Simple Summary
To improve the control of stored-grain pests and protect the environment, the use of natural enemies is attracting increasing attention. Cheyletus malaccensis and Cheyletus eruditus are effective natural enemies of common stored-grain pests, such as Liposcelis bostrychophila Badonnel. To evaluate the predation potential of these two types of predatory mites, this study was conducted by comparing the life histories of two predatory mites and the functional responses on Liposcelis bostrychophila. The results indicate that compared to C. eruditus, C. malaccensis had a shorter development time and longer adult survival time at 28 °C and 75% RH and showed higher predation ability against Liposcelis bostrychophila eggs. It has been proven from both its life history of artificial breeding and the predation ability that C. malaccensis has much greater biocontrol potential than C. eruditus against stored-grain pests.
Abstract
Cheyletus malaccensis Oudemans and Cheyletus eruditus (Schrank) are predators of stored-grain pests in China. The psocid Liposcelis bostrychophila Badonnel is prone to outbreaks in depots. To assess the potential of large-scale breeding with Acarus siro Linnaeus and the biological control potential of C. malaccensis and C. eruditus against L. bostrychophila, we determined the development times of different stages at 16, 20, 24, and 28 °C and 75% relative humidity (RH) while feeding on A. siro, as well as the functional responses of both species’ protonymphs and females to L. bostrychophila eggs at 28 °C and 75% RH. Cheyletus malaccensis had a shorter development time and longer adult survival time than C. eruditus at 28 °C and 75% RH and could establish populations faster than C. eruditus while preying on A. siro. The protonymphs of both species showed a type II functional response, while the females showed a type III functional response. Cheyletus malaccensis showed a higher predation ability than C. eruditus, and the females of both species had a higher predation ability than the protonymphs. Based on the observed development times, adult survival times, and predation efficiency, Cheyletus malaccensis has much greater biocontrol potential than C. eruditus.
1. Introduction
Cheyletus malaccensis Oudemans and Cheyletus eruditus (Schrank) (Acari: Cheyletidae) are predatory Cheyletus species that can adapt to a wide range of temperatures and humidity levels [1,2]. They are widely distributed in depots and feed commodity stores in Asia, Europe, and North America [3,4,5]. Both species prey on acarid grain mites and small arthropods, such as the eggs and larvae of stored-grain pests [6,7,8]. The two predatory mites present an environmentally friendly pest control method that is easy to use and does not rely on large instruments and equipment. Given these traits, the Cheyletus species can be employed to control stored products pests [9,10,11,12].
The booklouse Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae) is a common stored-grain pest that is prone to outbreaks in depots [13,14]. It causes agricultural and economic losses, threatens food security [15], and is hard to control because of its small size, rapid reproduction, and ability to survive for long periods without food [14,16,17]. Current measures against L. bostrychophila still rely on insecticide application [18]. However, chemical pesticides have led to problems, such as pesticide residue, pest resistance, and risk of environmental pollution [19,20,21]. Therefore, it is important to develop alternative control methods for stored-grain pests [19]. Biological control, such as the use of predatory mites, is an effective alternative [22,23].
The functional response is one of the most important measures of predator behavior and can reveal different aspects of prey–predator interactions and assess predator predation efficacy by estimating the attack rate and handling time [24,25,26]. There are three types of functional responses: Holling I, II, and III [27]. The functional responses of C. eruditus and C. malaccensis to different prey have been reported separately, and most reports have shown the Holling II response. For C. malaccensis, the prey studied include Aleuroglyphus ovatus (Acari: Sarcoptiformes), Lepidoglyphus destructor (Acaridida: Glycyphagidae), Megoura japonica (Hemiptera: Aphididae), Panonychus citri (Acari: Tetranychidae), and so on [28,29,30,31,32]. For C. eruditus, the functional responses to Tyrophagus putrescentiae (Acari: Acaridae) and A. ovatus were studied [31,32]. The comparisons of the function responses of C. malaccensis and C. eruditus on the same prey have not been reported. There is also a lack of comparative studies on the life history and population parameters of these two cheyletids under the same feeding conditions.
