Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Data
2.2. Environmental Variables
2.3. MaxEnt Modelling and Validation
2.4. Dynamic Change in Suitable Areas
3. Results
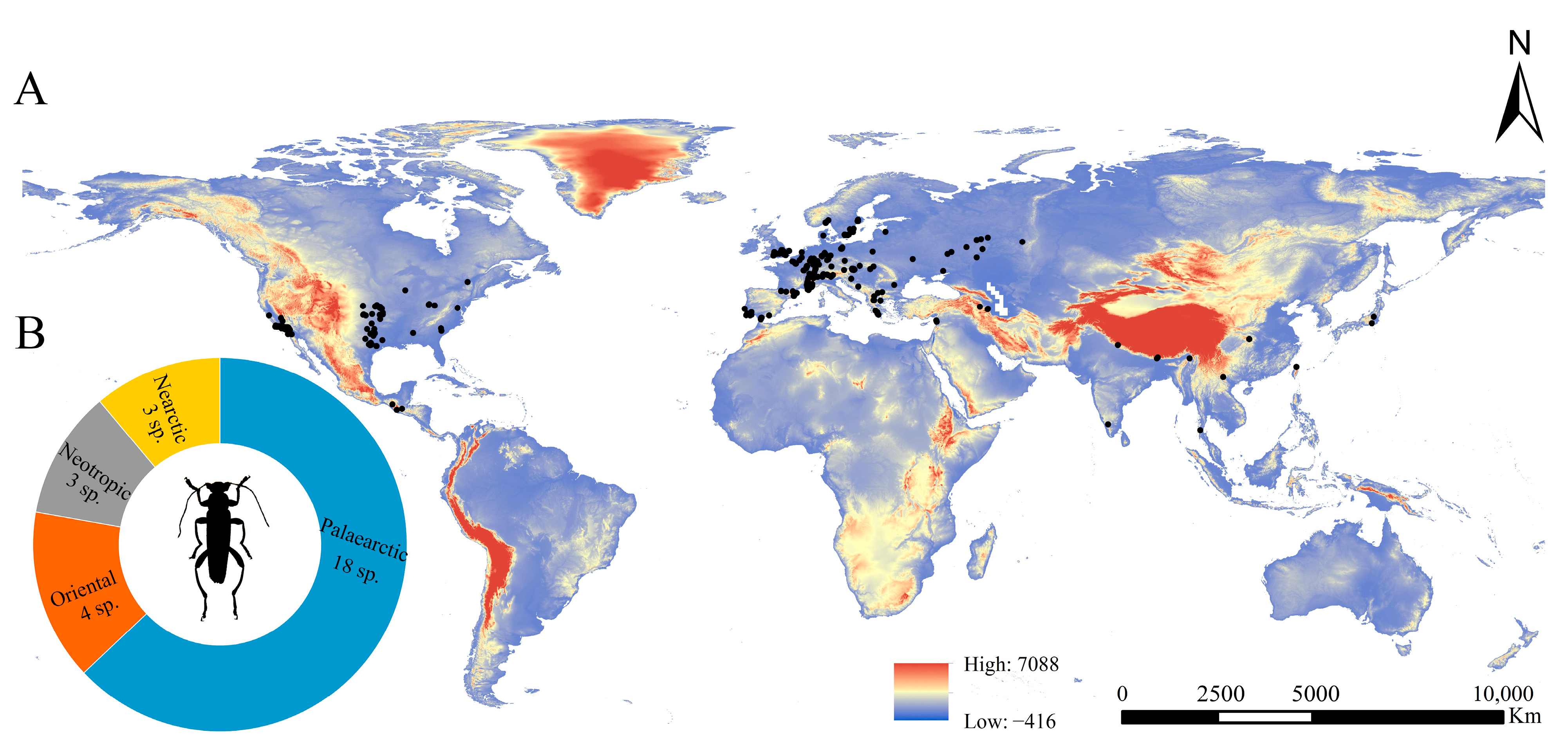
3.1. Geographical Distribution Pattern of Osphya
3.2. Potential Distribution of Osphya in Current Period
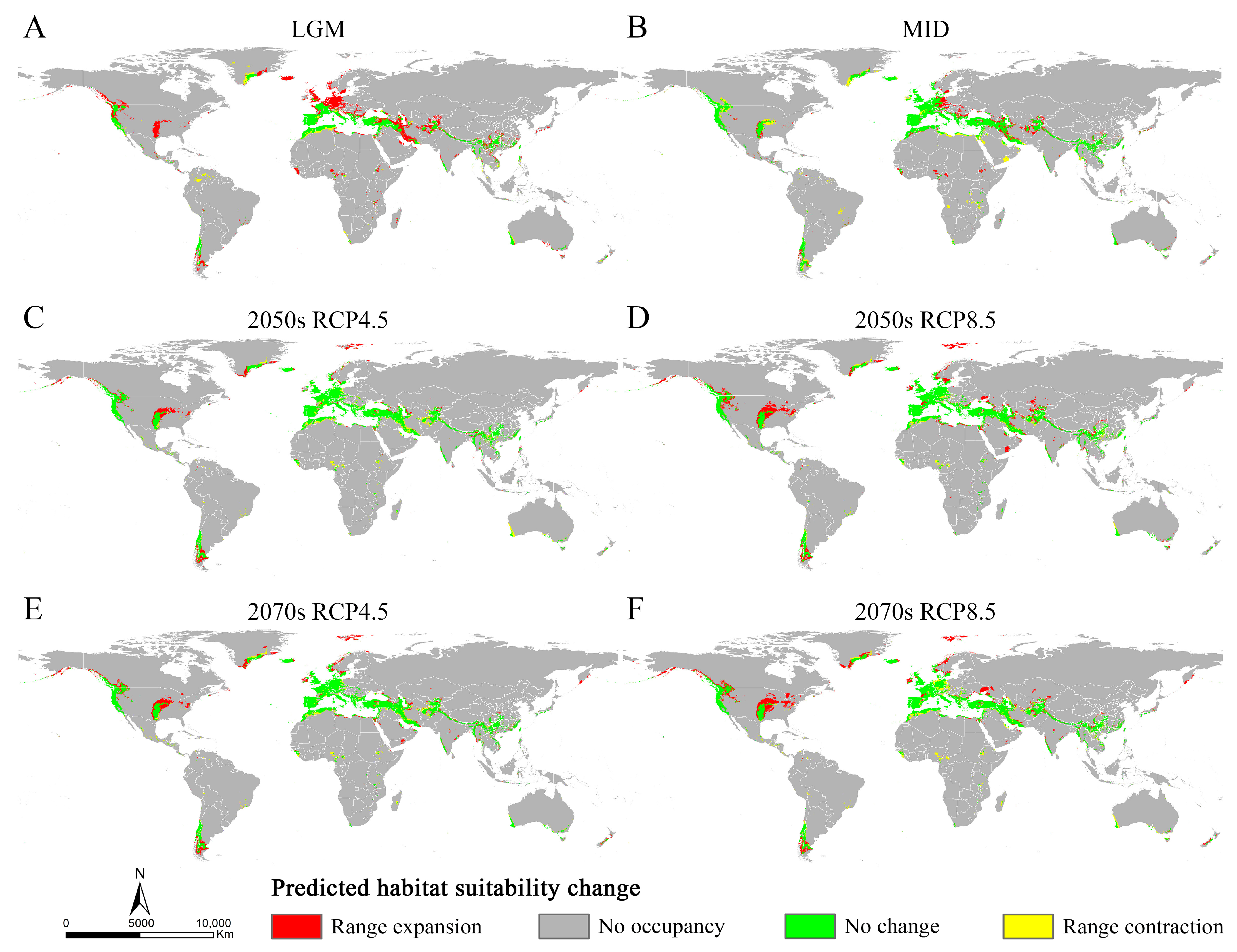
3.3. Potential Distribution of Osphya under Past and Future Climate Scenarios
3.4. Changes in Suitable Areas of Osphya from Past to Future
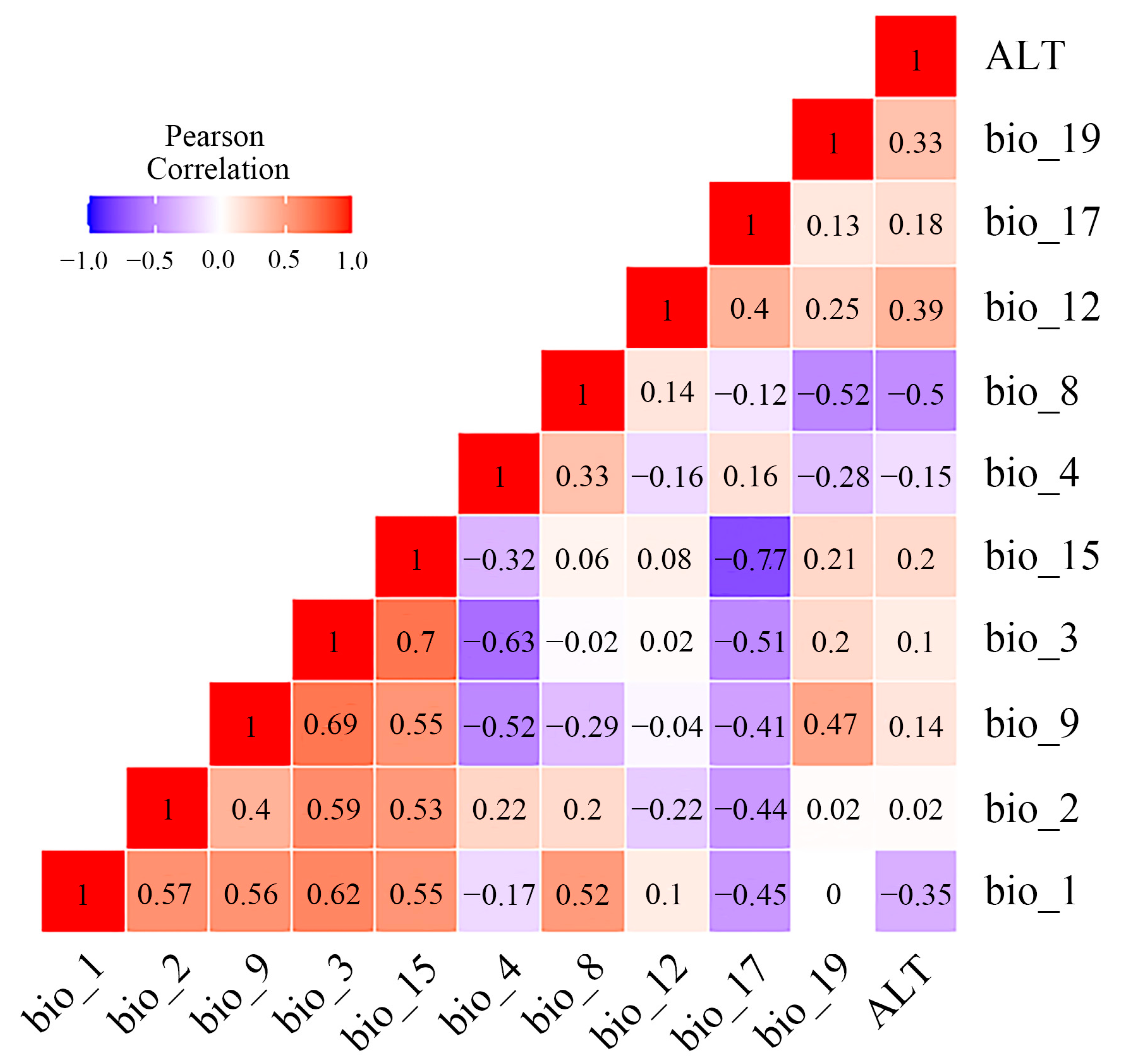
3.5. Determinants Affecting Geographical Distribution of Osphya
4. Discussion
4.1. Characteristics and Formation of Distribution Pattern of Osphya
4.2. Dynamic Changes in Potential Distribution of Osphya
4.3. Environmental Variables Affecting Suitability of Osphya
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amanda, B. Biogeography: Species Distribution. 2021. Available online: https://www.thoughtco.com/what-is-biogeography-1435311 (accessed on 13 April 2023).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, J.; Li, Y.; Jiang, L.Y.; Qiao, G.X. Congruent patterns between species richness and areas of endemism of the Greenideinae aphids (Hemiptera: Aphididae) revealed by global-scale data. Zool. J. Linn. Soc. 2018, 183, 791–807. [Google Scholar] [CrossRef]
