Greunke, Y.; Bohan, D.A.; Traugott, M.; Wallinger, C. Handling of Targeted Amplicon Sequencing Data Focusing on Index Hopping and Demultiplexing Using a Nested Metabarcoding Approach in Ecology. Sci. Rep. 2021, 11, 19510. [Google Scholar] [CrossRef] [PubMed]
- Guenay-Greunke, Y.; Trager, H.; Bohan, D.A.; Traugott, M.; Wallinger, C. Consumer Identity but Not Food Availability Affects Carabid Diet in Cereal Crops. J. Pest Sci. 2024, 97, 281–296. [Google Scholar] [CrossRef]
- Ullah, M.S.; Lim, U.T. Life History Characteristics of Frankliniella occidentalis and Frankliniella intonsa (Thysanoptera: Thripidae) in Con-Stant and Fluctuating Temperatures. J. Econ. Entomol. 2015, 108, 1000–1009. [Google Scholar] [CrossRef]
- Gai, H.T.; Zhì, J.R.; Sun, M. Effects of temperature on the survival and fecundity of Frankliniella occidentalis and Frankliniella intonsa (thysanoptera: Thripidae). J. Plant Prot. 2011, 38, 521–526. [Google Scholar]
- Qu, H.; Chuai, Z.; Zhang, W.; Zhang, J.; Zhao, J.; Li, H. Research progress on the occurrence and control of Frankliniella intonsa (Trybom). China Plant Prot. 2023, 43, 18–24, 30. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing. Found. Stat. Comput. Vienna Austria 2014.
- Payton, M.E.; Greenstone, M.H.; Schenker, N. Overlapping Confidence Intervals or Standard Error Intervals: What Do They Mean in Terms of Statistical Significance? J. Insect Sci. 2003, 3, 34. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bao, W.-F.; Yang, F.; Xu, B.; Yang, Y.-Z. The Specific Host Plant DNA Detection Suggests a Potential Migration of Apolygus lucorum from Cotton to Mungbean Fields. PLoS ONE 2017, 12, e0177789. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Reitz, S.R.; Germinara, G.S.; Wang, C.; Wang, L.; Yang, S.; Gao, Y.; Zhang, W.; Li, C. Host Preference of Thrips hawaiiensis for Different Ornamental Plants. J. Pest Sci. 2022, 95, 761–770. [Google Scholar] [CrossRef]
- Qin, J.; Wang, C. The Relation of Interaction between Insects and Plants to Evolution. Acta Entomol. Sin. 2001, 44, 360–365. [Google Scholar]
- Schoonhoven, L.M.; Van Loon, J.J.; Dicke, M. Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Hoogendoorn, M.; Heimpel, G.E. PCR-based Gut Content Analysis of Insect Predators: Using Ribosomal ITS-1 Fragments from Prey to Estimate Predation Frequency. Mol. Ecol. 2001, 10, 2059–2067. [Google Scholar] [CrossRef]
- Von Berg, K.; Traugott, M.; Symondson, W.O.C.; Scheu, S. The Effects of Temperature on Detection of Prey DNA in Two Species of Carabid Beetle. Bull. Entomol. Res. 2008, 98, 263–269. [Google Scholar] [CrossRef]
- Clissold, F.J.; Coggan, N.; Simpson, S.J. Insect Herbivores Can Choose Microclimates to Achieve Nutritional Homeostasis. J. Exp. Biol. 2013, 216, 2089–2096. [Google Scholar] [CrossRef]
- Kratina, P.; Greig, H.S.; Thompson, P.L.; Carvalho-Pereira, T.S.; Shurin, J.B. Warming Modifies Trophic Cascades and Eutrophication in Experimental Freshwater Communities. Ecology 2012, 93, 1421–1430. [Google Scholar] [CrossRef]
- Pumarino, L.; Alomar, O.; Agusti, N. Development of Specific ITS Markers for Plant DNA Identification within Herbivorous Insects. Bull. Entomol. Res. 2011, 101, 271–276. [Google Scholar] [CrossRef]
- Gou, Y.; Quandahor, P.; Zhang, Y.; Liu, C. Effects of Host Plants Nutrient on the Nutrient in Bradysia cellarum and Bradysia impatiens. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.K.; Pathania, V.S.; Thakur, S. Effect of Plant Nutrition in Insect Pest Management: A Review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]

| Plant | Temperature | Half-Life (h) | 95% Fiducial Limits | 84% Fiducial Limits |
|---|---|---|---|---|
| Cowpea | 16 °C | 19.8 ab | 3.7–35.9 | 8.3–31.3 |
| 26 °C | 29.6 a | 17.7–41.4 | 21.1–38.1 | |
| 32 °C | 14.4 ab | 4.1–24.6 | 7.0–21.7 | |
| Mango | 16 °C | 28.0 a | 15.2–40.8 | 18.9–37.1 |
| 26 °C | 52.3 c | 27.8–76.7 | 34.7–69.8 | |
| 32 °C | 8.0 b | 0.1–15.9 | 2.4–13.7 | |
| Pepper | 16 °C | 26.8 a | 9.8–43.7 | 14.6–38.9 |
| 26 °C | 22.0 ab | 6.2–37.8 | 10.7–33.4 | |
| 32 °C | 9.4 b | 5.2–13.6 | 6.4–12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Han, D.; Wen, J.; Liang, C.; Zhan, C.; You, Y.; Fu, Y.; Li, L.; Ye, Z. Influence of Temperature and Host Plant on the Digestion of Frankliniella intonsa (Trybom) Revealed by Molecular Detection. Insects 2024, 15, 806. https://doi.org/10.3390/insects15100806
Yang K, Han D, Wen J, Liang C, Zhan C, You Y, Fu Y, Li L, Ye Z. Influence of Temperature and Host Plant on the Digestion of Frankliniella intonsa (Trybom) Revealed by Molecular Detection. Insects. 2024; 15(10):806. https://doi.org/10.3390/insects15100806
Chicago/Turabian StyleYang, Keqing, Dongyin Han, Jian Wen, Changshou Liang, Canlan Zhan, Yiyangyang You, Yueguan Fu, Lei Li, and Zhengpei Ye. 2024. "Influence of Temperature and Host Plant on the Digestion of Frankliniella intonsa (Trybom) Revealed by Molecular Detection" Insects 15, no. 10: 806. https://doi.org/10.3390/insects15100806
APA StyleYang, K., Han, D., Wen, J., Liang, C., Zhan, C., You, Y., Fu, Y., Li, L., & Ye, Z. (2024). Influence of Temperature and Host Plant on the Digestion of Frankliniella intonsa (Trybom) Revealed by Molecular Detection. Insects, 15(10), 806. https://doi.org/10.3390/insects15100806






