Simple Summary
Wolbachia are obligate intracellular bacteria that occur in insects and filarial worms. Strains that infect insects have genomes that encode mobile genetic elements that are typically lost as obligate intracellular bacteria undergo genomic streamlining. These mobile elements include diverse lambda-like prophages that package an approximately 65 kb viral genome called Phage WO, which includes a unique eukaryotic association module, or EAM. The EAM encodes unusually large proteins thought to mediate interactions between the bacterium, its virus, and the host eukaryotic cell. We propose that in addition to conventional bacteriophages, Wolbachia also encode smaller gene transfer agents (GTAs). From two independent preparations, we recovered an identical 15.6 kb sequence that lacks an EAM. Its small size and gene composition suggest that the element is a GTA, which may participate in the horizontal transfer of random DNA from the Wolbachia genome. Although functional proof of GTA activity remains to be obtained, these data provide a useful framework for future analysis of mobile genetic elements encoded by Wolbachia genomes.
Abstract
Wolbachia are obligate intracellular bacteria that occur in insects and filarial worms. Strains that infect insects have genomes that encode mobile genetic elements, including diverse lambda-like prophages called Phage WO. Phage WO packages an approximately 65 kb viral genome that includes a unique eukaryotic association module, or EAM, that encodes unusually large proteins thought to mediate interactions between the bacterium, its virus, and the eukaryotic host cell. The Wolbachia supergroup B strain, wStri from the planthopper Laodelphax striatellus, produces phage-like particles that can be recovered from persistently infected mosquito cells by ultracentrifugation. Illumina sequencing, assembly, and manual curation of DNA from two independent preparations converged on an identical 15,638 bp sequence that encoded packaging, assembly, and structural proteins. The absence of an EAM and regulatory genes defined for Phage WO from the wasp, Nasonia vitripennis, was consistent with the possibility that the 15,638 bp sequence represents an element related to a gene transfer agent (GTA), characterized by a signature head–tail region encoding structural proteins that package host chromosomal DNA. Future investigation of GTA function will be supported by the improved recovery of physical particles, electron microscopic examination of potential diversity among particles, and rigorous examination of DNA content by methods independent of sequence assembly.
1. Introduction
Wolbachia pipientis (Rickettsiales; Anaplasmataceae) is an obligate intracellular alpha-proteobacterium that occurs in members of the Ecdysozoa, the group of molting animals that includes arthropods and nematodes. The abundance and diversity of Wolbachia’s hosts, horizontal transfer among Wolbachia genomes, and transinfection between species to produce novel infections for the reduction in vector-borne diseases have stimulated interest in the genetic manipulation of Wolbachia.
Genomes of Wolbachia that infect arthropods as reproductive parasites typically include one or more distinct prophage regions, or haplotypes, that vary in overall gene composition, but often share nearly identical copies of individual phage-related genes. In general, these prophages resemble the lysogenic form of bacteriophage lambda [1,2,3,4], with genomes of approximately 65 kb that include a unique eukaryotic association module, or EAM [3,4]. Electron microscopic observation of virus-like particles in Wolbachia-infected hosts is consistent with lytic replication of WO prophages and their potential value as transducing agents [1]. Experimental evaluation of phage production from Wolbachia genomes is challenging because conditions that induce lytic replication of WO prophages are unknown, and viral particles are recovered at low yields. These limitations likely reflect Wolbachia’s obligate intracellular lifestyle and genome streamlining, including the loss of bacterial genes involved in phage induction in free-living microbes [5,6].
Here, we describe the recovery of physical particles produced by supergroup B Wolbachia strain wStri, from the planthopper Laodelphax striatellus, maintained in the C/wStri1 mosquito cell line [7,8]. Two independent preparations from persistently infected cells converged on an identical 15.6 kb sequence encoding 22 genes, highly similar to the proximal region of WOVitA1 and the corresponding sequence in other well-defined WO phages. Unlike Phage WO, the 15.6 kb sequence, here called WOStri, lacked genes encoding the EAM and regulatory functions; in size and gene composition, WOStri resembled the head–tail gene cluster of a gene transfer agent (GTA) capable of packaging host DNA [9,10,11,12,13,14].
We note that precise linear correlation of DNA sequences recovered from physical particles with a corresponding prophage haplotype awaits progress in the annotation of the Wolbachia genomes from which WO phages have been recovered. Moreover, the possibility that Wolbachia genomes encode mobile genetic elements other than bacteriophages is supported by bioinformatics-based analyses [9,15,16]. These observations underscore the need for improving yields of virus-like particles produced by Wolbachia, and uncovering conditions that enhance their expression. We suggest that systematic implementation of experimental approaches that differentiate between prophages, GTAs, and other virus-like elements will provide new insights into the potential diversity of Wolbachia-encoded mobile elements and their genetic capabilities.
2. Materials and Methods
2.1. Cells and Culture Conditions
C/wStri1 cells were grown in E5 medium as described previously [7,8]. Recovery of approximately 100 ng of phage DNA after ultracentrifugation was guided by proteomic data predicting low phage abundance [17], an estimated harvest of 3 × 106 cells per ml, an assumed abundance of 100 Wolbachia and one phage per cell, and a phage genome size of 40 kb.
