Efficacy of Pyrethroid–Pyriproxyfen and Pyrethroid–Chlorfenapyr Long-Lasting Insecticidal Nets (LLINs) for the Control of Non-Anopheles Mosquitoes: Secondary Analysis from a Cluster Randomised Controlled Trial (cRCT)
Abstract
Simple Summary
Abstract
1. Background
2. Methods
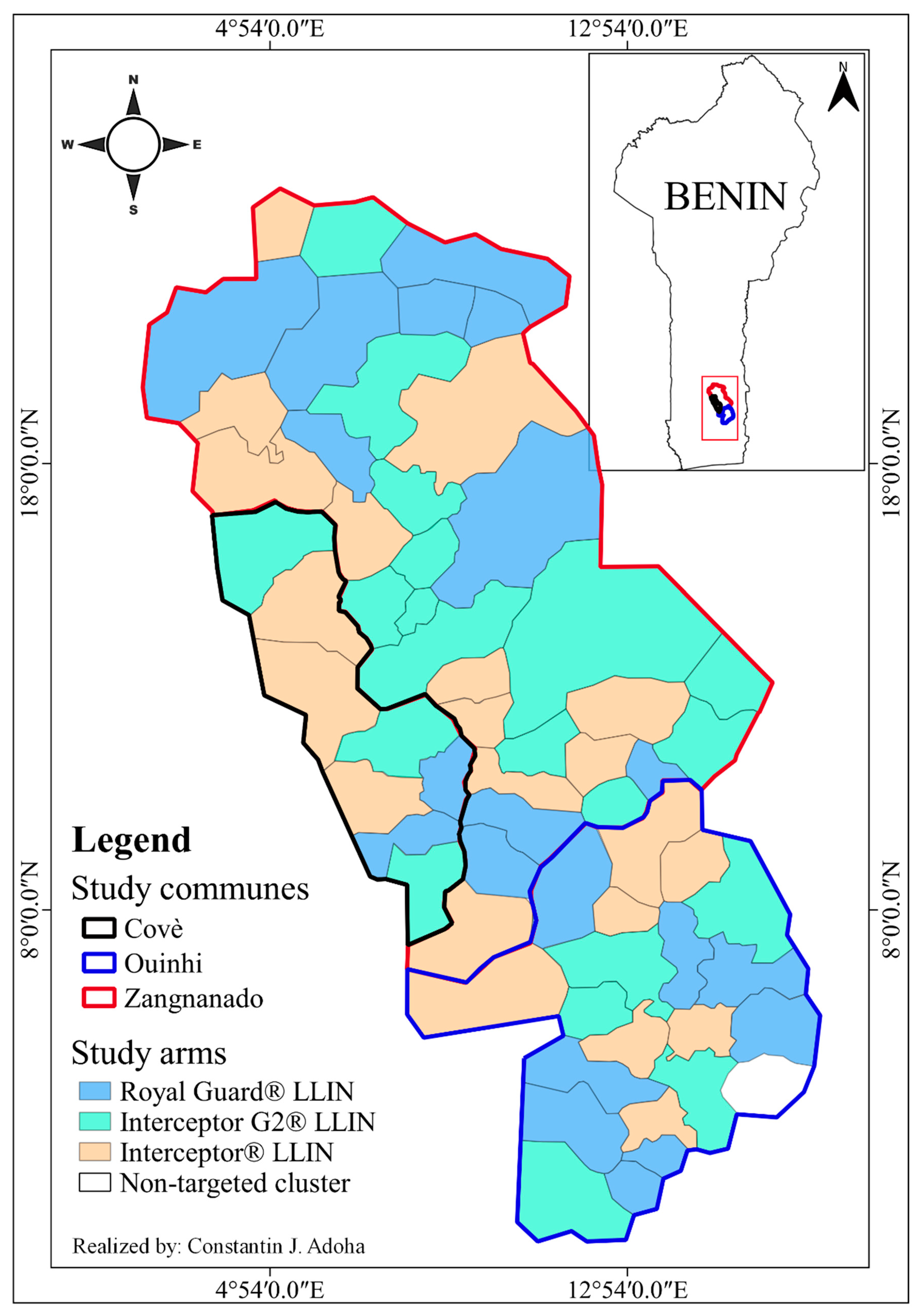
2.1. Study Area
2.2. Mosquito Sampling and Processing
2.3. Ethical Considerations
2.4. Data Management and Analysis
3. Results
3.1. Mosquito Species Composition
3.2. Density of Culex spp. and Mansonia spp. at Baseline in the Three Study Arms
3.3. Efficacy of Pyrethroid–Pyriproxyfen LLINs and Pyrethroid–Chlorfenapyr LLINs on Culex spp. Density Compared to Pyrethroid-Only LLINs
3.4. Efficacy of Pyrethroid–Pyriproxyfen LLINs, and Pyrethroid–Chlorfenapyr LLINs on the Mansonia spp. Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- N’Guessan, R.; Corbel, V.; Akogbéto, M.; Rowland, M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg. Infect. Dis. 2007, 13, 199–206. [Google Scholar] [CrossRef]
- Asidi, A.; N’Guessan, R.; Akogbeto, M.; Curtis, C.; Rowland, M. Loss of Household Protection from Use of Insecticide-Treated Nets against Pyrethroid-Resistant Mosquitoes, Benin. Emerg. Infect. Dis. 2012, 18, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Accrombessi, M.; Cook, J.; Dangbenon, E.; Yovogan, B.; Akpovi, H.; Sovi, A.; Adoha, C.; Assongba, L.; Sidick, A.; Akinro, B.; et al. Efficacy of pyriproxyfen-pyrethroid long-lasting insecticidal nets (LLINs) and chlorfenapyr-pyrethroid LLINs compared with pyrethroid-only LLINs for malaria control in Benin: A cluster-randomised, superiority trial. Lancet 2023, 401, 435–446. [Google Scholar] [CrossRef]
- Dutta, P.; Khan, S.A.; Khan, A.M.; Borah, J.; Sarmah, C.K.; Mahanta, J. The Effect of Insecticide-Treated Mosquito Nets (ITMNs) on Japanese Encephalitis Virus Seroconversion in Pigs and Humans. Am. J. Trop. Med. Hyg. 2011, 84, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, G.; Nguema, R.N.; Phiri, W.P.; Donfack, O.T.; Cortes, C.; Von Fricken, M.E.; Meyers, J.I.; Kleinschmidt, I.; Garcia, G.A.; Maas, C.; et al. Increased Biting Rate of Insecticide-Resistant Culex Mosquitoes and Community Adherence to IRS for Malaria Control in Urban Malabo, Bioko Island, Equatorial Guinea. J. Med. Entomol. 2019, 56, 1071–1077. [Google Scholar] [CrossRef]
- Harbach, R.E. The mosquitoes of the subgenus Culex in southwestern Asia and Egypt (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 1988, 24, 240. [Google Scholar]
