Morphology and Morphometry of the Reproductive Tract of the Cotton Boll Weevil after Prolonged Feeding on Alternative Diets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Insects
2.2. Experimental Design
2.3. Morphological and Morphometric Data
2.4. Data Analysis
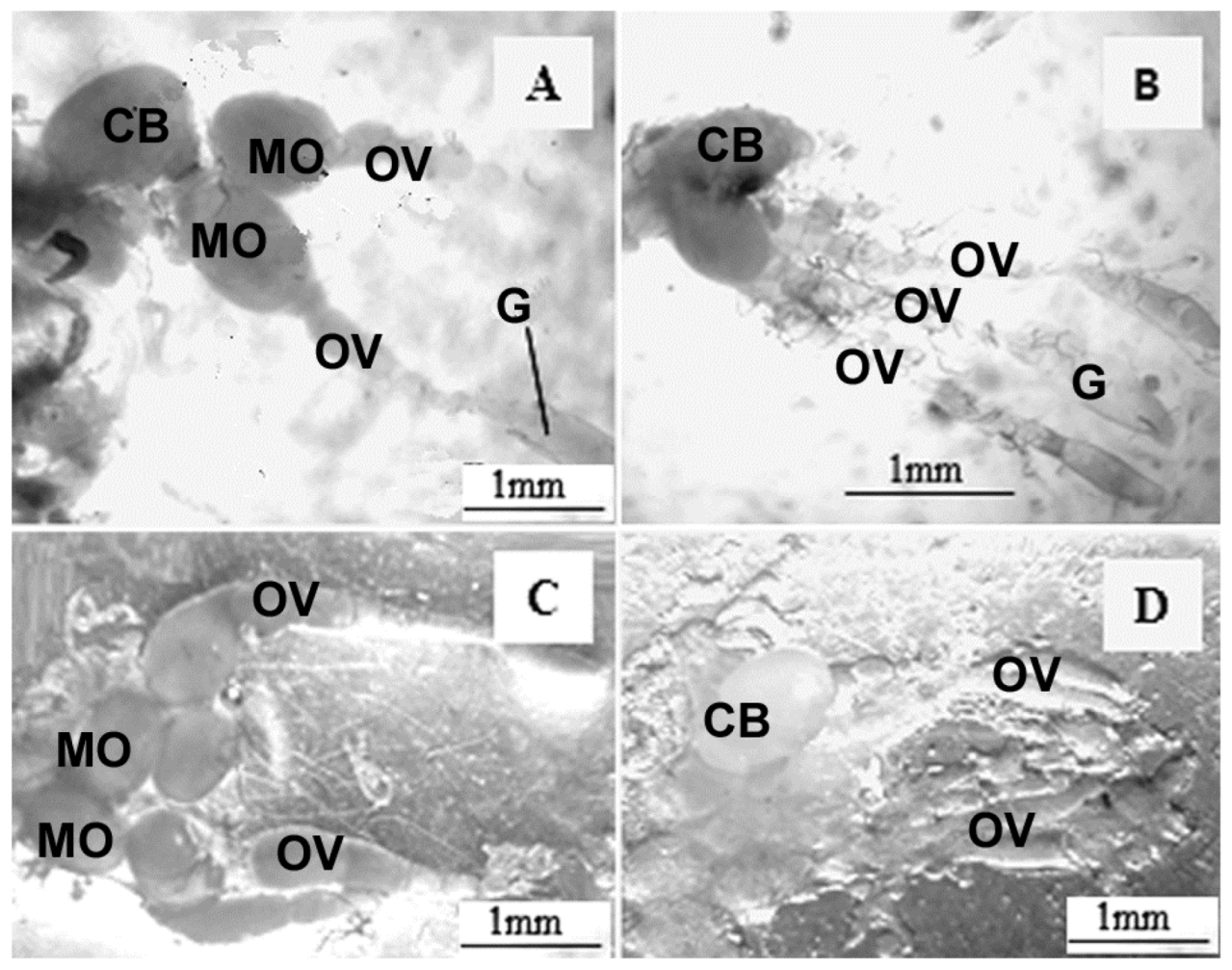
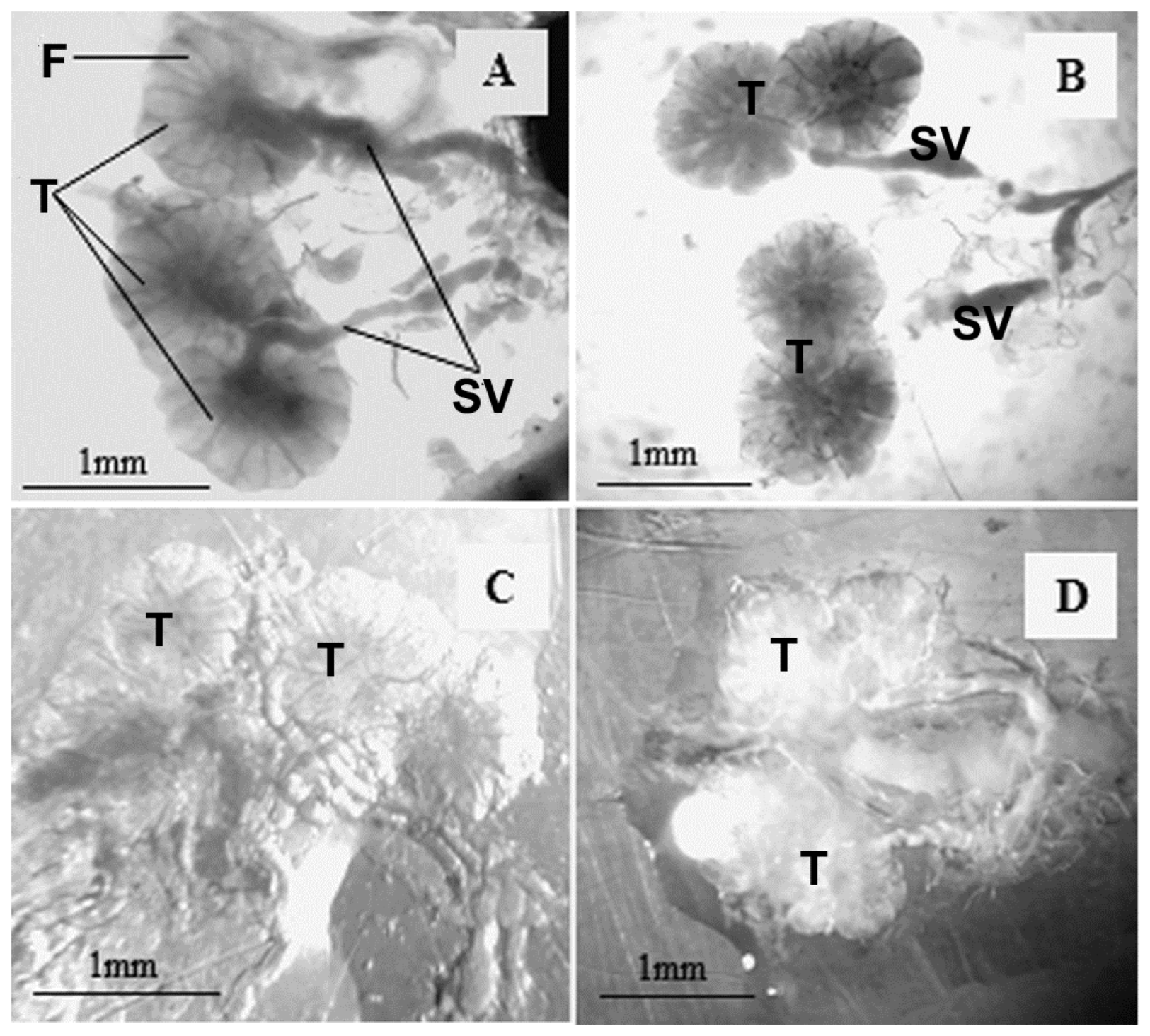
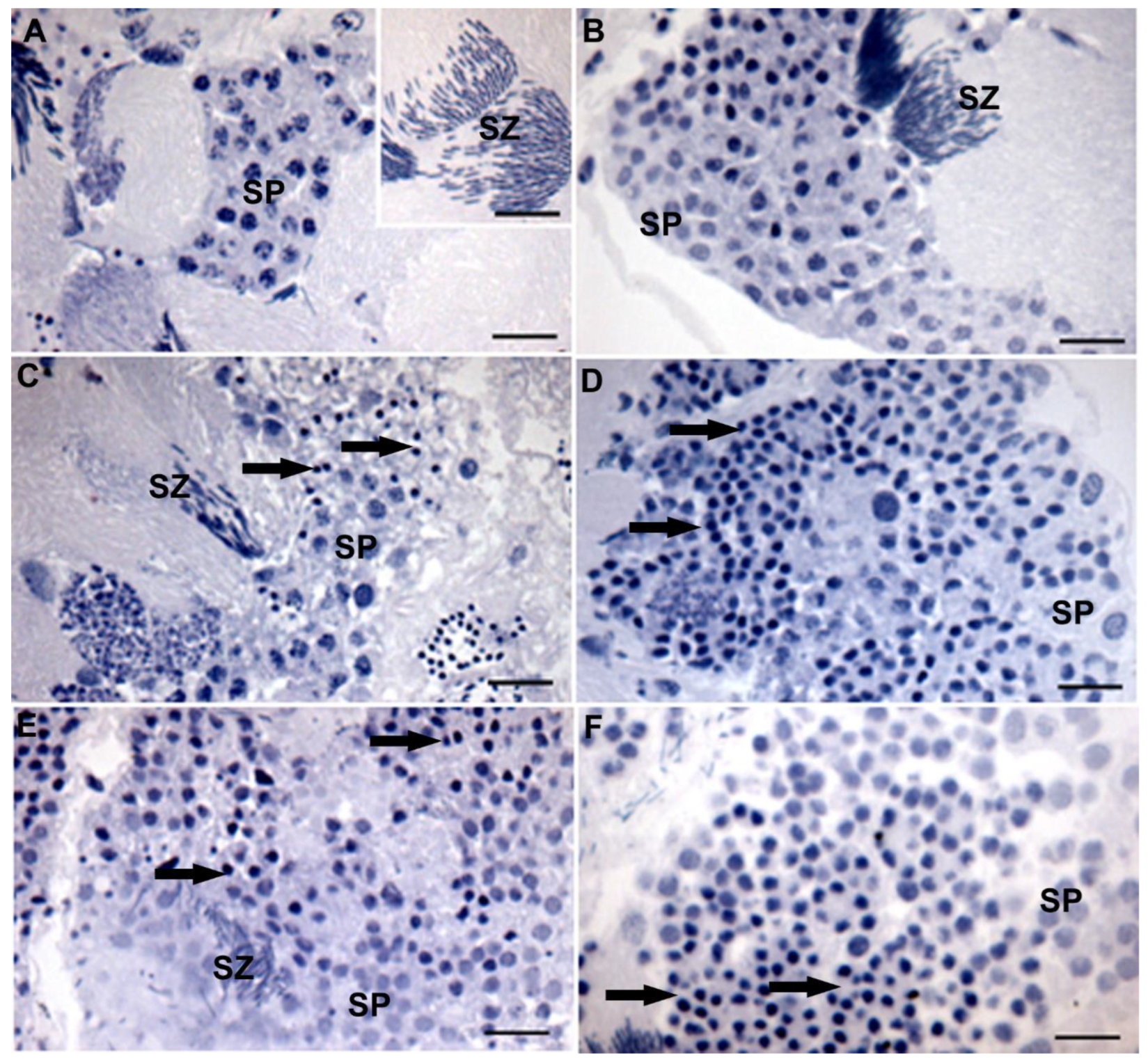
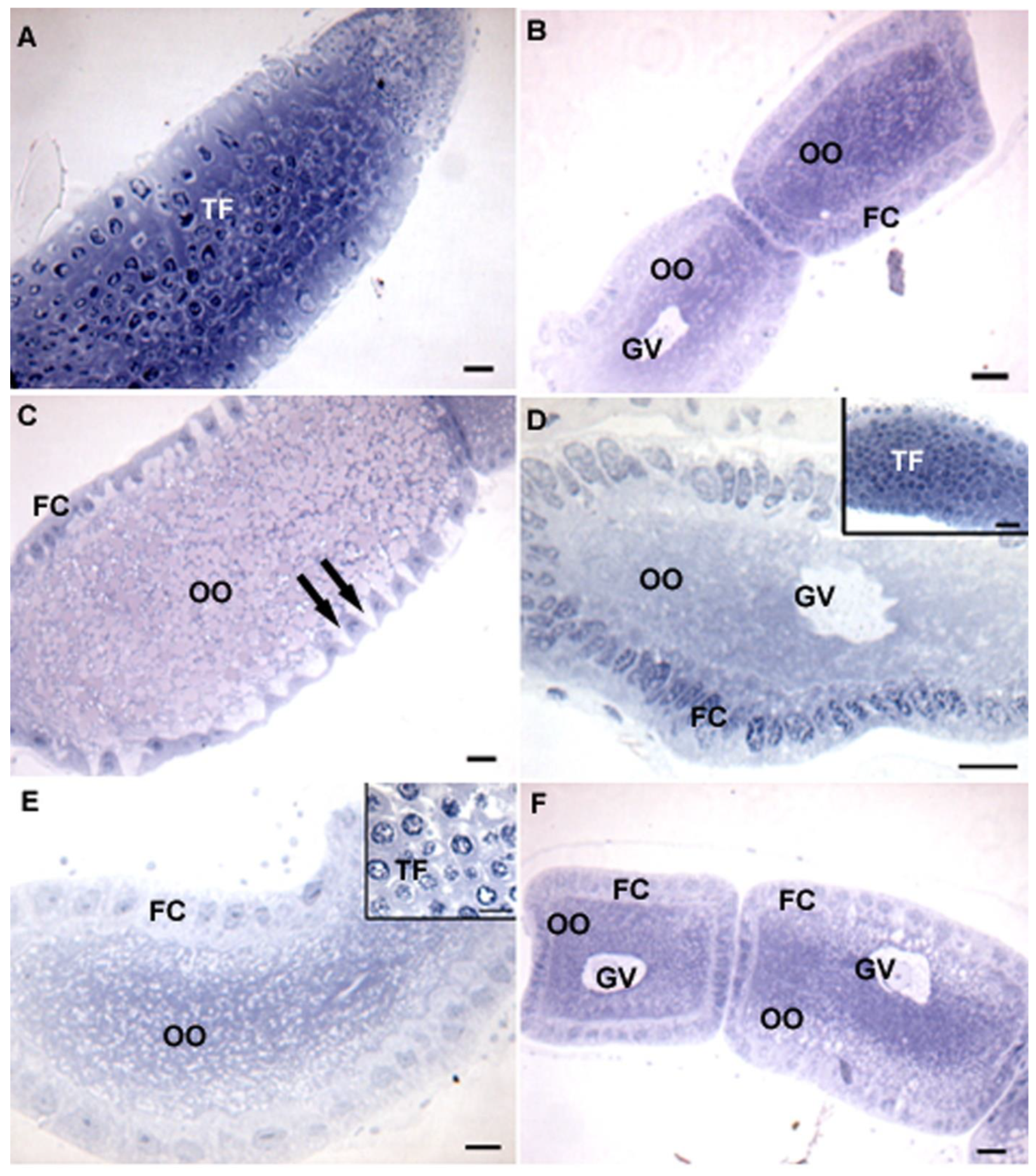
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, C.A.D.; Ramalho, F.S.; Miranda, J.E.; Almeida, R.P.; Rodrigues, S.M.M.; Albuquerque, F.A. Sugestões Técnicas para o Manejo Integrado de Pragas do Algodoeiro no Brasil; Circular Técnica No. 135; Embrapa Algodão: Campina Grande, Brazil, 2013; 12p. [Google Scholar]
- Hinds, W.E.; Yothers, W.W. Hibernation of the Mexican cotton boll weevil. USDA Bur. Entomol. Bull. 1909, 77, 1–100. [Google Scholar]
- Gaines, R.C. Ecological Investigations of the Boll Weevil, Tallulah, Louisiana, 1915–1959; Technical Bulletin No. 1208; USDA: Washington, DC, USA, 1950; pp. 1915–1958.