2.2. DNA Extraction and Purification
DNA was extracted from one to three liters of material pooled from 40 to 120 plates with a diameter of 100 mm. Cells were lysed by addition of 0.1 volume of chloroform with vigorous stirring on a magnetic stirrer (30 min at room temperature). Chloroform was removed by low-speed centrifugation, and the supernatant was supplemented with one-fifth volume of 2.5 M NaCl containing 37.5% polyethylene glycol 8000 (PEG) added with stirring at 4 °C. Individual preparations were stored at 4 °C to accumulate sufficient cell culture supernatant. Supernatants were combined, and care was taken to recover a light film of material that adhered to the bottom of glass storage bottles. PEG pellets were recovered after centrifugation in 250 mL bottles in a JA14 rotor (7500× g in a J2-21 Beckman centrifuge) and were resuspended in 20 mM Tris-HCl, pH 7.5 containing 10 mM MgCl2 and 10% glycerol (approximately 70 mL/L of cell culture supernatant). PEG was extracted from the resuspended material by stirring with an equal volume of chloroform, followed by centrifugation to remove chloroform. The aqueous supernatant was filtered through 5-micron filters, followed by a second filtration through a stacked series of 5-micron, 2.7-micron, and 0.22-micron filters. The filtrate was centrifuged in an SW41 rotor at 110,000× g for 16 h at 4 °C. DNA was extracted from pellets by conventional phenol extraction/ethanol precipitation (2016 sample) or with a PureLink (Invitrogen, Carlsbad, CA, USA) genomic DNA extraction kit (2020 sample) following the manufacturer’s instructions. Phage enrichment was verified by a positive PCR band for “orf7” defined for WO phages from wTai and wKue [18], using forward primer 5′-CTGSCTTCAAGKTGCTTTATTGC and reverse primer 5′-TCAAGAGAYCARATAACAGTAGC, coupled with absence of a PCR band corresponding to the single copy Wolbachia ribosomal protein gene pair using the S12F/S7R primers described previously [19]. Serial dilution of template estimated phage enrichment at 1000 to 10,000-fold.
2.3. Analysis
Samples were submitted to the University of Minnesota Genomics Center for Next-Gen Illumina paired-end sequencing. The 2016 library was prepared using NexteraXT reagent kits, and the 2000 library was created using Rubicon reagent kits. Both projects were sequenced on a MiSeq 300 bp PE stowaway run, and each library generated ~2 M reads, with a mean quality score ≥ Q30. FASTQ data were groomed and trimmed, yielding 302,155 sequences in 2016 and 560,466 sequences in 2020, with an average read quality score of 37 and GC content of 36%. Read length was approximately 300 bp. Assembly was performed using SPAdes (Galaxy version 3.15.3 + galaxy1; [20]). This analysis involved systematic comparison using increasing coverage criteria until the assembled reads were represented by a maximum of three contigs from the second independent preparation: 10.29_1, coverage 3425; 10.29_2, coverage 1043; and 11.1_1, coverage 4042, obtained from 10.29_1 and 10.29_2 by raising coverage stringency. At this step, we excluded the lower coverage 10.29_2 contig based on the presence of an IS110 transposase and a unique HP in reverse orientation relative to other genes in the three contigs. The 2020 data were then reanalyzed against the single 2016 contig that emerged from a high coverage analysis. An identical 15,638 nt core was represented by both data sets. The 2020 data varied at the flanking 5′ end of this core sequence, while the 2016 data varied at the flanking 3′ end. To facilitate description of the results here, we call the 15,638 nt core sequence “WOStri”, but acknowledge that in overall size and gene composition, it bears stronger resemblance to a GTA than to a conventional WO Phage [3,4].
Data were compared and manipulated using Geneious Prime (version 2022.0.2) [21,22] and Blast programs on NCBI (National Center for Biotechnology Information) [23,24], with a primary focus on the well-characterized prophage WOVitA1 (66,810 bp, HQ906662.1), alternatively known as Phage WO (KX522565.1). Queries were generated by Geneious Prime from translation products encoded by WOStri orfs. We evaluated alignments from the following complete, or nearly complete genomes: Wolbachia supergroup A strains wMel (NC_002978.6; taxid 163164), wRi (NC_012416.1; taxid 66084), wHa (NC_021089.1; taxid 1236909), and wAu (NZ_LK055284.1; taxid 225364); supergroup B strains wNo (NC_021084.1; taxid 1236908), wPip (NC_010981.1; taxid 570417), and wStriCN (NZ_ MUIX01000001.1 and NZ_MUIX01000002.1; taxid 368602); and supergroup E strain wFol (NZ_CP015510.2; taxid 169402). Note that the wStriCN genome [25] is represented by two contigs, NZ_MUIX01000001.1 and NZ_MUIX01000002.1; these are abbreviated “MUIX_01.1” and “MUIX_02.1” in the text below. The gene composition of the WOStri core has been deposited in Genbank (accession number: BankIt2634826 BSeq#1 OP690541, to be released in April 2023).
3. Results and Discussion
3.1. WOStri Resembles the Proximal Region of Phage WO
We compared DNA recovered from two independent preparations of physical particles from wStri, a supergroup B Wolbachia strain from the planthopper Laodelphax striatellus (Hemiptera, Delphacidae) maintained as a persistent infection in Aedes albopictus C/wStri1 mosquito cells [7,8]. Because an earlier proteomics investigation [17] showed evidence for the expression of phage peptides that map in the Undecim cluster [4], we anticipated recovery of a lambda-like genome resembling Phage WO (KX522565.1: 65,653 bp), encoded by prophage WOVitA1 (HQ906662.1: 66,810 bp) in Wolbachia that infect the wasp, Nasonia vitripennis [3,4]. Illumina data were groomed, trimmed, and assembled using SPAdes without a coverage cutoff, and contigs generated from the combined data sets were aligned to Phage WO using Geneious Prime (Figure 1).