- Manel, S.; Guerin, P.-E.; Mouillot, D.; Blanchet, S.; Velez, L.; Albouy, C.; Pellissier, L. Global determinants of freshwater and marine fish genetic diversity. Nat. Commun. 2020, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, H.Y.; Wang, Y.N.; Xi, H.C.; Yang, Y.X. Assessing the diversity and distribution pattern of the speciose genus Lycocerus (Coleoptera: Cantharidae) by the global-scale data. Front. Ecol. Evol. 2022, 10, 794750. [Google Scholar] [CrossRef]
- Currie, D.J.; Paquin, V. Large-scale biogeographical patterns of species richness of trees. Nature 1987, 329, 326–327. [Google Scholar] [CrossRef]
- Currie, D.J. Energy and large-scale patterns of animal-and plant-species richness. Am. Nat. 1991, 137, 27–49. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Porter, E.E. Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Glob. Ecol. Biogeogr. 2003, 12, 475–481. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. The relative roles of environment and history as controls of tree species composition and richness in Europe. J. Biogeogr. 2005, 32, 1019–1033. [Google Scholar] [CrossRef]
- Fine, P.V.; Ree, R.H. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 2006, 168, 796–804. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef]
- Jansson, R.; Davies, T.J. Global variation in diversification rates of flowering plants: Energy vs. climate Change. Ecol. Lett. 2008, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.M.; Qu, Y.H.; Song, G. Species diversification and phylogeographical patterns of birds in response to the uplift of the Qinghai-Tibet Plateau and Quaternary glaciations. Curr. Zool. 2014, 60, 149–161. