The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Scanning Electron Microscopy
2.3. Image Processing and Data Analysis
3. Results
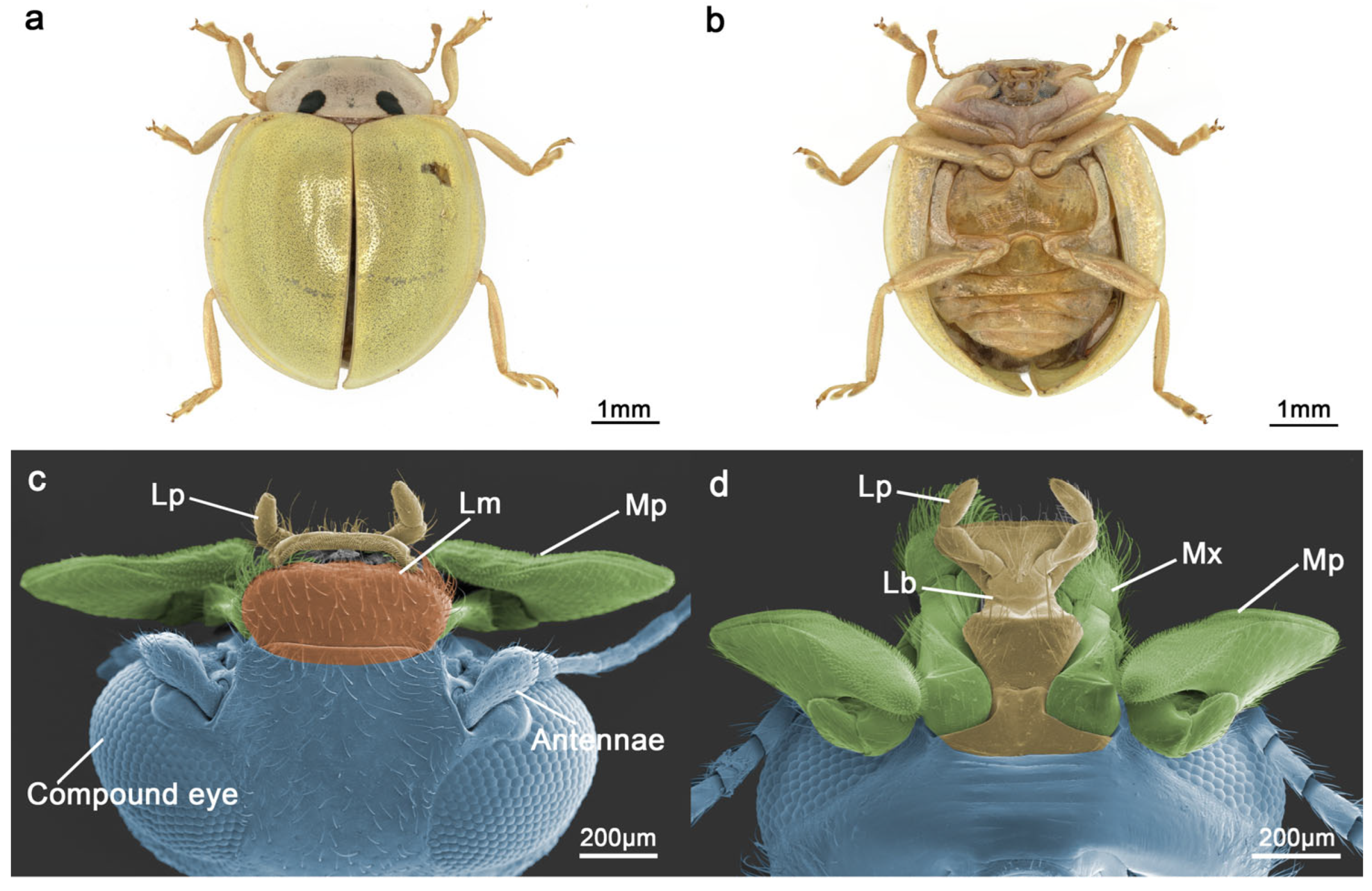
3.1. Gross Morphology of the Mouthparts
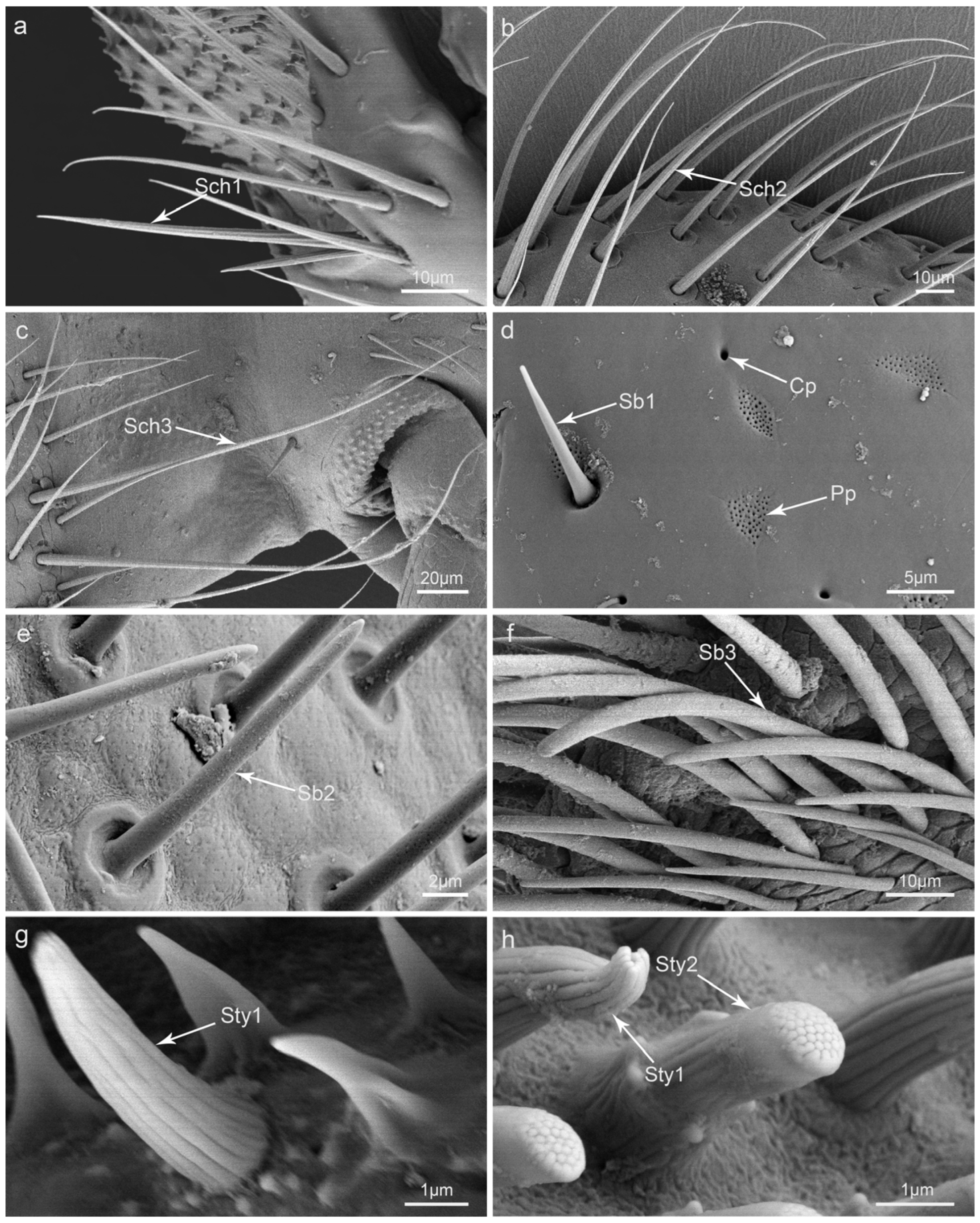
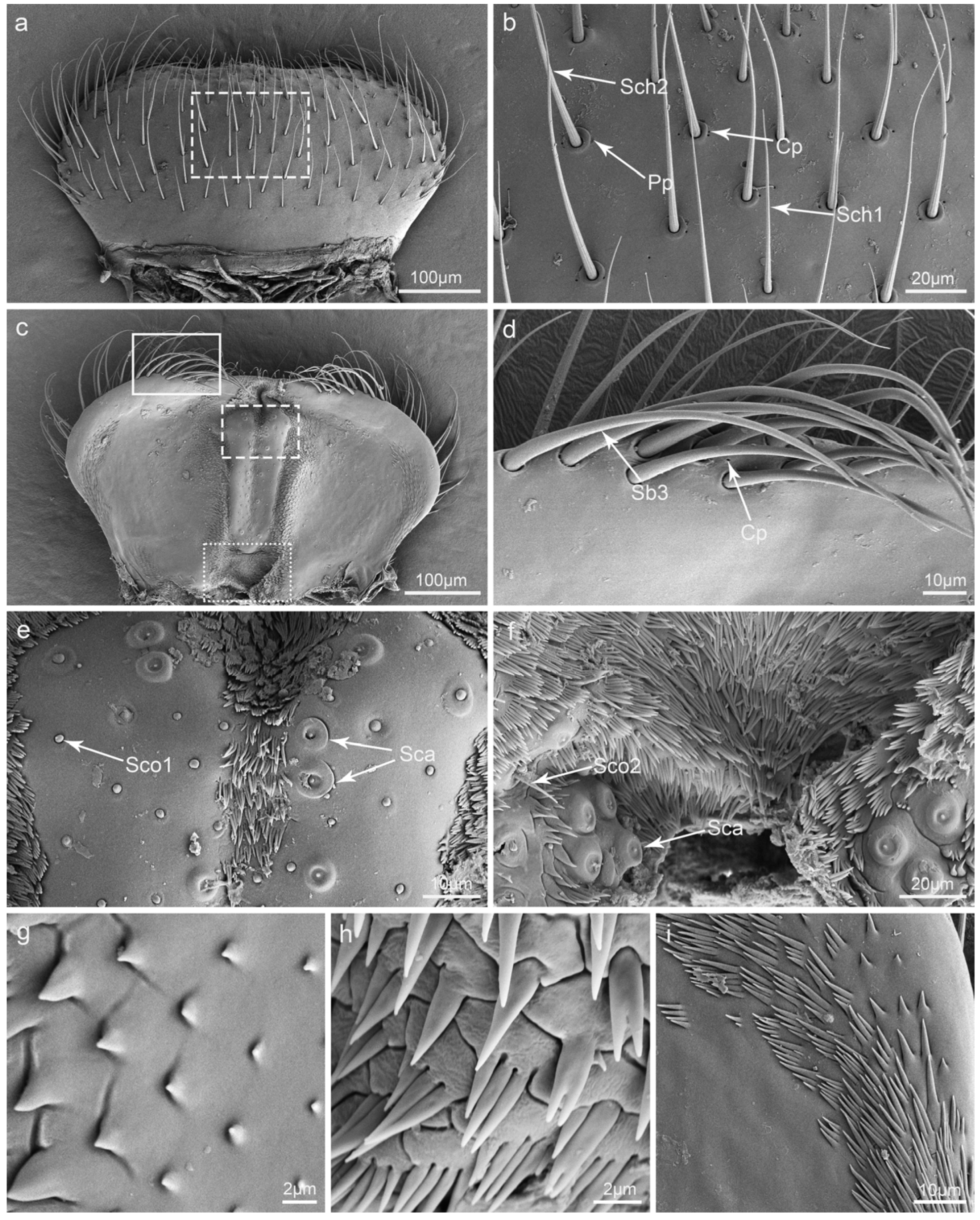
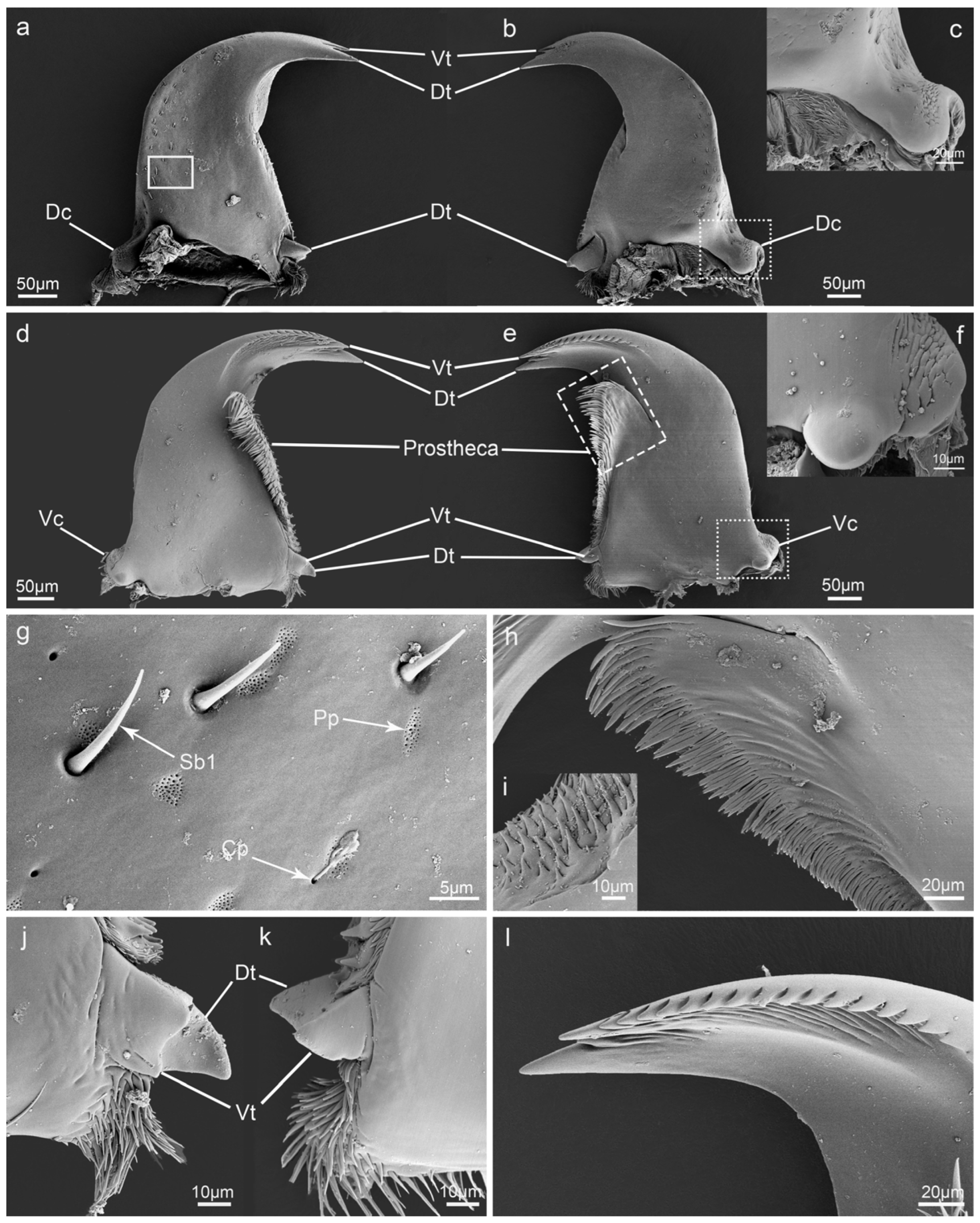
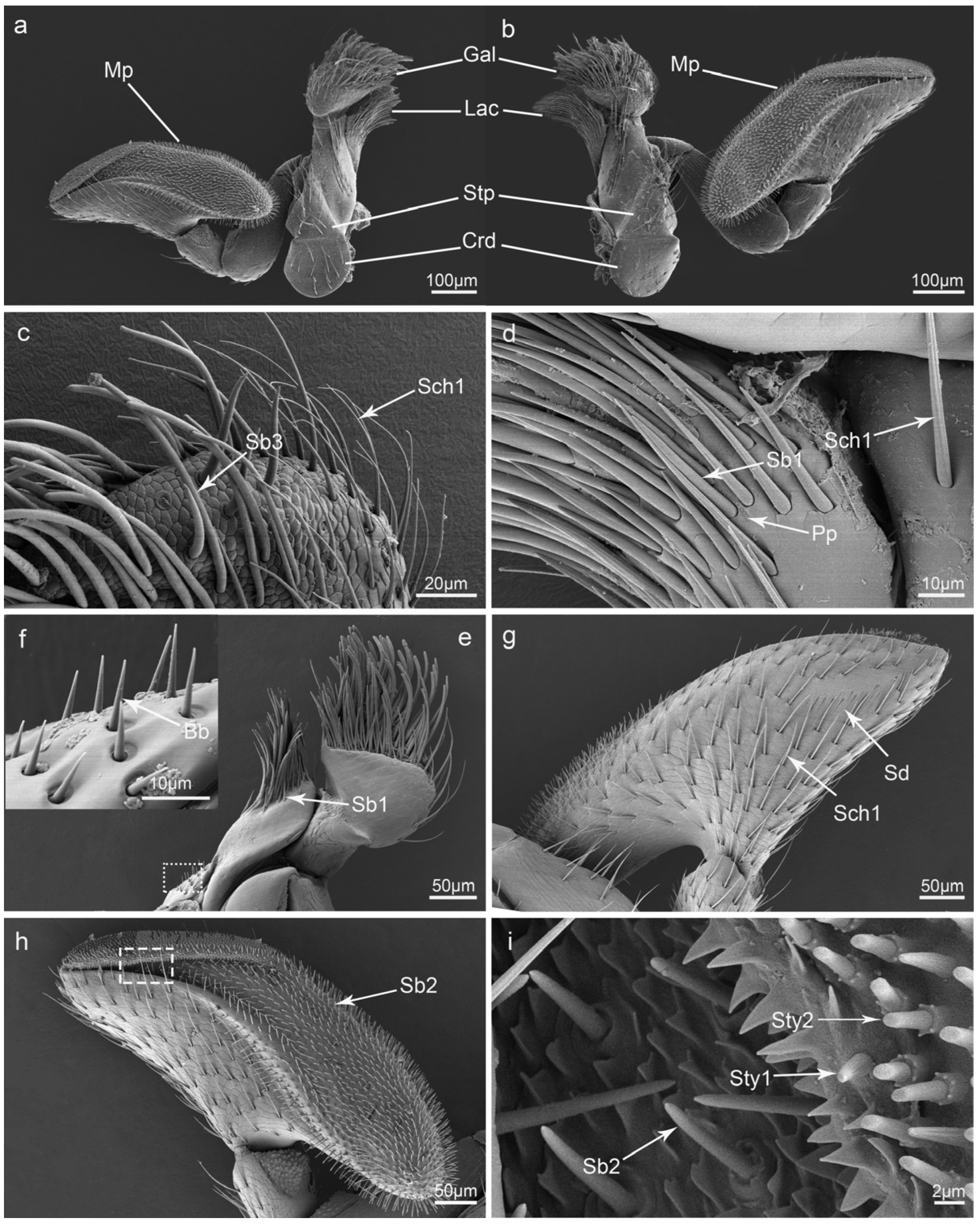
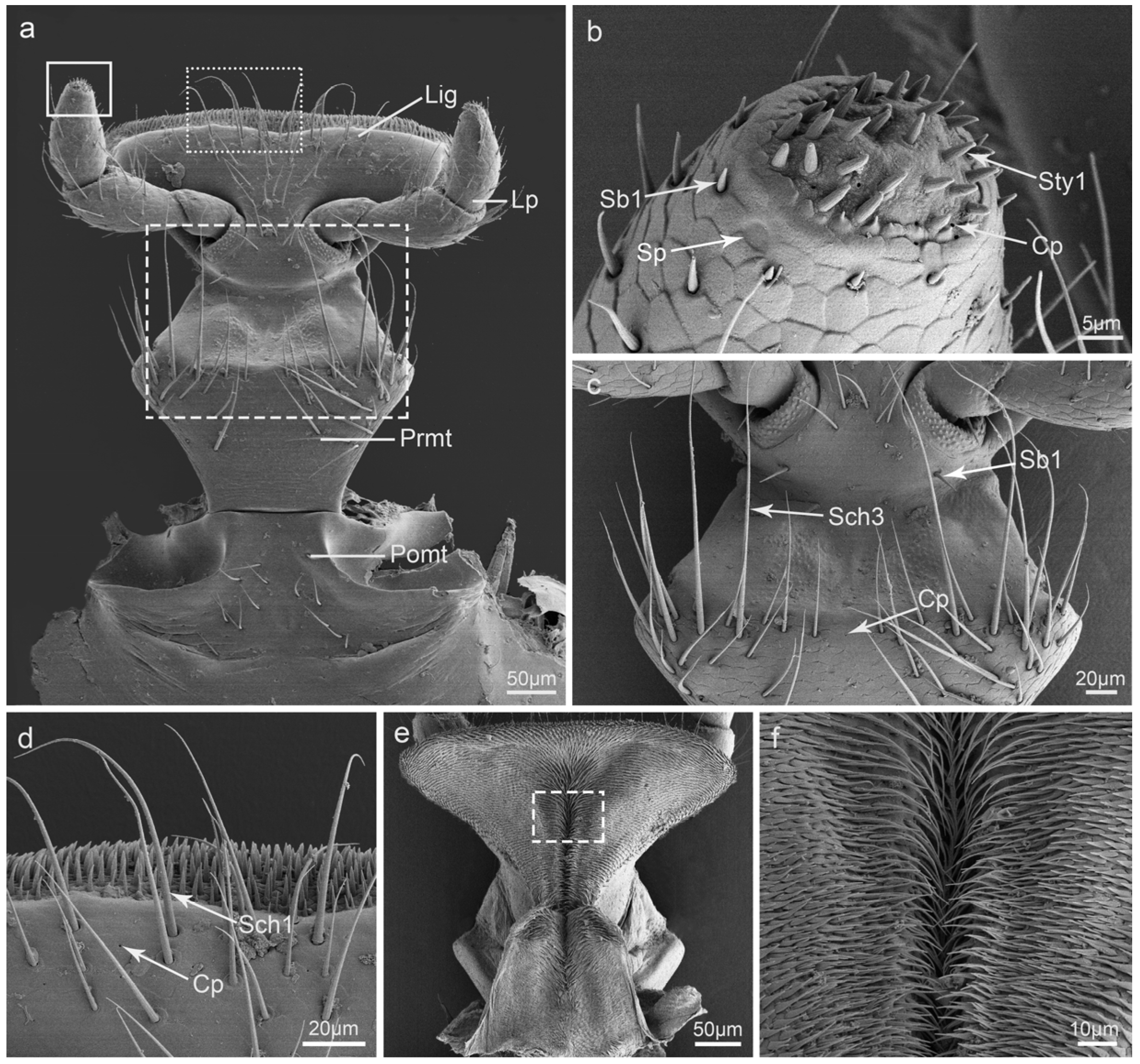
3.2. Types and Morphology of the Sensilla on the Mouthparts
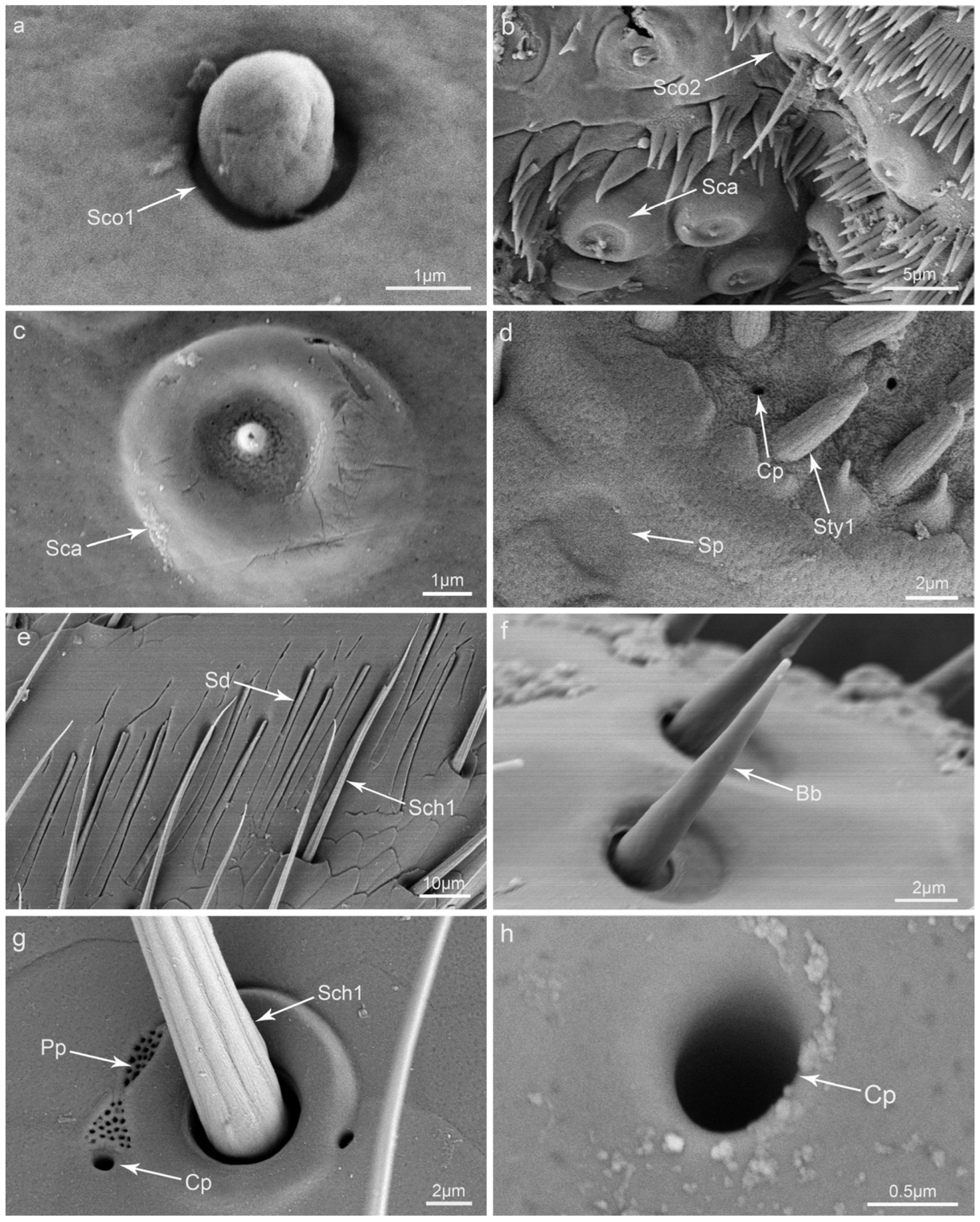
3.3. Glandular Structures on the Mouthparts
3.4. Labrum
3.5. Mandible
3.6. Maxillae
3.7. Labium
4. Discussion
4.1. Variation of Mouthpart Morphology between Different Diets
4.2. The Putative Function of the Sensilla
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betz, O.; Thayer, M.K.; Newton, A.F. Comparative morphology and evolutionary pathways of the mouthparts in spore-feeding Staphylinoidea (Coleoptera). Acta Zool. 2003, 84, 179–238. [Google Scholar] [CrossRef]
- Samways, M.J.; Osborn, R.; Saunders, T.S. Mandibles form relative to the main food type in ladybirds (Coleoptera: Coccinellidae). Biocontrol Sci. Technol. 1997, 7, 275–286. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Coccinella transversoguttata (Coccinellidae, Coleoptera), with reference to their feeding mechanism. J. Morphol. 2019, 280, 701–711. [Google Scholar] [CrossRef]
- Brożek, J.; Bourgoin, T. Morphology and distribution of the external labial sensilla in Fulgoromorpha (Insecta: Hemiptera). Zoomorphology 2013, 132, 33–65. [Google Scholar] [CrossRef]