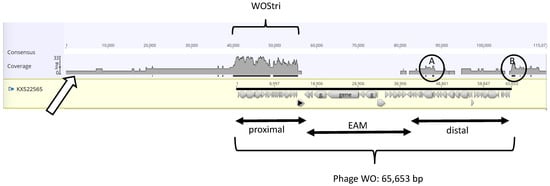
Figure 1.
Combined SPAdes 2016 and 2020 reads mapped to Phage WO (KX522565). Panel A. Geneious Prime “Map to reference” mapped 523 of 3499 contigs generated without a coverage cutoff to Phage WO (KX522565). Introduction of gaps to maximize the alignment lengthens the consensus from 65,653 nt to 115,077 nt. The open arrow indicates the coverage profile (Log scale, shaded in gray) of mapped reads, and black bars beneath the profile indicate coverage with 2 or more reads. Annotation of Phage WO is indicated by gray arrows beneath the black bar, with proximal, EAM, and distal regions indicated below the alignment. Note that short areas of two-fold coverage (circled) occur within (A) and just downstream (B) of the KX522565 sequence.
The reads predominantly mapped to a 17 kb proximal region of Phage WO, bracketed as WOStri. The 65,653 bp Phage WO genome, bracketed at the bottom of Figure 1, is represented by a solid black bar above a series of annotated genes denoted by gray arrowheads. The proximal, or 5′ region, extends from hypothetical protein (HP) gwv_1137 to recombinase (gvw_1156). Note that the gray-shaded read profile is shown on a log scale with a Geneious Prime coverage of “30” in the WOStri region, and that high coverage does not include the Phage WO recombinase (marked with a black triangle), the eukaryotic association module (EAM), or the distal portion of Phage WO. Two minor exceptions are represented by black bars identifying circled areas A and B, which had a Geneious Prime coverage of “2”.
Region A maps in the vicinity of gvw_1110 to gvw_1113 in Phage WO, encoding the P2 ortholog gpU, the tape measure protein (described in further detail in Section 3.5), and tail chaperone proteins gpGT and gpG. Circled region B maps downstream of the last annotated gene (gwv_1134). Within this 457 nt region are several short orfs measuring less than 200 nt. The sequence is represented by a series of 344 repeats, each measuring 5 bp and occurring as two to six copies. Blastn shows that a similar sequence is common among Wolbachia genomes but has not been identified in wStri. In wPip, the orthologous sequence occurs as four copies of a putative phage-related protein in the RNAse H-like superfamily. These isolated, low-abundance regions of phage-like genes fell out of the assembly as coverage stringency increased.
3.2. WOStri Gene Order and Sequence Are Conserved in Other WO Phages
By systematically increasing the coverage cutoff in SPAdes from 0 to 3000, we found that both data sets converged on an identical 15,638 nt sequence encoding 22 genes oriented in the same direction (Figure 2).
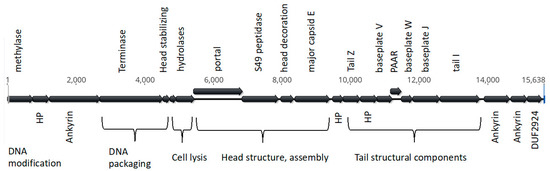
Figure 2.
Structure of WOStri. Open reading frames were produced in Geneious Prime. Genes clearly identifiable as phage-related are listed above, and unknown genes are labeled below the alignment.
We note that the methylase at the extreme 5′ end of WOStri (Figure 2) has an ortholog downstream of attL at the extreme 5′ end of the integrated WOVitA1 prophage that is not annotated in KX522565. N-terminal methylase variants with legitimate bacterial start codons encoded proteins ranging from 125 to 410 amino acids. The N-terminal domain of the longer orthologs included a ParB_N_Srx domain of about 100 residues, which may encode nuclease activity. With the exception of hydrolase/s (orfs6/7), orfs downstream of the methylase closely resembled a 14,262 region of Phage WO that includes a series of shared repeats measuring 50 bp or more (Figure 3A).
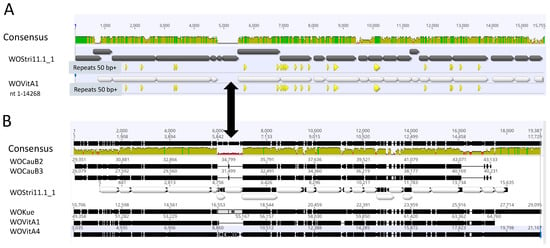
Figure 3.