- Labarthe, N.; Guerrero, J. Epidemiology of heartworm: What is happening in South America and Mexico? Vet. Parasitol. 2005, 133, 149–156. [Google Scholar] [CrossRef]
- Ughasi, J.; Bekard, H.E.; Coulibaly, M.; Adabie-Gomez, D.; Gyapong, J.; Appawu, M.; Wilson, M.D.; Boakye, D.A. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasites Vectors 2012, 5, 89. [Google Scholar] [CrossRef]
- Samy, A.M.; Elaagip, A.H.; Kenawy, M.A.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D.E. Climate Change Influences on the Global Potential Distribution of the Mosquito Culex quinquefasciatus, Vector of West Nile Virus and Lymphatic Filariasis. PLoS ONE 2016, 11, e0163863. [Google Scholar] [CrossRef]
- Ortega-Morales, A.; Zavortink, T.; Huerta-Jiménez, H.; Ibáñez-Bernal, S.; Siller-Rodríguez, Q. The mosquitoes (Diptera: Culicidae) of Hidalgo state, Mexico. Acta Trop. 2019, 189, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Boko-Collins, P.M.; Ogouyemi-Hounto, A.; Adjinacou-Badou, E.G.; Gbaguidi-Saizonou, L.; Dossa, N.I.; Dare, A.; Ibikounle, M.; Zoerhoff, K.L.; Cohn, D.A.; Batcho, W.; et al. Assessment of treatment impact on lymphatic filariasis in 13 districts of Benin: Progress toward elimination in nine districts despite persistence of transmission in some areas. Parasites Vectors 2019, 12, 276. [Google Scholar] [CrossRef]
- Adeleke, M.A.; Mafiana, C.F.; Idowu, A.B.; Sam-Wobo, S.O.; Idowu, O.A. Population dynamics of indoor sampled mosquitoes and their implication in disease transmission in Abeokuta, south-western Nigeria. J. Vector Borne Dis. 2010, 47, 33–38. [Google Scholar]
- Salako, A.S.; Ossè, R.; Padonou, G.G.; Dagnon, F.; Aïkpon, R.; Kpanou, C.; Sagbohan, H.; Sovi, A.; Sèzonlin, M.; Akogbeto, M.C. Population Dynamics of Anopheles gambiae s.l. and Culex quinquefasciatus in Rural and Urban Settings Before an Indoor Residual Spraying Campaign in Northern Benin. Vector Borne Zoonotic Dis. 2019, 19, 674–684. [Google Scholar] [CrossRef]
- Yovogan, B.; Sovi, A.; Padonou, G.G.; Adoha, C.J.; Akinro, B.; Chitou, S.; Dangbénon, E.; Akpovi, H.; Messenger, L.A.; Ossè, R.; et al. Pre-intervention characteristics of the mosquito species in Benin in preparation for a randomized controlled trial assessing the efficacy of dual active-ingredient long-lasting insecticidal nets for controlling insecticide-resistant malaria vectors. PLoS ONE 2021, 16, e0251742. [Google Scholar] [CrossRef] [PubMed]