- Carroll, S.C.; Rummel, D.R.; Segarra, E. Overwintering by the boll weevil (Coleoptera: Curculionidae) in conservation reserve program grasses on the Texas High Plains. J. Econ. Entomol. 1993, 86, 382–393. [Google Scholar] [CrossRef]
- Spurgeon, D.W. Seasonal patterns of host-free survival of the boll weevil (Coleoptera: Curculionidae) in the subtropics. J. Entomol. Sci. 2008, 43, 13–26. [Google Scholar] [CrossRef]
- Ramalho, F.S.; Wanderley, P.A. Ecology and management of the boll weevil in South American cotton. Am. Entomol. 1996, 42, 41–47. [Google Scholar] [CrossRef]
- Gabriel, D. Longevidade do bicudo do algodoeiro Anthonomus grandis Boh., criado em hospedeiros alternativos no laboratório. Arq. Do Inst. Biológico 2002, 69, 123–126. [Google Scholar]
- Ribeiro, P.A.; Sujii, E.R.; Diniz, I.R.; Medeiros, M.A.; Salgado-Laboriau, M.L.; Branco, M.C.; Pires, C.S.S.; Fontes, E.M.G. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae) under the tropical conditions of Central Brazil. Neotrop. Entomol. 2010, 39, 28–34. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Jones, G.D.; Eischen, F.; Coleman, R.J.; Adamczyk, J.J., Jr.; Liu, T.-X.; Setamou, M. Survival of boll weevil (Coleoptera: Curculionidae) adults after feeding on pollens from various sources. Insect Sci. 2007, 14, 503–510. [Google Scholar] [CrossRef]
- Showler, A.T.; Abrigo, V. Common subtropical and tropical nonpollen food sources of the boll weevil (Coleoptera: Curculionidae). Environ. Entomol. 2007, 36, 99–104. [Google Scholar] [CrossRef]
- Showler, A.T. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. Am. Entomol. 2009, 55, 234–242. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Raulston, J.R. Boll weevil (Coleoptera: Curculionidae) reproductive development as a function of temperature. Environ. Entomol. 1998, 27, 675–681. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Sappington, T.W.; Suh, C.P.-C. A system for characterizing reproductive and diapause morphology in the boll weevil (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 2003, 96, 1–11. [Google Scholar] [CrossRef]
- Paula, D.P.; Claudino, D.; Timbó, R.V.; Miranda, J.E.; Bemquerer, M.P.; Ribeiro, A.C.; Sujii, E.R.; Fontes, E.M.G.; Pires, C.S.S. Reproductive dormancy in boll-weevil from populations of the midwest of Brazil. J. Econ. Entomol. 2013, 106, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mendoza, J.; Throne, J.E.; Baker, J.E. Ovarian physiology and age-grading in the rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J. Stored Prod. Res. 2004, 40, 179–196. [Google Scholar] [CrossRef]
- Chang, Y.; Tauber, M.J.; Tauber, C.A. Reproduction and quality of F1 offspring in Chrysoperla carnea: Differential influence of quiescence, artificially-induced diapause, and natural diapause. J. Insect Physiol. 1996, 42, 521–528. [Google Scholar] [CrossRef]
- Wang, X.-P.; Xue, F.-S.; Hua, I.; Feng, G. Effects of diapause duration on future reproduction in the cabbage beetle, Colaphellus bowringi: Positive or negative? Physiol. Entomol. 2006, 31, 190–196. [Google Scholar] [CrossRef]