Overall sequence comparisons. (A) Pairwise alignment of WOStri 11.1_1 with residues 1–14,268 of KX522565. Green/yellow-green and red shading indicate Geneious designations of high, medium and low sequence identity. Gray (WOStri) and white (WOVitA1) horizontal arrows represent orfs and their direction of transcription. Triangles below the orfs indicate exact repeats of 50 nt or greater common to WOStri and the proximal region of KX522565. The double black arrow connecting Panels A and B indicates the variable region separating terminase/gpw and portal genes in Wolbachia prophages. (B) Sequence alignment of WOCauB2 (AB478515, bases 29,351–43,133); WOCauB3 (AB478516, bases 26,079–40,231); WOStri and WOKue (AB036666, bases 10,706–29,095); WOVitA1 (HQ906662, reversed, bases 49,358–64,760); and WOVitA4 (HQ906664; reversed, bases 3035–21,167). The region common to all six genomes was extracted and aligned in Geneious Prime.
The 15,638 nt region was conserved among other well-annotated WO phages, including WOVitA1, WOCauB2, WOCauB3, WOVitA1, as well as the smaller WOKue and WOVitA4 (Figure 3B). Each of the WOStri genes had one or more orthologs in wStri and, with a few individual exceptions, in other well-characterized Wolbachia genomes, as summarized in Table 1.

Table 1.
WOStri orthologs in wStri and other Wolbachia strains. Note that the prefix WP_ is omitted from accession numbers. The query was the translation product from the WOStri orf, with the exception of the methylase (orf1), where the query was the WOVitA1 ortholog.
Assemblies of the host Wolbachia genomes from which phages have been recovered are incomplete, and to date, none of the phage structures resulting from conventional molecular approaches and/or assembly of short reads has been correlated with a contiguous suite of genes in a host genome. In aggregate, wStri encodes nine putative phage regions, representing more than 10% of the genome [25]. Although all of these prophage regions include genes classified as “other than prophages and transposons”, we note that six of the nine putative prophages have estimated sizes ranging from 16.7 to 20.6 kb, comparable in size to WOStri and WOVitA4. The size of WOStri, absence of regulatory genes, and identities of structural genes involved in DNA packaging suggested that WOStri may be a gene transfer agent, similar to that in Rhodobacter capsulatus, a marine alpha-proteobacterium which is well studied because a GTA overproducer strain is available [10].
3.3. The WOStri Hydrolase Region Contains Evidence for a +1 Programmed Ribosomal Frameshift
Alignment with other WO Phages reveals a single variable region (Figure 3) between terminase/gpw (orfs 4/5) and portal (orf 8) genes, which may be absent, may encode a GH_25 hydrolase/muramidase, an alternative PD-(DE)XK nuclease, or a hypothetical protein and/or a RelE/ParE toxin-antitoxin module, which have been described elsewhere [26]. WOStri most closely resembles WOVitA4 (Figure 3B), which also encodes a GH_25 hydrolase/muramidase. Phage WO, WOCauB2, and WOCauB3, best known for their EAM regions [3,4], lack an intervening sequence, while WOKue encodes a PD-(D/E)XK nuclease.
The evolution of bacterial muramidases/hydrolases has been described earlier [36], and we suggest that in the context of a GTA, the hydrolase may contribute to host cell lysis. The wStri ortholog WP_241654108.1 (orf6, 64 amino acids), has a single putative hydrolase domain (residues 42–62); in WP_241654107.1 (orf7, 175 amino acids), the hydrolase domain extends from residues 5 to 160. We note that a +1 ribosomal frameshift (Figure 4A) potentially produces a translation fusion protein from orfs6/7.
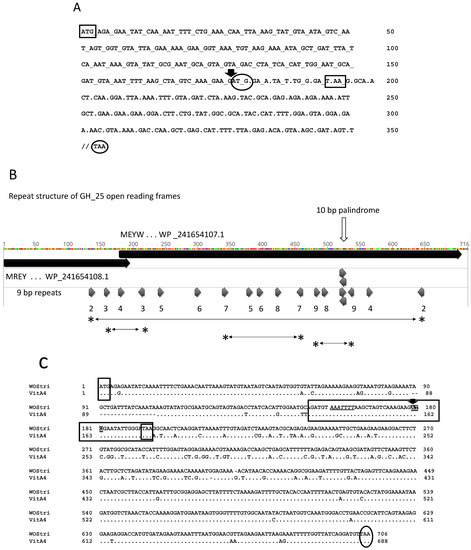
Figure 4.
Sequence support for a programmed + 1 ribosomal frameshift in hydrolase-encoding orfs. Panel (A) shows the sequence of the hydrolase Orf6 and Orf7 in the region of the potential frameshift. Rectangular boxes show initiation and termination codons of Orf6, and oval boxes show the start and stop codons of Orf7. The downward pointing arrow indicates the potential + 1 frameshift. Codons in the Orf6 reading frame are separated by underscores, and codons in the Orf7 reading frame are separated by periods. The 3′-end of Orf7 is not shown. Panel (B) shows a translation of GH_25 Orf6/Orf7 using Geneious Prime. Colors are the Geneious designations for residues and are not relevant here. Large solid black bars indicate the two translation products: N-terminal MREY… and MEYW…, with WP_ accessions. Short arrows below indicate positions of 9 and 10 bp repeats in the Orf6/Orf7 region, suggesting possible secondary structure in the mRNA. Horizontal lines flanked by asterisks show sequence flanked by repeat pairs 2, 3, 7, and 9, oriented in opposite directions. The white vertical arrow indicates a 10 nt palindrome. Panel (C) shows alignment of hydrolase reading frames in WOStri and WOVitA4. Dots indicate identities. Small rectangular boxes with only three nucleotides represent ATG start and TAA stop codons in Orf6. The region of exact identity immediately surrounding the out-of-frame Orf7 ATG (downward pointing arrow and white text on a black background) is shown in the long horizontal rectangle that terminates with the Orf6 TAA stop codon. Within the box, a slippery sequence AAATTTT upstream of the start codon is shown in italics and underlined. The oval represents the Orf7 stop codon.