- Ma, N.; Huang, J.; Hua, B.Z. Functional Morphology and Sexual Dimorphism of Mouthparts of the Short-Faced Scorpionfly Panorpodes kuandianensis (Mecoptera: Panorpodidae). PLoS ONE 2013, 8, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Karolyi, F.; Hansal, T.; Krenn, H.W.; Colville, J.F. Comparative morphology of the mouthparts of the megadiverse South African monkey beetles (Scarabaeidae: Hopliini): Feeding adaptations and guild structure. PeerJ 2016, 4, e1597. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.D.; Bahia, A.C.; Secundino, N.F.C.; Pimenta, P.F.P. Ultrastructural Analysis of Mouthparts of Adult Horn Fly (Diptera: Muscidae) from the Brazilian Midwest Region. J. Med. Entomol. 2020, 5, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. External morphology of antennal and rostral sensillae in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Hao, Y.N.; Dietrich, C.H.; Dai, W. Development of mouthparts in the cicada Meimuna mongolica (Distant): Successive morphological patterning and sensilla differentiation from nymph to adult. Sci. Rep. 2016, 1, 38151. [Google Scholar] [CrossRef]
- Pervez, A.; Yadav, M.; Bozdoğan, H. Antennal morphology and sensilla of the predaceous ladybirds, Menochilus sexmaculatus and Propylea dissecta. Eur. J. Environ. Sci. 2020, 10, 124–132. [Google Scholar] [CrossRef]
- Pervez, A. Antennal sensilla of an aphidophagous ladybird, Propylea dissecta. J. Appl. Biosci. 2008, 34, 168–171. [Google Scholar]
- Pervez, A.; Yadav, M.; Bozdoğan, H. Functional morphology of mouthparts and antennal sensillae of two co-generic aphidophagous ladybirds. Int. J. Trop. Insect Sci. 2022, 42, 2531–2546. [Google Scholar] [CrossRef]
- Cao, Y.K.; Huang, M. A SEM study of the antenna and mouthparts of Omosita colon (Linnaeus) (Coleoptera: Nitidulidae). Microsc. Res. Tech. 2016, 79, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Higgins, R.A.; Ramaswamy, S.B. Antennal morphology of the soybean Stemborer Dectes texanus texanus LeConte (Coleoptera: Cerambycidae). J. Kansas. Entomol. Soc. 2003, 76, 397–405. [Google Scholar]