In our previous work, two cheyletids preyed on the eggs and larvae of common stored-grain pests, especially L. bostrychophila [22,23]. In addition, Acarus siro Linnaeus (Acari: Acaridae) was proven to be a suitable feeding medium for the two species [22,23]. A recent sampling of stored-grain mites showed that the dominant predatory mites in grain depots have changed from C. eruditus to C. malaccensis in China [33,34,35,36]. There are various reasons for this shift in the dominant species, including population growth, prey range, and functional response. In such a situation, in order to choose a more suitable predatory mite to control stored grain pests in China in the future, the biological control potential of both mites needs to be evaluated and compared. Life history and functional response are two important evaluation criteria. In this study, we compared (1) the life history traits of the two mites on the feeding prey A. siro at 16, 20, 24, and 28 °C and 75% relative humidity (RH) to evaluate the potential of large-scale breeding with A. siro and (2) the functional responses of the two cheyletids on the eggs of L. bostrychophila to evaluate the predation efficiency. Based on the above information, we hope to provide fundamental information for effective large-scale artificial feeding and practical application.
2. Materials and Methods
2.1. Predator and Prey Sources
Cheyletus malaccensis was collected in Haikou, Hainan Province, China. Cheyletus eruditus was obtained from the Crop Research Institute, Prague, Czech Republic. Cheyletids were reared at 28 °C and 75% RH in the dark at the Institute of Grain Storage and Logistics Academy of National Food and Strategic Reserves Administration in Beijing, China. The Crop Research Institute provided the A. siro, and the mites were reared on whole wheat flour at 28 °C and 75% RH in the dark. Liposcelis bostrychophila was collected in Beijing (40°7′55.20″ N, 116°24′46.08″ E), China. Psocids were reared on a mixture of whole wheat flour and yeast (1:1) at 26 °C and 68% RH in the dark.
2.2. Life History Traits of Cheyletus malaccensis and Cheyletus eruditus
Female adult C. malaccensis and C. eruditus specimens were randomly selected from the feeding box. The mites were reared individually in plastic microrearing cells [37] with 15–20 A. siro adults as prey at 28 °C and 75% RH. After 24 h, 50 eggs were collected and designated as the F1 generation for further study. Eggs were individually placed inside microrearing cells and subjected to different temperatures (16, 20, 24, and 28 °C) at 75% RH in the dark. The data of C. malaccensis at 24 and 28 °C, with 75% RH come from our published articles [37]. Eggs were checked daily in each container. After the eggs hatched, 15 to 20 A. siro adults were added daily as food for each C. malaccensis and C. eruditus. Based on daily observations, the egg incubation periods and development times of larvae, protonymphs, deutonymphs (absent in males of C. malaccensis), and adult longevity (males and females) were determined. Twenty mites were included in each experiment for each predator mite species. Cheyletus eruditus can reproduce by gynogenesis, and males are rarely found [38,39]. Therefore, only the development times of C. eruditus females at different temperatures were determined. For C. malaccensis, the development times of both females and males were determined.
2.3. Functional Responses of Cheyletus malaccensis and Cheyletus eruditus
All functional response experiments were conducted at 28 °C and 75% RH in the dark. Functional responses of protonymphs and female adults of C. malaccensis and C. eruditus to eggs of L. bostrychophila were investigated. Before an experiment, each predator was starved for 24 h to standardize the degree of hunger [40]. Protonymph and adult female predatory mites were provided with 1, 3, 5, 7, or 10 prey per rearing cell. After 24 h, predators were removed, and the number of eggs consumed was recorded. Each experiment included 15 individual predatory mites.
The type of functional response curve was described by a logistic regression [41] as follows:
where Na is the number of prey consumed; N0 is the initial prey density; and P0, P1, P2, and P3 are the constant, linear, quadratic, and cubic coefficients, respectively. The type of functional response was determined by the values of P1 and P2, where significantly negative values (P1 < 0) exhibit a type II response and significantly positive values (P1 > 0) exhibit a type III response. Based on the logistical analysis results, the attack rat (a) and the handing time (Th) of type II were calculated using the Holling equation [42,43] as follows:
where Na is the number of prey consumed; a (number of prey/predator) is the attack rate; N0 is the number of prey offered; and Th is the handling time in hours. Predation ability was determined as a/Th.
Na = a N0/(1 + a Th N0)
For type III, the following model [44] was used:
where b is the attack rate for type III.