Although signals in the mRNA that might trigger a +1 ribosomal frameshift are not well understood, the sequence includes a 10 bp palindrome and several 9 bp repeats (Figure 4B), some of which are in reverse orientation and potentially generate secondary structure in mRNA transcripts [37]. A nucleotide alignment with the similar hydrolase coding region in WOVitA4 includes a region of identity spanning the position of the potential frameshift, with the potential XXXYYYN “slippery sequence” best associated with −1 ribosomal frameshifts, shown in underlined italics upstream of the ATG start codon (Figure 4C). Bacterial genes with orfs disrupted by programmed ribosomal frameshifting and programmed transcriptional realignment are apparently common, but difficult to identify computationally [38].
By way of comparison, the RcGTA gene responsible for bacterial lysis has not been unequivocally identified [39]. However, experimental evidence suggests that an unknown protein with a glycohydrolase domain encoded by VSH-1, a GTA in the anaerobic spirochete Brachyspira hyodysenteriae, hydrolyzes peptidoglycan [40]. We speculate that the muramidase/hydrolase motifs encoded between terminase/gpW and portal genes may play a role in WOStri release, and that variation at this locus may have physiological significance.
3.4. Evidence for Low-Level Packaging of wStri Genomic DNA Encoding EAM-like Proteins
We suggest that the 15,638 bp sequence we call WOStri represents an equivalent of the minority of RcGTA particles in which the head–tail encoding genome is packaged [11,14]. As a GTA, WOStri would have also packaged random pieces of the wStri genome, albeit at low frequency for any particular DNA fragment. Although not conclusive, examination of the low abundance reads is informative in the context of future experimental investigation. In general, these data are consistent with low coverage of wStri DNA. Moreover, early steps in the assembly generated contigs that encoded long orfs reminiscent of EAM proteins. These observations merit more rigorous future investigation.
Although we cannot rule out adventitious contamination that would be eliminated by rigorous DNAse treatment of purified particles, at the lowest coverage, the complete set of reads showed wide distribution across the two contigs that represent the wStri genome (Figure 5).
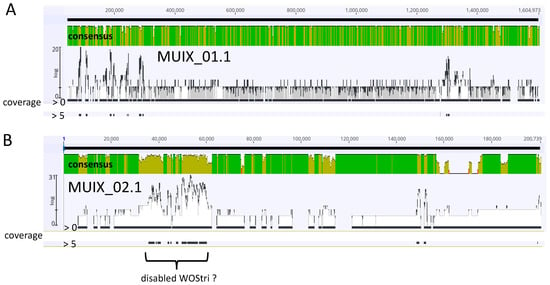
Figure 5.
Mapping of combined SPAdes contigs without coverage cutoff to MUIX_01.1 (Panel (A)) and MUIX_02.1 (Panel (B)) contigs representing an incomplete wStri genome. Green/yellow-green and red shading in consensus panels indicate Geneious designations of high, medium and low sequence identity. Of 3499 total reads, 1193 mapped to MUIX_01.1, and 515 mapped to MUIX_02.1. Profiles show consensus identities generated with Geneious Prime, with highlighted coverages set to >0 (highest possible representation of matches between contigs and wStri DNA) and to >5, represented by black bars below the top and lower consensus bars, respectively. Distribution of coverage bars emphasizes potential coverage of most of the wStri genome with the complete set of SPAdes contigs, and loss of matches as Geneious coverage criteria increase.
These reads are represented by the solid black bar immediately below each panel, labeled coverage “>0.” When coverage was raised to “5”, most of these reads were eliminated, while a region in MUIX_02.1 remained, possibly representing a rearranged WOStri (Figure 5B). The region encodes a discontinuous suite of the WOStri genes reported here, all oriented in the same direction, but including two copies of an IS110 family transposase (WP_063630491.1) and a duplication of baseplate assembly genes V and W. Moreover, the portal gene directly follows terminase/gpW genes, and a recombinase occurs immediately downstream of a DUF2924 domain-containing protein.
In addition to the possibility that wStri genomic DNA is packaged, long (>5000 nt), low abundance contigs sometimes encoded single orfs reminiscent of genes in the EAM or distal regions of Phage WO. These long, low-frequency contigs, presumably representing intermediates produced by the assembly algorithm, encoded three classes of proteins: ankyrin motif proteins, ATPases associated with diverse cellular activities (AAA-ATPases), and phage tape measure proteins (Table 2).

Table 2.
Analysis of low coverage contigs exceeding 5000 nt in length for large open reading frames (exceeding 2000 nt) potentially encoding EAM-like proteins. Entries in bold are common to both data sets. The final column indicates that ankyrin proteins may be represented by many additional putative orthologs at less than 50% coverage or identity.
These proteins have internal repetitive domains and participate in protein–protein interactions that involve the assembly of macromolecular structures. We did not find orfs that encoded proteins from host mosquito DNA, which would be expected if extraneous DNA contaminated physical particles recovered by ultracentrifugation.