- Guerra, A.A.; Garcia, R.D.; Tamayo, J.A. Physiological activity of the boll weevil during the fall and winter in subtropical areas of the Rio Grande Valley of Texas. J. Econ. Entomol. 1982, 75, 11–15. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P.-C. Termination of diapause in the boll weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 633–643. [Google Scholar] [CrossRef]
- Tatar, M. Reproductive aging in invertebrate genetic models. Ann. N. Y. Acad. Sci. 2010, 1204, 149–155. [Google Scholar] [CrossRef]
- Flurkey, K.; Harrison, D.E. Reproductive ageing: Of worms and women. Nature 2010, 468, 386–387. [Google Scholar] [CrossRef]
- Fay, R.; Barbraud, C.; Delord, K.; Weimerskirch, H. Paternal but not maternal age influences early-life performance of offspring in a long-lived seabird. Proc. R. Soc. B Biol. Sci. 2016, 283, 2015–2318. [Google Scholar] [CrossRef]
- Agee, H.R. Characters for determination of sex of the boll weevil. J. Econ. Entomol. 1964, 57, 500–501. [Google Scholar] [CrossRef]
- Brazzel, J.R.; Newsom, L.D. Diapause in Anthonomus grandis Boh. J. Econ. Entomol. 1959, 52, 603–611. [Google Scholar] [CrossRef]
- Stefanini, M.; Demartino, C.; Zamboni, L. Fixation of ejaculated spermatozoa for electron microscopy. Nature 1967, 216, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Sasa, A.; Gosik, R.; Witkowski, E.T.F.; Byrne, M.J.; Mazur, M.A. Sexual dimorphism in Anthonomus santacruzi (Coleoptera: Curculionidae): A biological control agent of Solanum mauritianum Scopoli (Solanaceae). Neotrop. Entomol. 2020, 49, 840–850. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. B Met. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Suh, C.P.-C.; Spurgeon, D.W. Host-free survival of boll weevils (Coleoptera: Curculionidae) previously fed vegetative-stage regrowth cotton. J. Entomol. Sci. 2006, 41, 277–284. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Sappington, T.W.; Rummel, D.R. Host-free survival of boll weevils (Coleoptera: Curculionidae) from two regions of Texas. Southwest. Entomol. 2008, 33, 151–152. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P.-C. Temperature influences on diapauses induction and survival in the boll weevil (Coleoptera: Curculionidae). J. Insect Sci. 2017, 17, 124. [Google Scholar] [CrossRef]
- Suh, C.P.-C.; Spurgeon, D.W.; Reardon, B.J. Reproductive and survival responses of overwintered boll weevils (Coleoptera: Curculionidae) to diet. J. Entomol. Sci. 2022, 55, 58–68. [Google Scholar] [CrossRef]
- Žďárek, J.; Čtvrtečka, R.; Hovorla, O.; Kostal, V. Activation of gonads and disruption of imaginal diapause in the apple blossom weevil, Anthonomus pomorum (Coleoptera: Curculionidae), with juvenoids in laboratory and fields trials. Eur. J. Entomol. 2000, 97, 25–31. [Google Scholar] [CrossRef]
- Walker, J.K., Jr.; Pickens, L.G. Egg deposition by boll weevils isolated from males during hibernation period and after spring emergence. J. Econ. Entomol. 1962, 55, 268–269. [Google Scholar] [CrossRef]