- Faucheux, M.J. Mouthpart sensilla of the adult yellow longicorn beetle Phoracantha recurva Newman, 1840 (Coleoptera, Cerambycidae, Cerambycinae). Bull. Inst. Sci. Rabat Sect. Sci. Vie. 2013, 35, 83–94. [Google Scholar]
- Liu, C.T.; Tong, X. Functional morphology of the mouthparts of longhorn beetle adult Psacothea hilaris (Coleoptera:Cerambycidae) and sensilla comparisons betwen the sexes. Arthropod. Struct. Dev. 2023, 77, 101312. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Luik, A.; Rahi, M.; Sammelselg, V. Antennal sensilla of the ground beetle Platynus dorsalis (Pontoppidan, 1763) (Coleoptera: Carabidae). Microsc. Res. Techniq. 2001, 55, 339–349. [Google Scholar] [CrossRef]
- Bartlet, E.; Romani, R.; Williams, I.H.; Isidoro, N. Functional anatomy of sensory structures on the antennae of Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). Int. J. Insect Morphol. Embryol. 1999, 28, 291–300. [Google Scholar] [CrossRef]
- Minelli, A.; Pasqual, C. The Mouthparts of Ladybirds: Structure and Function. Ital. J. Zool. 1977, 44, 183–187. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Hippodamia variegata (Coleoptera: Coccinellidae), with reference to their feeding mechanism. Zoomorphology 2020, 139, 199–212. [Google Scholar] [CrossRef]
- Ren, S.X.; Wang, X.M.; Pang, H.; Peng, Z.Q.; Zeng, T. Colored Pictorial Handbook of Ladybird Beetles in China; Beijing Science Press: Beijing, China, 2009. [Google Scholar]
- Wu, X.B.; Guo, X.L. Primary study on control of powdery by Ladybugs. J. Northeast. For. Univ. 1987, 15, 13–17. [Google Scholar]
- Guo, X.L.; Wu, X.B. Utility value and breeding of the fungivorous ladybugs. J. Gansu. Agric. Univ. 1990, 4, 394–400. [Google Scholar]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-,thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Abd El Ghany, N.M.; Abd El Aziz, S.E. Morphology of antennae and mouthpart sensillae in Lasioderma serricorne (fabricius) (Coleoptera: Anobiidae). J. Stored. Prod. Res. 2021, 90, 101754. [Google Scholar] [CrossRef]
