Simple Summary
Synthetic pesticides are among the main threatening factors for wild and managed bees. In recent decades, botanical biopesticides have been gained increasing interest and use in agriculture due to their high selectivity and short persistence in the environment. To date, however, little has been discovered or researched about the adverse effects of these substances on bees. This paper reviews studies in the literature reporting the lethal and sublethal effects of botanical biopesticides on social and solitary bees. Although botanical products are considered safer than chemical pesticides, some of them can cause lethal and several sublethal effects on bees. We suggest that more research is needed on this topic, especially increasing knowledge about certain groups of bees such as solitary bees.
Abstract
The recent global decline in insect populations is of particular concern for pollinators. Wild and managed bees (Hymenoptera, Apoidea) are of primary environmental and economic importance because of their role in pollinating cultivated and wild plants, and synthetic pesticides are among the major factors contributing to their decline. Botanical biopesticides may be a viable alternative to synthetic pesticides in plant defence due to their high selectivity and short environmental persistence. In recent years, scientific progress has been made to improve the development and effectiveness of these products. However, knowledge regarding their adverse effects on the environment and non-target species is still scarce, especially when compared to that of synthetic products. Here, we summarize the studies concerning the toxicity of botanical biopesticides on the different groups of social and solitary bees. We highlight the lethal and sublethal effects of these products on bees, the lack of a uniform protocol to assess the risks of biopesticides on pollinators, and the scarcity of studies on specific groups of bees, such as the large and diverse group of solitary bees. Results show that botanical biopesticides cause lethal effects and a large number of sublethal effects on bees. However, the toxicity is limited when comparing the effects of these compounds with those of synthetic compounds.
1. Introduction
Bees (Hymenoptera, Apoidea) are the main group of pollinating insects, represented by about 20,000 described species in the world, with the greatest biodiversity in Mediterranean and xeric climate regions of the globe [1,2]. Due to its biological and ethological characteristics, this group provides the ecological service of pollination for spontaneous and cultivated plants. In particular, the pollination service by animals includes 87% of the world’s spontaneous flowering plants [3], and about 75% of cultivated crops [4,5]. It has been estimated that crop pollination by insects has a value ranging from USD 195 billion to ~USD 387 billion annually worldwide [6]. Despite the difficulties in estimating the economic benefits of insect pollination for wild plants, it is assumed that this service is extremely important for the maintenance of the majority of terrestrial ecosystems.
In addition to being represented by large species richness, bees also include a wide range of life history, biological, and ethological traits [7]. The majority of bees are solitary, with females that build and provision the nest and raise their offspring by themselves without cooperation with other individuals [7]. The remaining insects are represented by parasitic bees, such as cleptoparasitic and social parasite bees, and social bees. Although they represent only a small fraction of the total species, social bees (and in particular the western honeybee, Apis mellifera L.) have always received the greatest attention from the public, scientists, and bee regulation and conservation programs [8]. Despite the role of major pollinators historically being mainly attributed to the western honeybee, subsequent studies showed that a greater biodiversity of wild bees increases and improves the pollination service [9].
The decline of insects, and bees in particular, has been documented by various studies in recent decades [10,11,12,13,14,15,16,17,18,19,20]. The reduction in abundance and richness of bees has been documented in different parts of the world at local, regional, and country levels [21,22]. The expansion and intensification of agriculture and livestock farming, exposure to pollutants, anthropization and habitat fragmentation, fires, and climate change are the factors that most threaten the diversity and populations of bees [23,24,25].
Despite these several factors related to the worldwide decline in pollinators, the impact of synthetic pesticides on non-target beneficial arthropods, and in particular pollinating insects, has represented a primary concern for at least the last decade [26,27]. From the 1990s up until now, a large number of scientific works has highlighted the toxicity and side effects of neonicotinoids for bees [28,29,30], and which have resulted in restrictions in the use of these products in Europe [31,32]. However, other families of chemical compounds (such as carbamates, organophosphates, and pyrethroids) are well known to be dangerous for these insects [27,33,34]. Conversely, there is less information on the toxicity of insecticides of biological origin for bees [35,36,37,38,39].
Biopesticides include a wide variety of compounds of natural origin, ranging from botanical compounds such as plant secondary metabolites and essential oils (EOs), to entomopathogenic viruses, bacteria, fungi, and nematodes [40,41]. Toxins and venoms produced by arthropods such as spiders and scorpions [42], or by other invertebrates [43], are also considered to be biopesticides. The U.S. Environmental Protection Agency (EPA) categorizes biopesticides in three different groups: (I) biochemical biopesticides, (II) plant-incorporated protectants (PIPs), and (III) biocontrol organisms [44]. Although there is no formal definition of biopesticides at the European level, two different groups are recognized, namely, (I) living organisms and (II) natural products, excluding PIPs, which are not included by the regulatory authorities of most of the other countries. Here, we divide biopesticides into four different groups according to their origin: (I) botanicals (alkaloids, essential oils, limonoids, etc.), (II) microbials (virus, bacteria, fungi), (III) animals (nematodes, toxins, and venoms from invertebrates), and (IV) genetic (toxins from GM plants, and RNAi based products). Biondi et al. [35] summarized the non-target effects of spinosyns on beneficial arthropods, including pollinators, while Erler et al. [38] reported from the literature the impacts of entomopathogenic organisms on social and solitary bees. The review by Cappa et al. [37] summarized the detrimental effects of biopesticides on insect pollinators (including social and solitary bees, Lepidoptera, Diptera Syrphidae, anthophilous Coleoptera, and wasps), including the effects of different classes of microbial biopesticides, and the effects of azadirachtin among the botanical biopesticides. Furthermore, Ntalli et al. [39] summarized the effects on honeybees of botanical treatments used in beekeeping to control the Apis mellifera arthropod pests. Giunti et al. [45] summarized the effects of essential oil-based biopesticides on non-target organisms, reporting some information on pollinator insects.