Na = N0{1 − exp [b N0(Th Na − 1)}
2.4. Statistical Analysis
Development times and adult longevity of different stages of C. malaccensis and C. eruditus were analyzed using IBM SPSS Statistics 20.0. One-way ANOVA and Tukey’s Honestly Significant Difference (HSD) multiple range tests were used with a significance level of 0.05. The means, standard errors, and variances of the population parameters were estimated using the bootstrap technique (10,000 samples) [45,46,47], which is contained in the TWOSEX-MSChart program [48,49,50]. Curve fitting was performed on functional response models using MATLAB (https://matlab.mathworks.com/, accessed on 1 April 2023).
3. Results
3.1. Life History of Cheyletus malaccensis and Cheyletus eruditus
Both C. malaccensis and C. eruditus completed their life cycles at temperatures ranging from 16 °C to 28 °C (Table 1, Figure 1). The incubation period of C. eruditus eggs ranged from 3.57 days (28 °C) to 11.5 days (16 °C). In C. malaccensis, for female eggs, the incubation period ranged from 2.25 days (28 °C) to 10.25 days (16 °C); for male eggs, it ranged from 1.83 days (28 °C) to 9.50 days (16 °C). The longest development times from egg to adult in both species were observed at 16 °C. For C. eruditus females, the development time was 49.38 days; for C. malaccensis females, it was 60.00 days; and for C. malaccensis males, it was 59.50 days. The shortest times were observed at 28 °C. For C. eruditus females, the development time was 15.71 days; for C. malaccensis females, it was 14.00 days; and for C. malaccensis males, it was 9.50 days. The development time from egg to adult for C. malaccensis was generally shorter than that of C. eruditus at the same temperature, except at 16 °C. The development times of the immature stages decreased with increasing temperature. In addition, the survival times of C. malaccensis were generally longer than those of C. eruditus, except at 16 °C. Female adult mites showed the most predatory potential. The survival times of C. malaccensis females were 58.00 days and 66.50 days at 24 and 28 °C, respectively; for C. eruditus females, the survival times were 19.50 days and 15.00 days at these temperatures. This represents about a three to four times difference in survival time. We also compared the population parameters of C. malaccensis and C. eruditus at different temperatures (Table 2). The net reproductive rate (R0) was significantly higher for C. malaccensis than C. eruditus at 24 and 28 °C. The fecundity of C. malaccensis was significantly higher than C. eruditus at 20, 24, and 28 °C. However, the intrinsic rate of increase (rm) and the finite rate of increase (λ) of C. eruditus were higher than those of C. malaccensis at 16 °C.

Table 1.
Development times (days) of different stages of Cheyletus eruditus and Cheyletus malaccensis reared at different temperatures in the laboratory (mean ± SD).
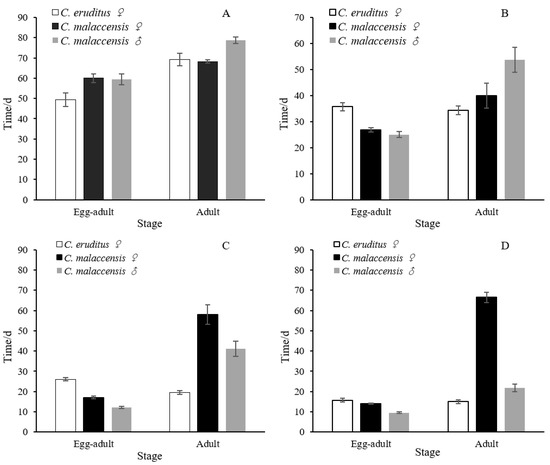
Figure 1.
Egg to adult development times and adult survival times of Cheyletus malaccensis (males and females) and Cheyletus eruditus (females) at different temperatures: (A) 16 °C, (B) 20 °C, (C) 24 °C, and (D) 28 °C.

Table 2.
Population parameters of Cheyletus malaccensis and Cheyletus eruditus at different temperatures.
3.2. Functional Responses
The functional responses of C. malaccensis and C. eruditus to the eggs of L. bostrychophila at 28 °C and 75% RH are shown in Figure 2. The consumption of eggs of L. bostrychophila by C. malaccensis protonymphs and females was higher than that by C. eruditus (Table 3). In addition, the consumption of eggs by females was higher than that by protonymphs in both species. C. malaccensis protonymphs and C. eruditus protonymphs and females consumed more L. bostrychophila eggs than the first-instar larvae.