- Accrombessi, M.; Akogbeto, M.C.; Dangbenon, E.; Akpovi, H.; Sovi, A.; Yovogan, B.; Adoha, C.; Assongba, L.; Ogouyemi-Hounto, A.; Padonou, G.G.; et al. Malaria Burden and Associated Risk Factors in an Area of Pyrethroid-Resistant Vectors in Southern Benin. Am. J. Trop. Med. Hyg. 2022, 107, 681–688. [Google Scholar] [CrossRef]
- Accrombessi, M.; Cook, J.; Ngufor, C.; Sovi, A.; Dangbenon, E.; Yovogan, B.; Akpovi, H.; Hounto, A.; Thickstun, C.; Padonou, G.G.; et al. Assessing the efficacy of two dual-active ingredients long-lasting insecticidal nets for the control of malaria transmitted by pyrethroid-resistant vectors in Benin: Study protocol for a three-arm, single-blinded, parallel, cluster-randomized controlled trial. BMC. Infect. Dis. 2021, 21, 194. [Google Scholar]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara. Ethiopian Zoogeographical Region; South African Institute for Medical Research: Johannesburg, South African, 1968; p. 343. [Google Scholar]
- Lupenza, E.; Gasarasi, D.B.; Minzi, O.M. Lymphatic filariasis, infection status in Culex quinquefasciatus and Anopheles species after six rounds of mass drug administration in Masasi District, Tanzania. Infect. Dis. Poverty 2021, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Uttah, E.C.; Wokem, G.N.; Okonofua, C. The Abundance and Biting Patterns of Culex quinquefasciatus Say (Culicidae) in the Coastal Region of Nigeria. Int. Sch. Res. Not. 2013, 8, e640691. [Google Scholar] [CrossRef]
- Galardo, A.K.R.; Hijjar, A.V.; Falcão, L.L.O.; Carvalho, D.P.; Ribeiro, K.A.N.; Silveira, G.A.; Neto, N.F.S.; Saraiva, J.F. Seasonality and Biting Behavior of Mansonia (Diptera, Culicidae) in Rural Settlements Near Porto Velho, State of Rondônia, Brazil. J. Med. Entomol. 2022, 59, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Reimer, L.J.; Thomsen, E.K.; Tisch, D.J.; Henry-Halldin, C.N.; Zimmerman, P.A.; Baea, M.E.; Dagoro, H.; Susapu, M.; Hetzel, M.W.; Bockarie, M.J.; et al. Insecticidal Bed Nets and Filariasis Transmission in Papua New Guinea. N. Engl. J. Med. 2013, 369, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Nsakashalo-Senkwe, M.; Mwase, E.; Chizema-Kawesha, E.; Mukonka, V.; Songolo, P.; Masaninga, F.; Rebollo, M.P.; Thomas, B.; Bockarie, M.J.; Betts, H.; et al. Significant decline in lymphatic filariasis associated with nationwide scale-up of insecticide-treated nets in Zambia. Parasite Epidemiol. Control. 2017, 2, 7–14. [Google Scholar] [CrossRef]