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.Y.; Tong, J.B.; Yang, Y.X. Habitat suitability of neotenic net-winged beetles (Coleoptera: Lycidae) in China using combined ecological models, with implications for biological conservation. Divers. Distrib. 2022, 28, 2806–2823. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Abellán, P. Effects of climate change on the distribution of threatened invertebrates in a Mediterranean hotspot. Insect Conserv. Diver. 2022, 15, 370–379. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Menéndez, R. How are insects responding to global warming? Tijdschr. Voor Entomolgie 2007, 150, 355. [Google Scholar]
- IPCC. Summary for policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Portner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Pean, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Liu, H.; Yuan, L.; Wang, P.; Pan, Z.; Tong, J.; Wu, G.; Yang, Y. First record of Osphya (Melandryidae: Osphyinae) from Chinese mainland based on morphological evidence and mitochondrial genome-based phylogeny of Tenebrionoidea. Diversity 2023, 15, 282. [Google Scholar] [CrossRef]
- Hunt, T.; Bergsten, J.; Levkanicova, Z.; Papadopoulou, A.; John, O.; Wild, R.; Hammond, P.M.; Ahrens, D.; Balke, M.; Caterino, M.S.; et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a Superradiation. Science 2007, 318, 1913–1916. [Google Scholar] [CrossRef]
- Jackson, S.T. Climate Change Throughout History. 2023. Available online: https://www.britannica.com/explore/savingearth/climate-change-throughout-history (accessed on 13 April 2023).