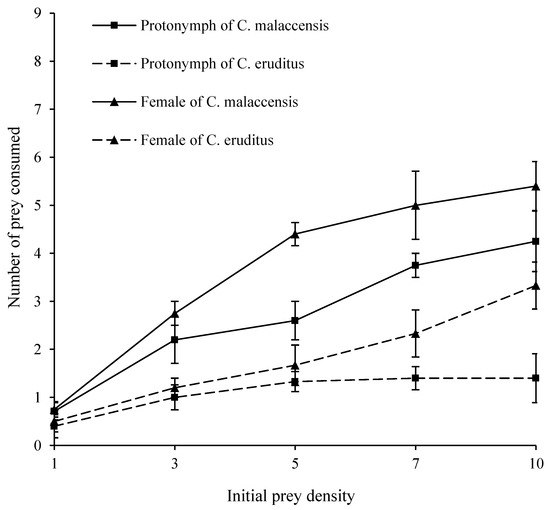
Figure 2.
Functional responses of protonymphs and females of Cheyletus malaccensis and Cheyletus eruditus on eggs of Liposcelis bostrychophila at 28 °C and 75% RH.

Table 3.
The consumption of Cheyletus malaccensis and Cheyletus eruditus preying on eggs of Liposcelis bostrychophila at 28 °C and 75% RH.
Based on logistic regression analyses (Table 4), the protonymphs of both species showed a type II functional response (P1 < 0). The females of C. eruditus exhibited a type II functional response (P1 < 0), while the females of C. malaccensis showed a type III functional response (P1 > 0, P2 < 0).

Table 4.
The functional responses of Cheyletus malaccensis and Cheyletus eruditus based on logistic regression.
The parameters of the functional responses of C. malaccensis and C. eruditus are shown in Table 5. A comprehensive analysis of the attack rate (a) and handling time (Th) indicated that C. malaccensis had a higher predation ability than C. eruditus, and the females of both species had a higher predation ability than the protonymphs. The predation ability (a/Th) of C. malaccensis females was 9.43 on eggs, whereas that of C. eruditus females was 8.90 on eggs. For the protonymphs, the predation ability of C. malaccensis was 7.39 on eggs, whereas that of C. eruditus was 1.29 on eggs. We compared the predation ability between C. malaccensis and C. eruditus and found that the predation ability of C. malaccensis females was 1.06 times that of C. eruditus on eggs. For C. malaccensis protonymphs, the predation ability was 5.73 times that of C. eruditus on eggs. Comparing the predation ability of the same species, that of C. malaccensis females was 1.28 times that of the protonymphs on eggs. The predation ability of C. eruditus females was 6.90 times that of the protonymphs on eggs.

Table 5.
Parameters of the functional responses of Cheyletus malaccensis and Cheyletus eruditus preying on eggs of Liposcelis bostrychophila at 28 °C and 75% RH.
4. Discussion
The development times of C. malaccensis and C. eruditus fed A. siro at different temperatures were determined. Both species completed development from egg to adult at temperatures ranging from 16 °C to 28 °C. The development times of the two cheyletids decreased with increasing temperature. These trends are consistent with previous studies on the development of C. malaccensis and C. eruditus [1,51,52]. According to our previous studies, the most suitable temperature for reproduction in the two cheyletids is 28 °C [22,37]. In this study, the development times of C. malaccensis were shorter than those of C. eruditus, but the adult survival times of C. malaccensis were longer. The R0 and the fecundity of C. malaccensis were significantly higher than C. eruditus at 20, 24, and 28 °C, while there was no significant difference between these two species at 16 °C. It was reported that the R0 of Neoseiulus californicus (Acari: Phytoseiidae) were 23.67 at 20 °C, 28.56 at 25 °C, and 19.73 at 30 °C [53], which were lower than the two cheyletids. These differences may be caused by the different areas, and the storage environment is also more stable than the field. Therefore, with more rapid development, C. malaccensis could establish populations faster than C. eruditus, which increased its effectiveness as a biocontrol agent. However, the daily temperature fluctuations also significantly affected the development times and longevity of the biocontrol agents studied, resulting in marked deviations and potentially erroneous predictions when compared to their constant temperature regimen counterparts [54,55]. In practical application, we need to adjust measures according to the actual conditions, such as temperature and climate.