- Villavaso, E.J. Function of the spermathecal muscle of the boll weevil Anthonomus grandis. J. Insect Physiol. 1975, 21, 1275–1278. [Google Scholar] [CrossRef]
- Villavaso, E.J. The role of the spermathecal gland of the boll weevil Anthonomus grandis. J. Insect Physiol. 1975, 21, 1457–1462. [Google Scholar] [CrossRef]
- Walker, J.K., Jr.; Bottrell, D.G. Infestations of boll weevils in isolated plots of cotton in Texas, 1960–1969. J. Econ. Entomol. 1979, 63, 1646–1650. [Google Scholar] [CrossRef]
- White, J.R.; Rummel, D.R. Emergence profile of overwintered boll weevils and entry into cotton. Environ. Entomol. 1978, 7, 7–14. [Google Scholar] [CrossRef]
| Sex | Reproductive Tract | Variation Source | DF | MS | F | p |
|---|---|---|---|---|---|---|
| Female | Morphological conditions | Diet consumed (DC) | 2 | 1666.67 | 5.00 | 0.01 |
| Boll weevil age (BWA) | 2 | 41,666.67 | 125.00 | <0.01 | ||
| DC × BWA | 4 | 1666.67 | 5.00 | <0.01 | ||
| Residue | 40 | |||||
| Male | Morphological conditions | Diet consumed (DC) | 2 | - | - | n.s. |
| Boll weevil age (BWA) | 2 | - | - | n.s. | ||
| DC × BWA | 4 | - | - | n.s. | ||
| Residue | 40 |
| Sex | Boll Weevil Age (Days) | Treatments | ||
|---|---|---|---|---|
| Cotton Squares | Banana Endocarp | Orange Endocarp | ||
| Female | 30 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA |
| 60 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | |
| 90 | 50.00 ± 22.36 bB | 0.00 ± 0.00 bB | 0.00 ± 0.00 bB | |
| Male | 30 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA |
| 60 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | |
| 90 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | |
| Sex | Morphometric Data | Variation Source | DF | MS | F | p |
|---|---|---|---|---|---|---|
| Female | Weight | Diet consumed (DC) | 2 | 1.41 × 10−5 | 0.46 | >0.05 |
| Boll weevil age (BWA) | 2 | 1.19 × 10−5 | 0.39 | >0.05 | ||
| DC × BWA | 4 | 3.19 × 10−5 | 1.04 | =0.40 | ||
| Residue | 45 | 3.07 × 10−5 | ||||
| Body length | Diet consumed (DC) | 2 | 0.45 × 10−1 | 0.43 | >0.05 | |
| Boll weevil age (BWA) | 2 | 0.92 × 10−1 | 0.87 | >0.05 | ||
| DC × BWA | 4 | 0.70 × 10−1 | 0.66 | >0.05 | ||
| Residue | 45 | 0.11 | ||||
| Ovariole length | Diet consumed (DC) | 2 | 4.44 | 25.11 | <0.01 | |
| Boll weevil age (BWA) | 2 | 0.99 | 5.61 | <0.01 | ||
| DC × BWA | 4 | 0.19 | 1.10 | =0.37 | ||
| Residue | 45 | 0.18 | ||||
| Width of most developed oocyte | Diet consumed (DC) | 2 | 0.39 × 10−1 | 12.67 | <0.01 | |
| Boll weevil age (BWA) | 2 | 0.23 × 10−4 | 0.01 | >0.05 | ||
| DC × BWA | 4 | 0.15 × 10−2 | 0.49 | >0.05 | ||
| Residue | 45 | 0.31 × 10−2 | ||||
| Male | Weight | Diet consumed (DC) | 2 | 0.59 × 10−5 | 2.71 | =0.08 |
| Boll weevil age (BWA) | 2 | 0.19 × 10−5 | 0.86 | >0.05 | ||
| DC × BWA | 4 | 0.51 × 10−5 | 2.35 | =0.07 | ||
| Residue | 45 | 0.22 × 10−5 | ||||
| Body length | Diet consumed (DC) | 2 | 0.48 | 7.09 | <0.01 | |
| Boll weevil age (BWA) | 2 | 0.15 | 2.19 | =0.12 | ||
| DC × BWA | 4 | 0.13 | 1.93 | =0.12 | ||
| Residue | 45 | 0.67 | ||||