- WHO. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Bottle Bioassays; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sabesan, S.; Kumar, N.P.; Krishnamoorthy, K.; Panicker, K.N. Seasonal abundance & biting behaviour of Mansonia annulifera, M. uniformis & M. indiana & their relative role in the transmission of malayan filariasis in Shertallai (Kerala state). Indian J. Med. Res. 1991, 6, 253–258. [Google Scholar]
- Mmbando, A.S.; Okumu, F.O.; Mgando, J.P.; Sumaye, R.D.; Matowo, N.S.; Madumla, E.; Kaindoa, E.; Kiware, S.S.; Lwetoijera, D.W. Effects of a new outdoor mosquito control device, the mosquito landing box, on densities and survival of the malaria vector, Anopheles arabiensis, inside controlled semi-field settings. Malar. J. 2015, 14, 494. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; N’Guessan, R.; Brengues, C.; Chandre, F.; Djogbenou, L.; Martin, T.; Akogbéto, M.; Hougard, J.M.; Rowland, M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007, 101, 207–216. [Google Scholar] [CrossRef]
- Yadouléton, A.; Badirou, K.; Agbanrin, R.; Jöst, H.; Attolou, R.; Srinivasan, R.; Padonou, G.; Akogbéto, M. Insecticide resistance status in Culex quinquefasciatus in Benin. Parasites Vectors 2015, 8, 17. [Google Scholar] [CrossRef]


| Culex spp. | Mansonia spp. | |||||
|---|---|---|---|---|---|---|
| Arms | N | Person-Nights | Mean Density (95% CI) | N | Person-Nights | Mean Density (95% CI) |
| Indoor | ||||||
| Std LLIN | 1988 | 80 | 24.9 (10.4–39.3) | 2346 | 80 | 29.3 (13.2–45.4) |
| Pyr-PPF LLIN | 2105 | 80 | 26.3 (19.6–33.0) | 2961 | 80 | 37.0 (21.2–52.8) |
| Pyr-CFP LLIN | 3148 | 80 | 39.4 (15.9–62.8) | 2542 | 80 | 31.8 (15.5–48.0) |
| Outdoor | ||||||
| Std LLIN | 2749 | 80 | 34.4 (21.5–47.2) | 2950 | 80 | 36.9 (17.5–56.3) |
| Pyr-PPF LLIN | 2653 | 80 | 33.2 (24.3–42.0) | 3478 | 80 | 43.5 (25.1–61.9) |
| Pyr-CFP LLIN | 3811 | 80 | 47.6 (20.7–74.6) | 2953 | 80 | 36.9 (16.9–56.9) |
| Locations/Period | Arms | N | Person Night | Mean Density (95% CI) | DR | p-Value | * DR | * p-Value |
|---|---|---|---|---|---|---|---|---|
| Indoor | ||||||||
| Overall | Std LLIN | 8541 | 640 | 13.3 (7.5–19.2) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 9631 | 640 | 15 (9.5–20.6) | 0.9 (0.4–2.4) | 0.8817 | 1 (0.4–2.0) | 0.7929 | |
| Pyr-CFP LLIN | 7647 | 640 | 11.9 (6.4–17.5) | 0.6 (0.2–1.5) | 0.2793 | 0.4 (0.2–1.0) | 0.0523 | |
| Year 1 | Std LLIN | 5360 | 320 | 16.8 (8.4–25.1) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 6840 | 320 | 21.4 (12.9–29.9) | 1.0 (0.4–3.0) | 0.9731 | 1.0 (0.4–2.5) | 0.9631 | |
| Pyr-CFP LLIN | 5291 | 320 | 16.5 (8.0–25.1) | 0.6 (0.2–1.7) | 0.3099 | 0.4 (0.2–1.1) | 0.0704 | |