Functional response is one of the most important behavioral responses of predators, and it can be used to simulate prey–predator relationships and evaluate the potential predation efficiency of predators [26]. In this study, protonymphs and females of C. malaccensis showed different types of functional responses on L. bostrychophila eggs, that is, type II (protonymphs) and type III (females), while C. eruditus exhibited the same type II functional response. The reason for this may be that the same predator can reveal different functional responses depending on the size and stage of its prey, its degree of hunger, and so on [24,25,26].
In this study, both C. malaccensis and C. eruditus were potential predators on the eggs of L. bostrychophila. In both species, the most active stage was the adult female stage. C. malaccensis had higher attack rates than those of C. eruditus. The predation ability of C. malaccensis was 1.06 times (females preying on eggs) to 5.71 times (protonymphs preying on eggs) higher than that of C. eruditus. This result is consistent with that when A. ovatus larvae were used as prey, where a predation ability of 110.68 was observed for C. malaccensis females and a predation ability 9.27 was observed for C. eruditus females [28,32]. In addition, the daily maximum predation (1/Th) of C. malaccensis protonymphs was higher than that of C. eruditus, while this finding was the opposite in females. All these show that C. malaccensis was more effective in the control of L. bostrychophila and pest mites.
The functional response of a predator to its prey can be influenced by the prey stage and size, predator stage and size, speed of movement, and starvation level of predator [56,57,58]. In this study, C. malaccensis usually had a higher consumption than C. eruditus. However, the daily maximum predation on eggs of C. malaccensis females (5.67) was 4.11 times lower than that of C. eruditus (23.33). The reason for the lower consumption of eggs by C. malaccensis may be that it prefers dynamic and large prey, similar to Amblyseius andersoni Chant (Acari: Phytoseiidae) and Euseius finlandicus (Oudemans) (Acari: Phytoseiidae) [59,60]. Moreover, eggs may be less nutritious, and females need to increase their intake of energy and nutrition to reproduce. The large and fast prey was more difficult to hunt, as it required more time to attack and digest [61]. The eggs of L. bostrychophila are larger than those of A. ovatus. Therefore, the predation ability values on A. ovatus were higher than those of L. bostrychophila.
To improve the control of stored-grain pests and protect the environment, integrated pest management has attracted increasing attention [62]. This study was conducted by comparing the life histories of two predatory mites and the functional responses on L. bostrychophila. However, many factors affected the results of the functional responses observed in laboratory conditions and in practical applications, such as the temperature, the state of the predator and prey, and the degree of hunger. In future studies, we will also need to carry out a wider range of temperatures and attempt real-world assays of efficacy in the wheat depots of China. All of this will provide a theoretical basis and verification for the biological control of predatory mites.
5. Conclusions
In conclusion, C. malaccensis had a shorter development time and longer adult survival time than C. eruditus at 28 °C and 75% RH and could establish populations faster than C. eruditus while preying on A. siro. The protonymphs of both species showed a type II functional response, while females of C. malaccensis showed a type III functional response. Based on the observed development times, adult survival times, and predation efficiency, C. malaccensis has a much greater biocontrol potential than C. eruditus under laboratory conditions. Based on the above information, this study provided fundamental information for the effective large-scale artificial feeding and releasing program of the two cheyletids. All these studies will help to determine the effective temperatures and seasons for both rearing and releasing these predatory mites for different ecological areas of grain storage.
Author Contributions
Conceptualization and funding acquisition, Y.W.; methodology and software W.S. and Y.W.; validation, L.X. and W.S.; writing—original draft preparation, W.S.; writing—review and editing, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Optional Research Project of the Academy of National Food and Strategic Reserves Administration (ZX2201, JY2308) and the National Key R&D Program of China (2016YFD0401004-2).
Data Availability Statement
Data may be requested from the corresponding authors.