3.5. Evaluation of EAM-like Proteins
Ankyrin repeat and latrotoxin-associated proteins included an exceptionally large (11,826 nt) orf encoding a 3941 residue protein reminiscent of the 2474 residue ankyrin/tetratricopeptide repeat protein in WOVitA1. This protein was identical to the ankyrin repeat protein WP_063631194.1 from the Korean strain of wStri (NZ_LRUH01000086.1), but is not encoded in the more complete wStri MUIX_01.1 and _02.1 assemblies, nor is it annotated among the selected Wolbachia strains examined here. However, orthologs with lower coverage/identity scores were common, including wStri WP_143688882.1, with 16 matches over 4357 residues, and WP_143688758.1, with 12 matches over 3082 residues.
Three AAA-ATPases are annotated in the distal portion of WOVitA1 (gwv_1124, gwv_1125, and gwv_1128). These proteins are characterized by a conserved 230 amino acid ATPase module which acts as a motor to drive macromolecular rearrangements in diverse cellular functions [41,42].
Finally, the tape measure protein (TMP), often recognized by the distribution of aromatic amino acids, is common in prophage genomes, typically measures about 1000 residues, and, like ankyrin proteins, contains partially repeated regions [43,44]. During infection, TMP controls phage tail length and facilitates DNA transit to the bacterial cytoplasm [45]. Uniquely among the large proteins considered here, tape measure proteins are encoded by eight genes distributed among the two wStri MUIX contigs, one of which (WP_077188535.1) shared complete identity with the WOVitA1 query. In contrast, however, wMel, wRi, wHa, wAu, and wPip genomes encoded only a single ortholog, while four were present in wFol.
The 2020 data set encoded a total of 27 orfs exceeding 2000 nt in length, again with no evidence of contaminating mosquito sequences. Of these, six were duplicates of ankyrin, AAA-ATPase, and TMP genes from the 2016 data set (indicated in bold font in Table 2). The remaining orfs were encoded by genes present as one to two copies in the wStri genome, and included non-phage proteins such as RNA polymerase, RecB, and a translational initiation factor. Recovery of contigs encoding EAM-like proteins only in the early stages of assembly may derive from the overrepresentation of some, but not all, regions of the wStri genome. For example, a GTA from flea-borne Bartonella grahamii is associated with packaged DNA that includes products generated by run-off replication of a chromosomal “high plasticity zone” thought to facilitate diversification of the host microbe [46,47].
4. Conclusions
We present an analysis that supports the possibility that GTA-like elements may be encoded in Wolbachia genomes and provide insights that serve as a baseline for further investigation of this possibility. In this context, it is important to note that GTA-like elements that do not transfer DNA, such as the defective phage PBSX from Bacillus subtilis, have been described [10], and that an understanding of GTA expression and function continues to evolve, as has recently been demonstrated for Bartonella GTA [48]. In addition, subtle differences in the structure of physical particles can correlate with packaging capability [14]. The sequence assembly of DNA recovered from particles produced by wStri in cultured cells was unexpectedly complex. Generation of a single consensus sequence required multiple iterations with progressively increased stringency, in which the longest contigs, some with orfs resembling genes encoded by the EAM in Phage WO, were not retained in the final assembly. Although we cannot eliminate an assembly artifact and/or potential contamination of phage-like particles with wStri genomic DNA, recovery of an identical 15.6 bp WOStri core sequence from two independent investigations, coupled with its strong similarity to the proximal portion of WOVitA1 and other WO phages, merits further analysis using more direct approaches that complement computational assembly, including direct sequencing of wStri chromosomal fragments from physical particles, as has been performed with RcGTA [10,11,12,13,14]. In addition, high-quality transmission electron microscopy of Wolbachia preparations will be important to evaluate the diversity of phage-like particles [14]. Finally, more rigorous purification of packaged DNA by cesium chloride gradient centrifugation, ideally under conditions that separate particles based on density and/or structural differences, will be desirable. These endeavors will require scaled-up procedures for particle recovery and, ideally, the identification of conditions that induce the expression of phages and/or GTAs in C/wStri-infected cells. Some GTAs may be inducible by nutrient depletion or quorum sensing [15], while DNA damage can elicit a lytic cycle in some bacteriophages. In pilot experiments, we have tested exposure to ultraviolet light, mitomycin C, and oxidizing agents, thus far without successful induction. Regardless of the precise nature of potential agents of gene transfer in Wolbachia, further exploration of their identities will contribute to the eventual genetic manipulation of this widespread intracellular bacterium.
Author Contributions
Conceptualization: A.M.F.; methodology: A.M.F. and E.M.C.; formal analysis and investigation: A.M.F. and E.M.C.; writing—original draft preparation: A.M.F.; writing—review and editing: A.M.F. and E.M.C.; funding acquisition: A.M.F.; resources: A.M.F.; supervision: A.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The gene composition of the WOStri core has been deposited in Genbank (accession number: BankIt2634826 BSeq#1 OP690541, to be released in April 2023).