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, H.H.; Wu, Y.X.; Zeng, L.D.; Huang, X.L. Spatial patterns and determinants of the diversity of Hemipteran insects in the Qinghai-Tibetan Plateau. Front. Ecol. Evol. 2019, 7, 165. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Porfirio, L.L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Bindoff, N.L.; Mackey, B. Improving the use of species distribution models in conservation planning and management under climate change. PLoS ONE 2014, 9, e113749. [Google Scholar] [CrossRef] [PubMed]
- Jarvie, S.; Svenning, J.-C. Using species distribution modelling to determine opportunities for trophic rewilding under future scenarios of climate change. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170446. [Google Scholar] [CrossRef]
- Zhao, H.; Xian, X.; Zhao, Z.; Zhang, G.; Liu, W.; Wan, F. Climate change increases the expansion risk of Helicoverpa zea in China according to potential geographical distribution estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Elith, J.; Ferrier, S.; Huettmann, F.; Leathwick, J. The evaluation strip: A new and robust method for plotting predicted responses from species distribution models. Ecol. Model. 2005, 186, 280–289. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- NTA. NT weed risk assessment report: Andropogon gayanus (gamba grass). In Natural Resources Division, Department of Natural Resources, Environment, The Arts and Sport; Northern Territory of Australia: Palmerston, NT, Australia, 2009. [Google Scholar]
- Wang, R.L.; Yang, H.; Luo, W.; Wang, M.T.; Lu, X.L.; Huang, T.T.; Zhao, J.P.; Li, Q. Predicting the potential distribution of the Asian citrus psyllid, Diaphorin acitri (Kuwayama), in China using the MaxEnt model. PeerJ 2019, 7, e7323. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Y.; Feng, L.; Zhao, Y.F.; Feng, L.; Wu, D.; Zhu, C.P. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Heinrichs, J.A.; Bender, D.J.; Gummer, D.L.; Schumaker, N.H. Assessing critical habitat: Evaluating the relative contribution of habitats to population persistence. Biol. Conserv. 2010, 143, 2229–2237. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, B.; Hu, Y.B.; Nie, Y.G.; Singh, P.B.; Khatiwada, R.R.; Yan, L.; Gu, X.D.; Wei, F.W. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10544. [Google Scholar] [CrossRef] [PubMed]
- Cord, A.; Rodder, D. Inclusion of habitat availability in species distribution models through multi-temporal remote sensing data? Ecol. Appl. 2011, 21, 3285–3298. [Google Scholar] [CrossRef]
- Jimenez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the 21st International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; ACM Press: New York, NY, USA, 2004; Volume 83, pp. 655–662. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Yee, T.W.; Mitchell, N.D. Generalized additive models in plant ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species MaxEnt model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Moreno-Amat, E.; Mateo, R.G.; Nieto-Lugilde, D.; Morueta-Holme, N.; Svenning, J.-C.; García-Amorena, I. Impact of model complexity on crosstemporal transferability in Maxent species distribution models: An assessment using paleobotanical data. Ecol. Model. 2015, 312, 308–317. [Google Scholar] [CrossRef]
- Jose, V.S.; Nameer, P.O. The expanding distribution of the Indian Peafowl (Pavocris tatus) as an indicator of changing climate in Kerala, southern India: A modeling study using MaxEnt. Ecol. Indic. 2020, 110, 105930. [Google Scholar] [CrossRef]
- Vaissi, S. Potential changes in the distributions of Near Eastern fire salamander (Salamandra infraimmaculata) in response to historical, recent and future climate change in the Near and Middle East: Implication for conservation and management. Glob Ecol. Conserv. 2021, 29, e01730. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using district-level occurrences in MaxEnt for predicting the invasion potential of an exotic insect pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Penado, A.; Rebelo, H.; Goulson, D. Spatial distribution modelling reveals climatically suitable areas for bumblebees in under sampled parts of the Iberian Peninsula. Insect Conserv. Divers. 2016, 9, 391–401. [Google Scholar] [CrossRef]