Acknowledgments
We greatly appreciate Peihuan He and Lu Liu for their generous support and help while conducting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palyvos, N.E.; Emmanouel, N.G. Reproduction, survival, and life table parameters of the predatory mite Cheyletus malaccensis (Acari: Cheyletidae) at various constant temperatures. Exp. Appl. Acarol. 2011, 54, 139–150. [Google Scholar] [CrossRef]
- Barker, P.S. Bionomics of Cheyletus-eruditus (Schrank) (Acarina, Cheyletidae), a predator of Lepidoglyphus-destructor (Schrank) (Acarina, Glycyphagidae), at 3 constant temperatures. Can. J. Zool. 1991, 69, 2321–2325. [Google Scholar] [CrossRef]
- Ardeshir, F. Cheyletid mites (Acari: Trombidiformes) in stored grains in Iran. Persian J. Acarol. 2017, 6, 11–24. [Google Scholar]
- Stejskal, V.; Hubert, J.; Aulicky, R.; Kucerova, Z. Overview of present and past and pest-associated risks in stored food and feed products: European perspective. J. Stored Prod. Res. 2015, 64, 122–132. [Google Scholar] [CrossRef]
- Sinha, R.N.; Wallace, H.A.H. Population dynamics of stored-product mites. Oecologia 1973, 12, 315–327. [Google Scholar] [CrossRef]
- Shen, Z.P. The biology of stored mites—Cheyletus malaccensis. Grain Storage 1997, 5, 50–51. [Google Scholar]
- Lin, P. Phylogenetic Status and Genetic Diversity of Cheyletus malaccensis Oudemans. Master’s Thesis, Nanchang University, Nanchang, China, 2008. [Google Scholar]
- Woodroffe, G.E. An ecological study of the insects and mites in the nests of certain birds in Britain. Bull. Entomol. Res. 1953, 44, 739–772. [Google Scholar] [CrossRef]
- Palyvos, N.E.; Athanassiou, C.G.; Kavallieratos, N.G. Acaricidal effect of a diatomaceous earth formulation against Tyrophagus putrescentiae (Astigmata: Acaridae) and its predator Cheyletus malaccensis (Prostigmata: Cheyletidae) in four grain commodities. J. Econ. Entomol. 2006, 99, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Munzbergova, Z.; Kucerova, Z.; Stejskal, V. Comparison of communities of stored product mites in grain mass and grain residues in the Czech Republic. Exp. Appl. Acarol. 2006, 39, 149–158. [Google Scholar] [CrossRef]
- Pekar, S.; Hubert, J. Assessing biological control of Acarus siro by Cheyletus malaccensis under laboratory conditions: Effect of temperatures and prey density. J. Stored Prod. Res. 2008, 44, 335–340. [Google Scholar] [CrossRef]
- Žďárková, E.; Horák, E. Preventive biological control of stored food mites in empty stores using Cheyletus eruditus (Schrank). Crop Prot. 1990, 9, 378–382. [Google Scholar] [CrossRef]
- Žďárková, E. Mass rearing of the predator Cheyletus eruditus (Schrank) (Acarina: Cheyletidae) for biological control of acarid mites infesting stored products. Crop Prot. 1986, 5, 122–124. [Google Scholar] [CrossRef]
- Cebolla, R.; Pekar, S.; Hubert, J. Prey range of the predatory mite Cheyletus malaccensis (Acari: Cheyletidae) and its efficacy in the control of seven stored-product pests. Biol. Control 2009, 50, 1–6. [Google Scholar] [CrossRef]
- Chen, W.X.; Wang, J.J.; Zhao, Z.M.; Ding, W. The advance research of Liposcelis bostrychophila Badonnel and L. entomophila (Enderlein)(Psopotera:Liposcelididae). Grain Storage 2003, 32, 3–7. [Google Scholar]
- Nayak, M.K.; Collins, P.J.; Throne, J.E.; Wang, J.J. Biology and Management of Psocids Infesting Stored Products. Annu. Rev. Entomol. 2014, 59, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Marco, G.; Pelta, R.; Carnes, J.; Iraola, V.; Zambrano, G.; Baeza, M.L. Occupational allergic asthma induced by Liposcelis decolor. Allergol. Int. 2016, 65, 210–211. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Pan, Y.M.; Xie, Y.X.; Lu, Y.J.; Cao, Y.F. Fumigant toxicity of four agents in combination against adults of Liposcelis bostrychophila Badonnel. Grain Storage 2016, 45, 7–11. [Google Scholar]