Acknowledgments
This research was supported by the University of Minnesota Agricultural Experiment Station, St. Paul, MN. AMF thanks Neal Binsfeld for the helpful discussion during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kent, B.N.; Bordenstein, S.R. Phage WO of Wolbachia: Lambda of the endosymbiont world. Trends Microbiol. 2010, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.N.; Funkhouser, L.J.; Setia, S.; Bordenstein, S.R. Evolutionary genetics of a temperate bacteriophage in an obligate intracellular bacteria (Wolbachia). PLoS ONE 2011, 6, e24984. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Bordenstein, S.R. Eukaryotic association module in phage WO genomes from Wolbachia. Nat. Commun. 2016, 7, 13155. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Bordenstein, S.R. Widespread phages of endosymbionts: Phage WO genomics and the proposed taxonomic classification of Symbioviridae. PLoS Genet. 2022, 18, e1010227. [Google Scholar] [CrossRef] [PubMed]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Fallon, A.M. DNA recombination and repair in Wolbachia: RecA and related proteins. Mol. Genet. Genom. 2021, 296, 437–456. [Google Scholar] [CrossRef]
- Fallon, A.M.; Baldridge, G.D.; Higgins, L.-A.; Witthuhn, B.A. Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell Dev. Biol. Anim. 2013, 49, 66–73. [Google Scholar] [CrossRef]
- Fallon, A.M. Conditions facilitating infection of mosquito cell lines with Wolbachia, an obligate intracellular bacterium. In Vitro Cell Dev. Biol. Anim. 2019, 55, 120–129. [Google Scholar] [CrossRef]
- Shakya, M.; Soucy, S.M.; Zhaxybayeva, O. Insights into origin and evolution of a-proteobacterial gene transfer agents. Virus Evol. 2017, 3, vex036. [Google Scholar] [CrossRef]
- Lang, A.S.; Zhaxbayeva, O.; Beatty, J.T. Gene transfer agents: Phage-like elements of genetic exchange. Nat. Rev. Microbiol. 2012, 10, 472–482. [Google Scholar] [CrossRef]
- Hynes, A.P.; Mercer, R.G.; Watton, D.E.; Buckley, C.B.; Lang, A.S. DNA packaging bias and differential expression of gene transfer agent genes within a population during production and release of the Rhodobacter capsulatus gene transfer agent, RcGTA. Mol. Microbiol. 2012, 85, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Hynes, A.P.; Shakya, M.; Mercer, R.G.; Grüll, M.P.; Bown, L.; Davidson, F.; Steffen, E.; Matchem, H.; Peach, M.E.; Berger, T.; et al. Functional and evolutionary characterization of a gene transfer agent’s multilocus “genome”. Mol. Biol. Evol. 2016, 33, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Redfield, R.J.; Soucy, S.M. Evolution of bacterial gene transfer agents. Front. Microbiol. 2018, 9, 2527. [Google Scholar] [CrossRef] [PubMed]
- Bárdy, P.; Füzik, T.; Hrebík, D.; Pantůček, R.; Beatty, J.T.; Plevka, P. Structure and mechanism of DNA delivery of a gene transfer agent. Nat. Commun. 2020, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Serbus, L.R. Gene Transfer Agents in Symbiotic Microbes. In Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects. Results and Problems in Cell Differentiation; Kloc, M., Ed.; Springer: Cham, Switzerland, 2020; Volume 69. [Google Scholar]
- George, E.E.; Tashyreva, D.; Kwong, W.K.; Okamoto, N.; Horák, A.; Husnik, F.; Lukeš, J.; Keeling, P.J. Gene transfer agents in bacterial endosymbionts of microbial eukaryotes. Genome Biol. Evol. 2022, 14, evac099. [Google Scholar] [CrossRef]
- Baldridge, G.D.; Markowski, T.W.; Witthuhn, B.A.; Higgins, L.; Baldridge, A.S.; Fallon, A.M. The Wolbachia WO bacteriophage proteome in the Aedes albopictus C/wStr1 cell line: Evidence for lytic activity? In Vitro Cell Dev. Biol. Anim. 2016, 52, 77–88. [Google Scholar] [CrossRef]
- Masui, S.; Kamoda, S.; Sasaki, T.; Ishikawa, H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J. Mol. Evol. 2000, 51, 491–497. [Google Scholar] [CrossRef]
- Fallon, A.M. Cytological properties of an Aedes albopictus mosquito cell line infected with Wolbachia strain wAlbB. In Vitro Cell Dev. Biol. Anim. 2008, 44, 154–161. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schaffer, A.A.; Yu, Y.-K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Bing, X.L.; Zhao, D.S.; Sun, J.T.; Zhang, J.; Hong, X.Y. Genomic analysis of Wolbachia from Laodelphax striatellus (Delphacidae, Hemiptera) reveals insights into its “Jekyll and Hyde” mode of infection pattern. Genome Biol. Evol. 2020, 12, 3818–3831. [Google Scholar] [CrossRef]
- Fallon, A.M. Muramidase, nuclease, or hypothetical protein genes intervene between paired genes encoding DNA packaging terminase and portal proteins in Wolbachia phages and prophages. Virus Genes 2022, 58, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.R.; Gao, L.A.; Shah, S.A.; López-Beltrán, A.; González-Delgado, A.; Martínez-Abarca, F.; Iranzo, J.; Redrejo-Rodríguez, M.; Zhang, F.; Toro, N. UG/Abi: A highly diverse family of prokaryotic reverse transcriptases associated with defense functions. Nucleic Acid Res. 2022, 50, 6084–6101. [Google Scholar] [CrossRef]