| Testis area | Diet consumed (DC) | 2 | 0.47 | 49.34 | <0.01 | |
| Boll weevil age (BWA) | 2 | 0.28 | 29.68 | <0.01 | ||
| DC × BWA | 4 | 0.42 | 0.45 | >0.05 | ||
| Residue | 45 | 0.95 | ||||
| Testis diameter | Diet consumed (DC) | 2 | 0.30 | 39.83 | <0.01 | |
| Boll weevil age (BWA) | 2 | 0.18 | 29.94 | <0.01 | ||
| DC × BWA | 4 | 0.42 | 0.56 | >0.05 | ||
| Residue | 45 | 0.75 |
| Morphometric Data | Treatments | ||
|---|---|---|---|
| Banana Endocarp | Orange Endocarp | Cotton Squares | |
| Weight | 16.00 ± 0.40 a | 16.22 ± 0.39 a | 15.67 ± 0.44 a * |
| Body length | 4.08 ± 0.08 a | 4.11 ± 0.09 a | 4.01 ± 0.05 a |
| Ovariole length | 1.96 ± 0.11 b | 2.02 ± 0.11 b | 2.85 ± 0.11 a |
| Width of most developed oocyte | 0.30 ± 0.01 b | 0.30 ± 0.01 b | 0.38 ± 0.02 a |
| Boll weevil age (days) | |||
| 30 | 60 | 90 | |
| Weight | 16.11 ± 0.41 a | 16.11 ± 0.38 a | 15.67 ± 0.44 a |
| Body length | 4.15 ± 0.08 a | 4.05 ± 0.07 a | 4.01 ± 0.08 a |
| Ovariole length | 2.51 ± 0.12 a | 2.28 ± 0.12 ab | 2.04 ± 0.16 b |
| Width of most developed oocyte | 0.32 ± 0.02 a | 0.32 ± 0.02 a | 0.32 ± 0.01 a |
| Morphometric Data | Treatments | ||
|---|---|---|---|
| Banana Endocarp | Orange Endocarp | Cotton Squares | |
| Weight | 15.80 ± 0.40 a | 15.56 ± 0.35 a | 14.78 ± 0.33 a * |
| Body length | 4.14 ± 0.08 a | 4.07 ± 0.06 a | 3.85 ± 0.05 b |
| Testis area | 0.56 ± 0.04 b | 0.56 ± 0.03 b | 0.85 ± 0.03 a |
| Testis diameter | 0.91 ± 0.03 b | 0.87 ± 0.03 b | 1.12 ± 0.02 a |
| Boll weevil age (days) | |||
| 30 | 60 | 90 | |
| Weight | 15.78 ± 0.42 a | 15.22 ± 0.37 a | 15.20 ± 0.32 a |
| Body length | 4.13 ± 0.08 a | 3.98 ± 0.06 a | 3.97 ± 0.07 a |
| Testis area | 0.78 ± 0.04 a | 0.68 ± 0.04 b | 0.52 ± 0.04 c |
| Testis diameter | 1.08 ± 0.03 a | 0.96 ± 0.03 b | 0.87 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, T.d.S.; Silva, C.A.D.d.; Martins, C.F.; Silva, L.L.d.; Zanuncio, J.C.; Serrão, J.E. Morphology and Morphometry of the Reproductive Tract of the Cotton Boll Weevil after Prolonged Feeding on Alternative Diets. Insects 2023, 14, 571. https://doi.org/10.3390/insects14060571
Carvalho TdS, Silva CADd, Martins CF, Silva LLd, Zanuncio JC, Serrão JE. Morphology and Morphometry of the Reproductive Tract of the Cotton Boll Weevil after Prolonged Feeding on Alternative Diets. Insects. 2023; 14(6):571. https://doi.org/10.3390/insects14060571
Chicago/Turabian StyleCarvalho, Thiele da Silva, Carlos Alberto Domingues da Silva, Celso Feitosa Martins, Laryssa Lemos da Silva, José Cola Zanuncio, and José Eduardo Serrão. 2023. "Morphology and Morphometry of the Reproductive Tract of the Cotton Boll Weevil after Prolonged Feeding on Alternative Diets" Insects 14, no. 6: 571. https://doi.org/10.3390/insects14060571
APA StyleCarvalho, T. d. S., Silva, C. A. D. d., Martins, C. F., Silva, L. L. d., Zanuncio, J. C., & Serrão, J. E. (2023). Morphology and Morphometry of the Reproductive Tract of the Cotton Boll Weevil after Prolonged Feeding on Alternative Diets. Insects, 14(6), 571. https://doi.org/10.3390/insects14060571