- Rees, D.P.; Walker, A.J. The effect of temperature and relative-humidity on population-growth of 3 Liposcelis species (Psocoptera, Liposcelidae) infesting stored products in tropical countries. Bull. Entomol. Res. 1990, 80, 353–358. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational Approaches to Managing Stored-Product Insects. Annu. Rev. Entomol. 2009, 55, 375–397. [Google Scholar] [CrossRef]
- Forget, G.; Goodman, T.; Villiers, A.D. Impact of Pesticide Use on Health in Developing Countries. In Proceedings of the Symposium Held in Ottawa, Ottawa, Canada, 17–20 September 1990; International Development Research Centre: Ottawa, ON, Canada, 1993. [Google Scholar]
- Perry, A.S.; Yamamoto, I.; Ishaaya, I.; Perry, R. Insecticides in Agriculture and Environment. Crop Prot. 1998, 17, 221–249. [Google Scholar]
- He, P.H.; Zhang, T.; Wu, Y.; Li, Y.Y.; Li, Z.H.; Li, F.J.; Stejskal, V.; Zuzana, K.; Radek, A.; Jiang, Y.J.; et al. Predation Ability of Cheyletus eruditus (Schrank) (Acari: Cheyletidae) on 9 Species of Stored Grain Insect Pests. J. Chin. Cereals Oils Assoc. 2016, 31, 112–117. [Google Scholar]
- Liu, L. A Preliminary Study on the Growing Development and Predatory Ability of Cheyletus malaccensis Oudemans. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2018. [Google Scholar]
- Poncio, S.; Montoya, P.; Cancino, J.; Nava, D.E. Determining the Functional Response and Mutual Interference of Utetes anastrephae (Hymenoptera: Braconidae) on Anastrepha obliqua (Diptera: Tephritidae) Larvae for Mass Rearing Purposes. Ann. Entomol. Soc. Am. 2016, 109, 518–525. [Google Scholar] [CrossRef]
- Yazdani, M.; Keller, M. The shape of the functional response curve of Dolichogenidea tasmanica (Hymenoptera: Braconidae) is affected by recent experience. Biol. Control 2016, 97, 63–69. [Google Scholar] [CrossRef]
- Costa, J.F.; Matos, C.H.C.; de Oliveira, C.R.F.; da Silva, T.G.F.; Neto, I. Functional and numerical responses of Stethorus tridens Gordon (Coleoptera: Coccinellidae) preying on Tetranychus bastosi Tuttle, Baker & Sales (Acari: Tetranychidae) on physic nut (Jatropha curcas). Biol. Control 2017, 111, 1–5. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Zhu, P.P.; Fan, Y.X.; Mo, W.F.; Xin, T.R.; Xia, B.; Zou, Z.W. Functional response of adult Cheyletus malaccensis (Acari: Cheyletidae) to different developmental stages of Aleuroglyphus ovatus (Acari: Acaridae). J. Stored Prod. Res. 2019, 84, 101525. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Lin, J.Z. The Predatory Effect of Cheyletus malaccensis On Lepidoglyphus destructor. Entomol. J. East China 1996, 39, 65–68. [Google Scholar]
- Yin, J.D.; Li, Y.H.; Li, X.E.; Mo, W.F.; Cong, Z.; Jing, W.; Xin, T.R.; Zou, Z.W.; Xia, B. Predation of Cheyletus malaccensis (Acari: Cheyletidae) on Megoura japonica (Hemiptera: Aphididae) under five different temperatures. Int. J. Acarol. 2019, 45, 176–180. [Google Scholar] [CrossRef]
- Xia, B.; Gong, Z.Q.; Zou, Z.W.; Zhu, Z.M. The predation of Cheyletus eruditus (Acari: Cheyletidae)on Tyrophagus putrescentiae (Acari: Acaridae). J. Nanchang Univ. (Nat. Sci.) 2003, 27, 334–337. [Google Scholar]
- Xia, B.; Luo, D.M.; Zou, Z.W.; Xu, R.; Zhu, Z.M. Predation of Cheyletus eruditus on Aleuroglyphus ovatus. Chin. Bull. Entomol. 2007, 44, 549–552. [Google Scholar]
- Chen, Q.Z. A Preliminary report on the faunal investigation of the warehouse pests in Tibet Autonomous Region. J. Zhengzhou Grain Coll. 1990, 3, 29–41. [Google Scholar]
- Chen, Q.Z. Investigation and Research on Storage Pests in China. Grain Sci. Technol. Econ. 1994, 5, 6–9. [Google Scholar]
- Li, X.D.; Li, G.C.; Hao, L.J. Investigation of stored grain mites in Henan Province. J. Zhengzhou Grain Coll. 1988, 4, 64–69. [Google Scholar]
- Lukas, J.; Stejskal, V.; Jarosik, V.; Hubert, J.; Zd’arkova, E. Differential natural performance of four Cheyletus predatory mite species in Czech grain stores. J. Stored Prod. Res. 2007, 43, 97–102. [Google Scholar] [CrossRef]