- López-Martínez, V.; Sánchez-Martínez, G.; Jiménez-García, D.; Pérez-Dela, O.N.B.; Coleman, T.W. Environmental suitability for Agrilus auroguttatus (Coleoptera: Buprestidae) in Mexico using MaxEnt and database records of four quercus (Fagaceae) species. Agric. For. Entomol. 2016, 18, 409–418. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Saatchi, S.; Buermann, W.; Steege, H.T.; Mori, S.; Smith, T.B. Modelling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sens. Environ. 2008, 112, 2000–2017. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Zhang, H.T.; Luo, D.; Mu, X.D.; Xu, M.; Wei, H.; Luo, J.R.; Zhang, J.E.; Hu, Y.C. Predicting the potential suitable distribution area of the apple snail Pomacea canaliculata in China based on multiple ecological niche models. J. Appl. Ecol. 2016, 27, 1277–1284. [Google Scholar]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Wieprecht, S.; Zhang, S.H.; Wu, Y.J. Evaluating the ecological influence of hydraulic projects: A review of aquatic habitat suitability models. Renew. Sustain. Energy Rev. 2017, 68, 748–762. [Google Scholar] [CrossRef]
- Mazurov, S.G.; Egorov, L.V.; Ruchin, A.B.; Artaev, O.N. Biodiversity of Coleoptera (Insecta) in Lipetsk Region (Russia). Diversity 2022, 14, 825. [Google Scholar] [CrossRef]
- Egorov, L.V.; Alekseev, S.K.; Ruchin, A.B.; Sazhnev, A.S.; Artaev, O.N.; Esin, M.N.; Lobachev, E.A.; Lukiyanov, S.V.; Semenov, A.V.; Lukyanova, Y.A.; et al. Biodiversity of Coleoptera (Insecta) in the middle and lower Volga Regions (Russia). Diversity 2022, 14, 1128. [Google Scholar] [CrossRef]
- Lewis, G. On the Cistelidae and other heteromerous species of Japan. Ann. Mag. Nat. Hist. 1895, 15, 250–278. [Google Scholar] [CrossRef]
- Champion, G.C. Melandryidae. In Biologia Centrali-Americana; Insecta, Coleoptera; R. H. Porter: London, UK, 1889; Volume 4, pp. 75–103. [Google Scholar]
- Champion, G.C. Notes on Melandryidae (2-3). Entomol. Mon. Magazi. 1916, 52, 1–10, 32–40, 52–59, 75–83, 99–108, 144–156. [Google Scholar]
- Champion, G.C. Some Indian Coleoptera (2). Entomol. Mon. Magazi. 1920, 56, 68–77. [Google Scholar]
- Champion, G.C. Some Indian Coleoptera (7). Entomol. Mon. Magazi. 1922, 58, 31–34, 68–76. [Google Scholar]
- Pic, M. Coleopteres exotiques nouveaux oupeuconnus (Suite). L ‘Echang. Rev. Linn. 1910, 26, 74–78. [Google Scholar]
- Pic, M. Notes diverses, descriptions et diagnoses (Suite). L ‘Echang. Rev. Linn. 1921, 37, 1–6. [Google Scholar]
- Pic, M. Diagnoses de coleopteres exotiques (Suite). L ‘Echang. Rev. Linn. 1921, 37, 10–15. [Google Scholar]
- Pic, M. Nouveaux coleopteres exotiques. Bull. Natl. d’ Histoire Nat. Paris. 1926, 32, 354–359. [Google Scholar] [CrossRef]
- Pic, M.H. Sauter’s Formosa-Ausbeute: Heteromera [ex parte] (Col.). Entomol. Mitteil. 1927, 16, 48–49. [Google Scholar]
- Pic, M. Coléoptères de l’Indochine. Mélang. Exotico.-Entomol. 1927, 49, 1–36. [Google Scholar]
- Pic, M. Notes diverses, nouveautes (Suite). L ‘Echang. Rev. Linn. 1937, 53, 1. [Google Scholar]
- Nikitsky, N.B.; Pollock, D.A. Melandryidae Leach, 1815. In Catalogue of Palaearctic Coleoptera; Tenebrionoidea; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2008; Volume 5, pp. 64–73. [Google Scholar]
- Konvička, O. Osphya lehnertae sp. nov. from Greece (Coleopteta: Melandryidae). Klapalekiana 2014, 50, 161–166. [Google Scholar]
- Konvička, O. Osphya brusteli sp. nov. from the Balkan Peninsula (Coleopteta: Melandryidae). Acta Musei Sil. Sci. Nat. 2016, 65, 271–277. [Google Scholar] [CrossRef]
- Recalde Irurzun, J.I.; Konvička, O.; Torres, J.L. On Osphya valdalitidae (Kraatz, 1868) and the Iberian Osphyinae (Coleopteta: Melandryidae). Heterop. Rev. Entomol. 2017, 17, 41–52. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Ji, W.; Han, K.; Lu, Y.Y.; Wei, J.F. Predicting the potential distribution of the vine mealy bug, Planococcus ficus under climate change by MaxEnt. Crop. Prot. 2020, 137, 105268. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Xu, J.; Shrestha, K.K.; Kindt, R. Ensemble forecast of climate suitability for the Trans-Himalayan Nyctaginaceae species. Ecol. Model. 2014, 282, 18–24. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Young, N.; Carter, L.; Evangelista, P.; Jarnevich, C. A MaxEnt Model v3.3.3e Tutorial (ArcGIS v10). 2011. Available online: https://coloradoview.org/wp-content/coloradoviewData/trainingData/a-maxent-model-v8.pdf (accessed on 20 December 2022).