- Arriaga-Varela, E.; Tomaszewska, W.; Huo, L.Z.; Seidel, M. On Neotropical Merophysiinae with descriptions of a new genus and new species (Coleoptera: Endomychidae). ZooKeys 2018, 736, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Chen, L.Y.; Liu, M.K.; Wang, W.K.; Sabatelli, S.; Di Giulio, A.; Audisio, P. Scanning Electron Microscope Study of Antennae and Mouthparts in the Pollen-Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects 2021, 12, 659. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, S.F.; Liu, F.; Zhang, Z.; Xu, F.Y.; Yin, S.Y.; Kong, X.B. Sensilla on antennae and mouthparts of adult spruce bark beetle Ips typographus (Coleoptera: Curculionidae). Microsc. Res. Tech. 2021, 84, 1484–1497. [Google Scholar] [CrossRef]
- Mayer, G.; Huag, J.T.; Maas, A.; Waloszek, D. Functional aspects of the gammaridean mandibles with special reference to the lacinia mobilis (Crustacea, Amphipoda). Zool. Anz. 2013, 252, 536–547. [Google Scholar] [CrossRef]
- Richter, S.; Edgecombe, G.D.; Wilson, G.D.F. The Lacinia mobilis and Similar Structures—A Valuable Character in Arthropod Phylogenetics? Zool. Anz. 2002, 241, 339–361. [Google Scholar] [CrossRef]
- Ricci, C.; Stella, J. Relationship between morphology and function in some Palaearctic Coccinellidae. In Ecology and Effectiveness of Aphidophaga; Dixon, A.F.G., Ed.; Academic Publishing: The Hague, The Netherlands, 1988; pp. 21–25. [Google Scholar]
- Cai, C.Y.; Newton, A.F.; Thayer, M.K.; Leschen, R.A.B.; Huang, D. Specialized proteinine rove beetles shed light on insect—Fungal associations in the Cretaceous. Proc. R. Soc. 2016, 283, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Youn, Y.N. The Asian ladybird, Harmonia axyridis, as biological control agents: I. Predacious behavior and feeding ability. Korean J. Appl. Entomol. 2000, 39, 59–71. [Google Scholar]