| Year 2 | Std LLIN | 3181 | 320 | 9.9 (5.3–14.6) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 2791 | 320 | 8.7 (5.3–12.2) | 0.9 (0.4–2.1) | 0.766 | 0.8 (0.4–1.8) | 0.6691 | |
| Pyr-CFP LLIN | 2356 | 320 | 7.4 (4.3–10.4) | 0.6 (0.3–1.5) | 0.2994 | 0.5 (0.2–1.0) | 0.0613 | |
| Outdoor | ||||||||
| Overall | Std LLIN | 13,627 | 640 | 21.3 (12.6–30.0) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 16,837 | 640 | 26.3 (17.1–35.5) | 1.0 (0.4–2.5) | 0.9745 | 1.0 (0.5–2.3) | 0.9579 | |
| Pyr-CFP LLIN | 12,986 | 640 | 20.3 (11.3–29.3) | 0.6 (0.3–1.6) | 0.3461 | 0.5 (0.2–1.1) | 0.0826 | |
| Year 1 | Std LLIN | 8726 | 320 | 27.3 (15.3–39.3) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 11,803 | 320 | 36.9 (22.7–51.1) | 1.0 (0.4–2.8) | 0.9646 | 1.1 (0.4–2.6) | 0.8947 | |
| Pyr-CFP LLIN | 8621 | 320 | 26.9 (15.1–38.8) | 0.6 (0.2–1.8) | 0.4093 | 0.5 (0.2–1.2) | 0.1341 | |
| Year 2 | Std LLIN | 4901 | 320 | 15.3 (8.6–22.0) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 5034 | 320 | 15.7 (9.2–22.3) | 1.0 (0.4–2.4) | 0.9279 | 1.0 (0.5–2.1) | 0.9833 | |
| Pyr-CFP LLIN | 4365 | 320 | 13.6 (6.7–20.6) | 0.6 (0.3–1.6) | 0.3311 | 0.5 (0.2–1.1) | 0.0692 |
| Locations/ Periods | Arms | N | Person-Nights | Mean | DR | p Value | * DR | * p Value |
|---|---|---|---|---|---|---|---|---|
| Indoor | ||||||||
| Overall | Std LLIN | 15,979 | 640 | 25.0 (15.4–34.5) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 18,166 | 640 | 28.4 (16.6–40.1) | 0.5 (0.1–2.3) | 0.392 | 0.4 (0.1–1.2) | 0.0982 | |
| Pyr-CFP LLIN | 15,614 | 640 | 24.4 (15.1–33.7) | 0.5 (0.1–2.4) | 0.416 | 0.5 (0.1–1.5) | 0.2061 | |
| Year 1 | Std LLIN | 10,226 | 320 | 32.0 (19.6–44.4) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 12,610 | 320 | 39.4 (23.0–55.8) | 0.6 (0.1–2.7) | 0.5113 | 0.4 (0.1–1.4) | 0.1628 | |
| Pyr-CFP LLIN | 10,266 | 320 | 32.1 (20.1–44.0) | 0.6 (0.1–2.9) | 0.5623 | 0.5 (0.2–1.8) | 0.3207 | |
| Year 2 | Std LLIN | 5753 | 320 | 18.0 (10.4–25.6) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 5556 | 320 | 17.4 (9.1–25.6) | 0.5 (0.1–2.7) | 0.4007 | 0.3 (0.1–1.3) | 0.1108 | |
| Pyr-CFP LLIN | 5348 | 320 | 16.7 (6.9–26.5) | 0.4 (0.1–2.4) | 0.3261 | 0.4 (0.1–1.5) | 0.1524 | |
| Outdoor | ||||||||
| Overall | Std LLIN | 25,832 | 640 | 40.4 (25.3–55.4) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 28,233 | 640 | 44.1 (26.5–61.8) | 0.6 (0.1–2.6) | 0.454 | 0.4 (0.1–1.5) | 0.1825 | |
| Pyr-CFP LLIN | 22,971 | 640 | 35.9 (22.7–49.1) | 0.6 (0.1–2.9) | 0.5494 | 0.6 (0.2–2.2) | 0.4674 | |