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Boys, W.A.; Siepielski, A.; Smith, B.D.; Patten, M.A.; Bried, J.T. Predicting the distributions of regional endemic dragonflies using a combined model approach. Insect Conserv. Diver. 2021, 14, 52–66. [Google Scholar] [CrossRef]
- Lobo, J.M. More complex distribution models or more representative data? Biodivers. Inform. 2008, 5, 15–19. [Google Scholar] [CrossRef]
- Shabani, F.; Tehrany, M.; Solhjouy, F.S.; Kumar, L. A comparative modeling study on non-climatic and climatic risk assessment on Asian Tiger Mosquito (Aedes albopictus). PeerJ 2018, 6, e4474. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Yin, S.G.; Pan, Y.Z.; Tian, B.; Gong, X. Climatic Refugia and Geographical Isolation Contribute to the Speciation and Genetic Divergence in Himalayan-Hengduan Tree Peonies (Paeonia delavayi and Paeonia ludlowii). Front. Genet. 2021, 11, 595334. [Google Scholar] [CrossRef] [PubMed]
- Britannica, T. “Pangea.” Encyclopedia Britannica. 2021. Available online: https://www.britannica.com/place/Pangea (accessed on 1 April 2023).
- Deep Time Maps. Maps of Ancient Earth. 2020. Available online: https://deeptimemaps.com/ (accessed on 1 April 2023).
- Hou, Z.E.; Li, S.Q. Tethyan changes shaped aquatic diversification. Biol. Rev. 2018, 93, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Renema, W.; Bellwood, D.R.; Braga, J.C.; Bromfifield, K.; Hall, R.; Johnson, K.G.; Lunt, P.; Meyer, C.P.; McMonagle, L.B.; Morley, R.J.; et al. Hopping hotspots: Global shifts in marine biodiversity. Science 2008, 321, 654–657. [Google Scholar] [CrossRef]
- Leprieur, F.; Descombes, P.; Gaboriau, T.; Cowman, P.F.; Parravicini, V.; Kulbicki, M.; Melián, C.J.; de Santana, C.N.; Heine, C.; Mouillot, D.; et al. Plate tectonics drive tropical reef biodiversity dynamics. Nat. Commun. 2016, 7, 11461. [Google Scholar] [CrossRef]
- Bosboom, R.E.; Dupont-Nivet, G.; Houben, A.J.P.; Brinkhuis, H.; Villa, G.; Mandic, O.; Stoica, M.; Zachariasse, W.J.; Guo, Z.; Li, C.; et al. Late Eocene Sea retreat from the Tarim Basin (west China) and concomitant Asian paleoenvironmental change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 299, 385–398. [Google Scholar] [CrossRef]
- Bosboom, R.; Dupont-Nivet, G.; Grothe, A.; Brinkhuis, H.; Villa, G.; Mandic, O.; Stoica, M.; Kouwenhoven, T.; Huang, W.; Yang, W.; et al. Timing, cause and impact of the late Eocene stepwise sea retreat from the Tarim Basin (west China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 403, 101–118. [Google Scholar] [CrossRef]
- Carrapa, B.; DeCelles, P.G.; Wang, X.; Clementz, M.T.; Mancin, N.; Stoica, M.; Kraatz, B.; Meng, J.; Abdulov, S.; Chen, F. Tectono-climatic implications of Eocene Paratethys regression in the Tajik basin of central Asia. Earth. Earth Planet. Sci. Lett. 2015, 424, 168–178. [Google Scholar] [CrossRef]
- Royden, L.H.; Burchfiel, B.C.; Van der Hilst, R.D. The geological evolution of the Tibetan Plateau. Science 2008, 321, 1054–1058. [Google Scholar] [CrossRef]
- Van Hinsbergen, D.J.J.; Lippert, P.C.; Dupont-Nivet, G.; McQuarrie, N.; Doubrovine, P.V.; Spakman, W.; Torsvik, T.H. Greater India Basin hypothesis and a two-stage Cenozoic collision between India and Asia. Proc. Natl. Acad. Sci. USA 2012, 109, 7659–7664. [Google Scholar] [CrossRef]
- Takhtajan, A. Floristic Regions of the World; University of California Press: Los Angeles, CA, USA, 1986; p. 522. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; ARG Gantner Verlag KG: Vaduz, Germany, 2001; Volume 2, pp. 1–526. [Google Scholar]
- Enghoff, H. Phylogenetic biogeography of a Holarctic group: The Julian millipedes. Cladistic subordinateness as an indicator of dispersal. J. Biogeogr. 1993, 20, 525–536. [Google Scholar] [CrossRef]