- Wang, J.Z.; Wang, Y.; Sun, S.L. Sequence of predatory behavior of the adult lady bird beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae) on aphids. Entomol. Knowl. 2000, 37, 195–197. [Google Scholar]
- Barbier, R.; Ferran, A.; Lelannic, J.; Lestrat, A. The fine-structure and function of the sensory organs on the maxillary palps of adult ladybird Semiadalia undecimnotata Schn. (Coleoptera, Coccinellidae). Bull. Soc. Zool. Fr. 1989, 114, 119–128. [Google Scholar]
- Baker, G.T.; Monroe, W.A. Sensory receptors on the adult labial and maxillary palpi and galea of Cicindela sexguttata (Coleoptera: Cicindelidae). J. Morphol. 1995, 226, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Zhu, X.; Qiao, H.L.; Liu, S.; Xu, C.Q.; Xu, R.; Zhan, W.H.; Li, J.L.; Guo, K.; Chen, J. Ultrastructure of sensilla on the maxillary and labial palps of the adult Xylotrechus grayii (Coleoptera: Cerambycidae). Microsc. Res. Techniq. 2018, 81, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo-and hygroreceptors. Ann. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Eilers, E.J.; Talarico, G.; Hansson, B.S.; Hilker, M.; Reinecke, A. Sensing the underground–ultrastructure and function of sensoryorgans in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PLoS ONE 2012, 7, e41357. [Google Scholar] [CrossRef]
- Honomichl, K.; Guse, G.W. Digitiform sensilla on the maxillary palp of Coleoptera. Acte. Zool. 1981, 62, 17–25. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Albert, P.J.; Bellamy, F.W. Ultrastructure and function of digitiform sensilla on the labial palp of a larval elaterid (Coleoptera). Can. J. Zool. 1977, 55, 569–578. [Google Scholar] [CrossRef]