| Year 1 | Std LLIN | 16,247 | 320 | 50.8 (31.1–70.5) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 19,198 | 320 | 60.0 (35.5–84.5) | 0.7 (0.1–3.0) | 0.6036 | 0.5 (0.2–1.7) | 0.2878 | |
| Pyr-CFP LLIN | 14,694 | 320 | 45.9 (29.4–62.5) | 0.8 (0.2–3.4) | 0.7284 | 0.8 (0.2–2.5) | 0.6568 | |
| Year 2 | Std LLIN | 9585 | 320 | 30.0 (17.9–42.0) | 1 (Ref) | 1 (Ref) | ||
| Pyr-PPF LLIN | 9035 | 320 | 28.2 (14.4–42.0) | 0.5 (0.1–2.9) | 0.4099 | 0.3 (0.1–1.6) | 0.1674 | |
| Pyr-CFP LLIN | 8277 | 320 | 25.9 (11.3–40.5) | 0.4 (0.1–2.7) | 0.3752 | 0.4 (0.1–2.0) | 0.2919 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adoha, C.J.; Sovi, A.; Yovogan, B.; Akinro, B.; Accrombessi, M.; Dangbénon, E.; Odjo, E.M.; Sagbohan, H.W.; Kpanou, C.D.; Padonou, G.G.; et al. Efficacy of Pyrethroid–Pyriproxyfen and Pyrethroid–Chlorfenapyr Long-Lasting Insecticidal Nets (LLINs) for the Control of Non-Anopheles Mosquitoes: Secondary Analysis from a Cluster Randomised Controlled Trial (cRCT). Insects 2023, 14, 417. https://doi.org/10.3390/insects14050417
Adoha CJ, Sovi A, Yovogan B, Akinro B, Accrombessi M, Dangbénon E, Odjo EM, Sagbohan HW, Kpanou CD, Padonou GG, et al. Efficacy of Pyrethroid–Pyriproxyfen and Pyrethroid–Chlorfenapyr Long-Lasting Insecticidal Nets (LLINs) for the Control of Non-Anopheles Mosquitoes: Secondary Analysis from a Cluster Randomised Controlled Trial (cRCT). Insects. 2023; 14(5):417. https://doi.org/10.3390/insects14050417
Chicago/Turabian StyleAdoha, Constantin J., Arthur Sovi, Boulais Yovogan, Bruno Akinro, Manfred Accrombessi, Edouard Dangbénon, Esdras M. Odjo, Hermann Watson Sagbohan, Casimir Dossou Kpanou, Gil G. Padonou, and et al. 2023. "Efficacy of Pyrethroid–Pyriproxyfen and Pyrethroid–Chlorfenapyr Long-Lasting Insecticidal Nets (LLINs) for the Control of Non-Anopheles Mosquitoes: Secondary Analysis from a Cluster Randomised Controlled Trial (cRCT)" Insects 14, no. 5: 417. https://doi.org/10.3390/insects14050417
APA StyleAdoha, C. J., Sovi, A., Yovogan, B., Akinro, B., Accrombessi, M., Dangbénon, E., Odjo, E. M., Sagbohan, H. W., Kpanou, C. D., Padonou, G. G., Messenger, L. A., Agbangla, C., Ngufor, C., Cook, J., Protopopoff, N., & Akogbéto, M. C. (2023). Efficacy of Pyrethroid–Pyriproxyfen and Pyrethroid–Chlorfenapyr Long-Lasting Insecticidal Nets (LLINs) for the Control of Non-Anopheles Mosquitoes: Secondary Analysis from a Cluster Randomised Controlled Trial (cRCT). Insects, 14(5), 417. https://doi.org/10.3390/insects14050417