- Enghoff, H. Historical biogeography of the Holarctic: Area relationships, ancestral areas, and dispersal of nonmarine animals. Cladistics 1995, 11, 223–263. [Google Scholar] [CrossRef]
- Marusik, Y.M.; Koponen, S. A survey of spiders (Araneae) with Holartcic distribution. J. Arachnol. 2005, 33, 300–305. [Google Scholar] [CrossRef]
- Noonan, G.R. Distribution of insects in the Northern Hemisphere: Continental drift and epicontinental seas. Bull. Entomol. Soc. Am. 1986, 31, 80–84. [Google Scholar] [CrossRef]
- Andersen, N.M.; Spence, J.R. Classification and phylogeny of the Holarctic water strider genus Limnoporus Stål (Hemiptera: Gerridae). Can. J. Zool. 1992, 70, 753–785. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.; Katariya, V. The Mediterranean: A biodiversity hotspot under threat. In The 2008 Review of the IUCN Red List of Threatened Species; Vie, J.C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- NatureServe. States of the Union: Ranking America’s Biodiversity. 2023. Available online: https://www.treehugger.com/top-states-for-biodiversity-1203613 (accessed on 1 April 2023).
- Bruce, A.S.; Lynn, S.K.; Jonathan, S.A. Biodiversity in the United States; Oxford University Press: New York, NY, USA, 2000; p. 399. ISBN 0-19-512519-3. [Google Scholar]
- Liu, T.; Liu, H.Y.; Yang, Y.X. Uncovering the determinants of biodiversity hotspots in China: Evidence from the drivers of multiple diversity metrics on insect assemblages and implications for conservation. Sci. Total Environ. 2023, 880, 163287. [Google Scholar] [CrossRef]
- Ito, T.; Yu, C.C.; Nakamura, K.; Chung, K.F.; Yang, Q.E.; Fu, C.X.; Qi, Z.C.; Kokubugata, G. Unique parallel radiations of high-mountainous species of the genus Sedum (Crassulaceae) on the continental island of Taiwan. Mol. Phylogenetics Evol. 2017, 113, 9–22. [Google Scholar] [CrossRef]
- Chou, Y.W.; Thomas, P.I.; Ge, X.J.; LePage, B.A.; Wang, C.N. Refugia and phylogeography of Taiwania in East Asia. J. Biogeogr. 2011, 38, 1992–2005. [Google Scholar] [CrossRef]
- He, J.K.; Gao, Z.F.; Su, Y.Y.; Lin, S.L.; Jiang, H.S. Geographical and temporal origins of terrestrial vertebrates endemic to Taiwan. J. Biogeogr. 2018, 11, 2458–2470. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hayashi, F.; Yang, D. Systematics and biogeography of the fishfly genus Parachauliodes (Megaloptera: Corydalidae) endemic to the east Asian islands. Syst. Entomol. 2008, 33, 560–578. [Google Scholar] [CrossRef]
- Feng, G.; Mao, L.F.; Sandel, B.; Swenson, N.G.; Svenning, J.C. High plant endemism in China is partially linked to reduced glacial-interglacial climate change. J. Biogeogr. 2016, 43, 145–154. [Google Scholar] [CrossRef]
- Lei, F.M.; Qu, Y.H.; Lu, J.L.; Liu, Y.; Yin, Z.H. Conservation on diversity and distribution patterns of endemic birds in China. Biodivers. Conserv. 2003, 12, 239–254. [Google Scholar] [CrossRef]
- Lv, J.M.; Ren, J.Z.; Ju, J.H. The interdecadal variability of East Asia Monsoon and its effect on the rainfall over China. J. Trop. Meteorol. 2004, 10, 14–22. [Google Scholar]
- Huang, X.L.; Lei, F.M.; Qiao, G.X. Areas of endemism and patterns of diversity for aphids of the Qinghai-Tibetan Plateau and the Himalayas. J. Biogeogr. 2008, 35, 230–240. [Google Scholar] [CrossRef]
- Lei, F.M.; Qu, Y.H.; Song, G.; Alström, P.; Fjeldså, J. The potential drivers in forming avian biodiversity hotspots in the East Himalaya Mountains of Southwest China. Integr. Zool. 2015, 10, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.S.; Stokes, C.R.; Batchelor, C.L. Evolution of the Laurentide and Innuitian ice sheets prior to the Last Glacial Maximum (115 ka to 25 ka). Earth-Sci. Rev. 2022, 224, 103875. [Google Scholar] [CrossRef]