- Ortloff, A.; Zanetti, N.; Centeno, N.; Silva, R.; Bustamante, F.; Olave, Á. Ultramorphological characteristics of mature larvae of Nitidula carnaria (Schaller 1783) (Coleoptera: Nitidulidae), a beetle species of forensic importance. Forensic Sci. Int. 2014, 239, e1–e9. [Google Scholar] [CrossRef]
- Sevarika, M.; Rondoni, G.; Conti, E.; Romani, R. Antennal sensory organs and glands of the harlequin ladybird, Harmonia axyridis. Entomol. Exp. Appl. 2021, 169, 111–124. [Google Scholar] [CrossRef]
- Skilbeck, C.A.; Anderson, M. The fine structure of glandular units on the antennae of two species of the parasitoid, Aleochara (Coleoptera: Staphylinidae). Int. J. Ins. Morph. Embryol. 1994, 23, 319–328. [Google Scholar] [CrossRef]
- Di Giulio, A.; Rossi Stacconi, M.V.; Romani, R. Fine structure of the antennal glands of the ant nest beetle Paussus favieri (Coleoptera, Carabidae, Paussini). Arthr. Struct. Dev. 2009, 38, 293–302. [Google Scholar] [CrossRef]
- Faucheux, M.J.; Kundrata, R. Perforated plates corresponding to integumental glands on the antennae of adult male Drilus mauritanicus Lucas 1849 (Coleoptera: Elateridae: Agrypninae: Drilini). Ann. Société Entomol. Fr. (N.S.) 2015, 1, 1–3. [Google Scholar]
- Leung, E.S.; Zacharuk, R.Y. Fine structure of integumental glands in the labial palp of adult Graphoderus occidentalis Horn (Coleoptera: Dytiscidae). Can. J. Zool. 1986, 64, 2788–2800. [Google Scholar] [CrossRef]
- Drilling, K.; Dettner, K.; Klass, K.D. The distribution and evolution of exocrine compounds glands in Erotylinae (Insecta: Coleoptera: Erotylidae). Ann. Société Entomol. Fr. 2013, 1, 36–52. [Google Scholar] [CrossRef]