- Sun, W.W.; Cui, M.; Xia, L.Y.; Yu, Q.; Cao, Y.; Wu, Y. Age-Stage, Two-Sex Life Tables of the Predatory Mite Cheyletus malaccensis Oudemans at Different Temperatures. Insects 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.H. Study on the life history of Cheyletus eruditus (Schrank). Jiangxi Plant Prot. 1999, 3, 14–15. [Google Scholar]
- Hughes, A.M. The Mites of Stored Food and Houses; Her Majesty’s Stationery Office: London, UK, 1976. [Google Scholar]
- Dehkordi, S.D.; Sahragard, A. Functional Response of Hippodamia variegata (Coleoptera: Coccinellidae) to Different Densities of Aphis gossypii (Hemiptera: Aphididae) in an Open Patch Design. J. Agric. Sci. Technol. 2013, 15, 651–659. [Google Scholar]
- Juliano, S.A. Nonlinear Curve Fitting: Predation and Functional Response Curves; Chapman & Hall: London, UK, 1993; pp. 178–196. [Google Scholar]
- Holling, C.S. The Components of Predation as Revealed by a Study of Small-Mammal Predation of the European Pine Sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of Simple Types of Predation and Parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Hassanpour, M.; Nouri-Ganbalani, G.; Mohaghegh, J.; Enkegaard, A. Functional response of different larval instars of the green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae), to the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). J. Food Agric. Environ. 2009, 7, 424–428. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Brandstätter, E. Confidence intervals as an alternative to significance testing. Methods Psychol. Res. Online 1999, 4, 43–46. [Google Scholar]
- Huang, Y.B.; Chi, H. Assessing the Application of the Jackknife and Bootstrap Techniques to the Estimation of the Variability of the Net Reproductive Rate and Gross Reproductive Rate: A Case Study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agric. For. 2012, 61, 37–45. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2022; Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 11 January 2022).
- Toldi, M.; Faleiro, D.C.C.; Da Silva, G.L.; Ferla, N.J. Life cycle of the predatory mite Cheyletus malaccensis (Acari: Cheyletidae) fed on Poultry Red Mite Dermanyssus gallinae (Acari: Dermanyssidae). Syst. Appl. Acarol. 2017, 22, 1422–1430. [Google Scholar] [CrossRef]
- Žd’árková, E. Biological control of storage mites by Cheyletus eruditus. Integr. Pest Manag. Rev. 1998, 3, 111–116. [Google Scholar] [CrossRef]
- Gotoh, T.; Yamaguchi, K.; Mori, K. Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2004, 32, 15–30. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of Constant and Fluctuating Temperatures on Development Rates and Longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Chailleux, A.; Mohl, E.K.; Alves, M.T.; Messelink, G.J.; Desneux, N. Natural enemy-mediated indirect interactions among prey species: Potential for enhancing biocontrol services in agroecosystems. Pest Manag. Sci. 2014, 70, 1769–1779. [Google Scholar] [CrossRef]
- Su, J.; Zhu, A.D.; Han, G.D.; Dong, F.; Chen, J.; Zhang, J.P. Re-adaptation from alternative prey to target prey increased predation of predator on target mite. Syst. Appl. Acarol. 2019, 24, 467–476. [Google Scholar] [CrossRef]
- Uiterwaal, S.F.; Mares, C.; DeLong, J.P. Body size, body size ratio, and prey type influence the functional response of damselfly nymphs. Oecologia 2017, 185, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.L.; Nojima, S.; Kikuchi, T. Mechanics of prey size preference in the gastropod Neverita didyma preying on the bivalve Ruditapes philippinarum. Mar. Ecol.-Prog. Ser. 1987, 40, 87–93. [Google Scholar] [CrossRef]
- Blackwood, J.S.; Schausberger, P.; Croft, B.A. Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ. Entomol. 2001, 30, 1103–1111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).