- Labrie, S.J.; Tremblay, D.M.; Moisan, M.; Villion, M.; Magadán, A.H.; Campanacci, V.; Cambillau, C.; Moineau, S. Involvement of the major capsid protein and two early-expressed phage genes in the activity of the lactococcal abortive infection mechanism AbiT. Appl. Environ. Microbiol. 2012, 78, 6890–6899. [Google Scholar] [CrossRef]
- Kampfraath, A.A.; Klasson, L.; Anvar, S.Y.; Vossen, R.H.; Roelofs, D.; Kraaijeveld, K.; Ellers, J. Genome expansion of an obligate parthenogenesis-associated Wolbachia poses an exception to the symbiont reduction model. BMC Genom. 2019, 20, 106. [Google Scholar] [CrossRef]
- Fallon, A.M. Computational evidence for antitoxins associated with RelE/ParE, RatA, Fic, and AbiEii-family toxins in Wolbachia genomes. Mol. Genet. Genom. 2020, 295, 891–909. [Google Scholar] [CrossRef]
- Häuser, R.; Blasche, S.; Dokland, T.; Haggård-Ljungquist, E.; von Brunn, A.; Salas, M.; Casjens, S.; Molineux, I.; Uetz, P. Bacteriophage protein-protein interactions. Adv. Virus Res. 2012, 83, 219–298. [Google Scholar] [CrossRef]
- Christie, G.E.; Calendar, R. Bacteriophage P2. Bacteriophage 2016, 6, e1145782. [Google Scholar] [CrossRef] [PubMed]
- Ghequire, M.G.; De Mot, R. The tailocin tale: Peeling off phage tails. Trends Microbiol. 2015, 23, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Schol, D. Phage tail-like bacteriocins. Annu. Rev. Virol. 2017, 4, 453–467. [Google Scholar] [CrossRef]
- Cianfanelli, F.R.; Alcoforado Diniz, J.; Guo, M.; De Cesare, V.; Trost, M.; Coulthurst, S.J. VgrG and PAAR proteins define distinct versions of a functional Type VI secretion system. PLoS Pathog. 2016, 12, e1005735. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.A.; Funkhouser-Jones, L.J.; Brileya, K.; Reysenbach, A.-L.; Bordenstein, S.R. Antibacterial gene transfer across the tree of life. eLife 2014, 3, e04266. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.; Sattelberger, E.; Inman, R.B.; Calendar, R.; Loessner, M.J. Genome and proteome of Listeria monocytogenes phage PSA: An unusual case for programmed + 1 translational frameshifting in structural protein synthesis. Mol. Microbiol. 2003, 50, 303–317. [Google Scholar] [CrossRef]
- Sharma, V.; Firth, A.E.; Antonov, I.; Fayet, O.; Atkins, J.F.; Borodovsky, M.; Baranov, P.V. A pilot study of bacterial genes with disrupted orfs reveals a surprising profusion of protein sequence recoding mediated by ribosomal frameshifting and transcriptional realignment. Mol. Biol. Evol. 2011, 28, 3195–3211. [Google Scholar] [CrossRef] [PubMed]
- Fogg, P.C.M.; Westbye, A.B.; Beatty, J.T. One for all or all for one: Heterogeneous expression and host cell lysis are key to gene transfer agent activity in Rhodobacter capsulatus. PLoS ONE 2012, 7, e43772. [Google Scholar] [CrossRef]
- Matson, E.G.; Thompson, M.G.; Humphrey, S.B.; Zuerner, R.L.; Stanton, T.B. Identification of genes of VSH-1, a prophage-like gene transfer agent of Brachyspira hyodysenteriae. J. Bacteriol. 2005, 187, 5885–5892. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, Y. AAA+ ATPases in protein degradation: Structures, functions and mechanisms. Biomolecules 2020, 10, 629. [Google Scholar] [CrossRef]
- Yedidi, R.S.; Wendler, P.; Enenkel, C. AAA-ATPases in protein degradation. Front. Mol. Biosci. 2017, 4, 42. [Google Scholar] [CrossRef]
- Mahony, J.; Alqarni, M.; Stockdale, S.; Spinelli, S.; Feyereisen, M.; Cambillau, C.; Sinderen, D.V. Functional and structural dissection of the tape measure protein of lactococcal phage TP901-1. Sci. Rep. 2016, 8, 36667. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, M.; Bergeron, A.; Poisson, G. The evolution of the tape measure protein: Units, duplications and losses. BMC Bioinform. 2011, 12 (Suppl. 9), S10. [Google Scholar] [CrossRef]
- Zinke, M.; Schröder, G.F.; Lange, A. Major tail proteins of bacteriophages of the order Caudovirales. J. Biol. Chem. 2022, 298, 101472. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.C.; Frank, A.C.; Calteau, A.; Vinnere Pettersson, O.; Granberg, F.; Eriksson, A.S.; Näslund, K.; Holmberg, M.; Lindroos, H.; Andersson, S.G. Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii. PLoS Genet. 2009, 5, e1000546. [Google Scholar] [CrossRef]
- Guy, L.; Nystedt, B.; Toft, C.; Zaremba-Niedzwiedzka, K.; Berglund, E.C.; Granberg, F.; Naslund, C.; Eriksson, A.-S.; Andersson, S.G.E. A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella. PLoS Genet. 2013, 9, e1003393. [Google Scholar] [CrossRef] [PubMed]
- Québatte, M.; Dehio, C. Bartonella gene transfer agent: Evolution, function, and proposed role in host adaptation. Cell. Microbiol. 2019, 21, e13068. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).