- Yan, F.S.; Qin, J.D.; Xiang, X.F. The chemoreceptors on the maxillary palps of the adult lady bird beetle Coccinella septempunctata. Acta Entomol. Sinica 1987, 30, 146–158. [Google Scholar]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Faucheux, M.J. Antennal sensilla of Pachychila (Pachychila) impunctata Fairmaire, 1860 (Coleoptera: Tenebrionidae: Pimeliinae: Tentyriini). Bull. Soc. Sci. Nat. L’ouest Fr. 2023, 45, 54–62. [Google Scholar]
- Liu, C.T.; Tong, X. Morphological comparison of the sensilla on the maxillary and labial palps between male and female adults of Moechotypa diphysis (Coleoptera: Cerambycidae). Microsc. Res. Techniq. 2023, 9, 1079–1090. [Google Scholar] [CrossRef]
- Altner, H.; Routil, C.; Loftus, R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981, 215, 289–308. [Google Scholar] [CrossRef]
- Ren, L.L.; Wu, Y.; Shi, J.; Zhang, L.; Luo, Y.Q. Antenna morphology and sensilla ultrastructure of Tetrigus lewisi Candèze (Coleoptera: Elateridae). Micron 2014, 60, 29–38. [Google Scholar] [CrossRef]
- Mclver, S.B. Mechanoreception. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 71–132. [Google Scholar]
- Xu, L.; Zhang, L.; Yang, Y.C.; Ren, L.L.; Wang, T.; Zong, S.X. Morphology of antennal, maxillary palp and labial palp sensilla in different larval instars of the asian long-horned beetle Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). Acti. Zool. 2015, 1, 20–31. [Google Scholar] [CrossRef]
- Faucheux, M.J. The tactile antennae with an olfactive apex of Adelostoma sulcatum Duponchel, 1827 (Coleoptera: Tenebrionidae: Pimeliinae: Adelostomini): Characteristics in liaison with the life in termite nests. Bulletin de l’Institut Scientifique, Rabat, Section Sciences de la Vie 2023, 45, 55–65. [Google Scholar]
| Type | Shape | Socket | Surface | Pore | Length (μm) | Diameter (μm) | Distribution | |
|---|---|---|---|---|---|---|---|---|
| Sensilla | Sch1 | Peg | Convex | Grooved | No | 52.8–60.2 | 2.2–3.6 | Lm, Md, Mx, Lb |
| Sch2 | Hair, peg | Convex | Grooved | No | 95.2–110.0 | 3.4–5.6 | Lm, Mx | |