- Milankov, V.; Ludoški, J.; Francuski, L.; Ståhls, G.; Vujić, A. Genetic and phenotypic diversity patterns in Merodon albifrons Meigen, 1822 (Diptera: Syrphidae): Evidence of intraspecific spatial and temporal structuring. Biol. J. Linn. Soc. 2013, 110, 257–280. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.Y.; Chen, L.D.; Peng, Y.S.; Wang, X. Changes in potential geographical distribution of Tsoongioden dronodorum since the Last Glacial Maximum. Chin. J. Plant Ecol. 2020, 44, 44–55. [Google Scholar] [CrossRef]
- Selwood, K.E.; Zimmer, H.C. Refuges for biodiversity conservation: A review of the evidence. Biol. Conserv. 2020, 245, 108502. [Google Scholar] [CrossRef]
- Tzedakis, P.C.; Frogley, M.R.; Heaton, T.H.E. Duration of last interglacial conditions in northwest Greece. Quat. Res. 2002, 58, 53–55. [Google Scholar] [CrossRef]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Monteith, J.L. Agricultural meteorology: Evolution and application. Agric. For. Meteorol. 2000, 103, 5–9. [Google Scholar] [CrossRef]
- Britannica, T. “Mediterranean Climate”. Encyclopedia Britannica. 2019. Available online: https://www.britannica.com/science/Mediterranean-climate (accessed on 1 April 2023).
- Copper, J.C. “Taiwan”. Encyclopedia Britannica. 2021. Available online: https://www.britannica.com/place/Taiwan (accessed on 1 April 2023).
- Scheffers, B.R.; Meester, L.D.; Bridge, T.; Hoffmann, A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef] [PubMed]
- Nikitsky, N.B. Fam. Ischaliidae, stat. n.—False Fire-red Beetles. In Keys to Insects of the Far East of the USSR in Six Volumes; Coleoptera or Beetles, Part 2; Ler, P.A., Ed.; Nauka Publishers: Moscow, Russia, 1992; Volume 3, pp. 497–498. [Google Scholar]
- Koster, R.D.; Dirmeyer, P.A.; Guo, Z.C.; Gordon, B.; Edmond, C.; Peter, C.; Gordon, C.T.; Shinjiro, K.; Eva, K.; David, L. Regions of strong coupling between soil moisture and precipitation. Science 2004, 305, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huo, Z.G.; Wang, L.; Jiang, Y.Y. Effects of climate change on the occurrence of crop insect pests in China. Chin. J. Ecol. 2012, 31, 1499–1507. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Thomas, C.D. A northward shift of range margins in British Odonata. Glob. Chang. Biol. 2005, 11, 502–506. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 30, 313–323. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Baker, R.; Sansford, C.E.; Jarvis, C.H.; Cannon, R.; Macleod, A.; Walters, K. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agric. Ecosyst. Environ. 2000, 82, 57–71. [Google Scholar] [CrossRef]
- Ding, W.; Li, H.; Wen, J. Climate change impacts on the potential distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae). Insects 2022, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Saupe, E.E.; Barve, V.; Myers, C.E.; Soberón, J.; Barve, N.; Hensz, C.M.; Peterson, A.T.; Owens, H.L.; Lira-Noriega, A. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Model. 2012, 238, 11–22. [Google Scholar] [CrossRef]
- Lobo, J.M. The use of occurrence data to predict the effects of climate change on insects. Curr. Opin. Insect Sci. 2016, 17, 62–68. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, H.; Wang, Y.; Yang, Y. Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere. Insects 2023, 14, 476. https://doi.org/10.3390/insects14050476
Liu T, Liu H, Wang Y, Yang Y. Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere. Insects. 2023; 14(5):476. https://doi.org/10.3390/insects14050476
Chicago/Turabian StyleLiu, Tong, Haoyu Liu, Yongjie Wang, and Yuxia Yang. 2023. "Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere" Insects 14, no. 5: 476. https://doi.org/10.3390/insects14050476
APA StyleLiu, T., Liu, H., Wang, Y., & Yang, Y. (2023). Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere. Insects, 14(5), 476. https://doi.org/10.3390/insects14050476