| Sch3 | Hair, peg | Convex | Grooved | No | 91.4–193.0 | 3.1–5.2 | Mx, Lb | |
| Sb1 | Coniform | Concave | Smooth | No | 2.3–12.2 | 1.4–2.6 | Md, Mx, Lp | |
| Sb2 | Coniform | Concave | Smooth | No | 15.3–29.7 | 1.6–2.6 | Mp | |
| Sb3 | Hair, cylindrical | Concave | Smooth | No | 68.2–105.8 | 3.4–5.0 | Epi, Gal | |
| Sty1 | Conical | Convex | Grooved | Apical pore | 3.6–5.0 | 1.3–1.7 | Mp, Lp | |
| Sty2 | Cylindrical | Convex | Grooved | Apical pore | 2.6–4.2 | 1.5–1.8 | Mp | |
| Sco1 | Coniform | Convex | Smooth | No | – | 1.5–2.4 | Epi | |
| Sco2 | Coniform | Convex | Rugose | No | 5.7–11.4 | 4.2–6.0 | Epi | |
| Sca | Round | Convex | Papilliform | Multiporous | – | 4.4–6.6 | Epi | |
| Sp | Round | Concave | Smooth | No | – | 1.1–3.2 | Lp | |
| Sd | Finger | Concave | Smooth | No | 31.5–39.9 | 1.8–2.3 | Mp | |
| Bb | Conical | Concave | Smooth | No | 7.0–11.1 | 1.1–1.8 | Lac | |
| Glandular structures | Pp | Irregular | Concave | No | Multiporous | - | 1.6–5.1 | Lm, Md, Mx, Lb |
| Cp | Hole | Concave | - | Uniporous | - | 0.4–0.7 | Lm, Md, Mx, Lb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Lu, Y.; Bai, M.; Sun, Y.; Hao, Y. The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae). Insects 2024, 15, 46. https://doi.org/10.3390/insects15010046
Wang K, Lu Y, Bai M, Sun Y, Hao Y. The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae). Insects. 2024; 15(1):46. https://doi.org/10.3390/insects15010046
Chicago/Turabian StyleWang, Ke, Yuanyuan Lu, Ming Bai, Yuanxing Sun, and Yanan Hao. 2024. "The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae)" Insects 15, no. 1: 46. https://doi.org/10.3390/insects15010046
APA StyleWang, K., Lu, Y., Bai, M., Sun, Y., & Hao, Y. (2024). The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae). Insects, 15(1), 46. https://doi.org/10.3390/insects15010046