In the present review, we analysed the impacts on bees of botanicals biopesticides used or potentially used in agriculture, summarizing the studies in the literature and reporting the lethal and sublethal effects of these products on the different groups of Apoidea Anthophila, such as social (honeybees, bumblebees, stingless bees) and solitary bees. We also reported a critical analysis on the detrimental effects of these eco-friendly products on bees, divided into different categories according to Acheuk et al. [46].
2. Botanical Biopesticides
Botanical pesticides have been applied for more than 150 years in Europe and North America, going back much earlier than the discovery and the wide spread use of the main classes of synthetic pesticides in the first half of the twentieth century [47]. The extensive use of synthetic pesticides, with their consequent negative effects on human and environmental health, has led to a recent and increasing demand for botanical and organic pesticides as eco-friendly alternatives to synthetic pesticides [46]. Botanical pesticides, and in general biopesticides, have a higher selectivity, cause less pest resistance, and have low environmental persistence in comparison with the synthetic compounds [46,48]. For these reasons, these products can be good candidates for modern and sustainable agriculture. Despite the growing interest of the scientific community in botanical pesticides during recent years [37], their commercial use is still restricted compared to the synthetic alternatives, particularly in developing countries [49].
The great biosynthetic ability of plants enables a wide diversity and versatility of botanical compounds, which can be divided into seven different classes: (1) alkaloids, (2) essential oils, (3) fatty acids, (4) limonoids, (5) phenolics, (6) polyketides, and (7) pyrethrins [46].
Alkaloids represent a wide and highly diverse group of chemical compounds found in several botanical species: the most important examples are anabasine from Anabasis aphylla L. (Amaranthaceae), nicotine from Nicotiana (Solanaceae) species, or zygacine from Melanthiaceae species. These compounds show high insecticidal activities at low doses, and sublethal effects such as antifeeding, deterrence, and malformations [50]. Nicotine is a non-systemic insecticide that can be used for the control of a large number of pests in a confined environment [40]. Used as a fumigant, nicotine has neurotoxic action on insects, but it also shows high toxicity for birds, aquatic organisms, bees, and humans [51]. A mixture of alkaloids can be found in sabadilla, a traditional insecticidal preparation used in Central and South America and commercially used since the 1970s [40]. Sabadilla powder is prepared from Schoenocaulon officinale Gray (Melanthiaceae) and contains a mixture of veratridine, cevadine, and other alkaloids, and it is effective against thrips [40]. Ryania extracts from the stem of Ryania speciosa Vahl (Salicaceae) contain the alkaloid ryanodine, a highly toxic bioinsecticide that has been used in the USA for the control of Cydia pomonella L. (Tortricidae) and Ostrinia nubilalis Hübner (Crambidae) [40].
Essential oils (EOs) are contained in about 17,500 aromatic plant species and can be extracted mainly by steam distillation for various industrial applications, including plant protection from pests [52]. These products have been used since ancient times and can be obtained from plants belonging to the families Asteraceae (e.g., Artemisia spp.), Lamiaceae (e.g., Mentha spp., Origanum spp., Rosmarinus officinalis L., Salvia spp., Thymus spp.), Lauraceae (e.g., Laurus nobilis L.), and Myrtaceae (e.g., Eucalyptus spp., Myrtus communis L.) [52,53]. EOs are produced as secondary metabolites by these plants for protection against microorganisms, insects, herbivores, and allelopathic interactions [53,54]. The major constituents of EOs are low-molecular-weight terpenoids (monoterpenes and sesquiterpenes) and phenolics [52]. EOs have been recently tested on pests with successful results, but their high volatility, poor solubility in water, and easy environmental degradation make their commercialization difficult. For these reasons, some techniques have been studied to improve their efficacy, such as encapsulation in nanoparticles (NPs) (e.g., polyethylene glycol, PEG) [55], microencapsulation in SiO2 [56], and the use of plant powders containing EOs [57].
Fatty acids have also been used in some commercial biopesticides where they have a stabilizing function. However, some of them can have a secondary toxic effect on insect pests, for example, conjugated linoleic acid (CLA) or pelargonic acid [46]. Furthermore, preliminary studies on the fatty amid pellitorine have shown promising results for the control of mosquitoes [58] and Coleoptera post-harvest pests [59].
Limonoids are natural compounds mainly present in plants of the Rutaceae (Citrus spp.) and Meliaceae (Neem tree, Azadirachta indica A. Juss.) families. Azadirachtin from the Neem tree is one of the most widely used and studied biopesticides [49], isolated from all the parts of the plants, in particular from seeds [40]. Considered as a safe and selective product, Azadirachtin is very effective against several groups of pests, causing acute toxicity and anti-feeding and physiological effects [60], and it can also be used as a fungicide [40]. However, several studies question its safety as regards beneficial insects [61,62,63].
Phenolics, the largest group of plant secondary metabolites, perform various essential functions, from the regulation of physiological processes to defence against herbivores [64]. Abundantly present in Thymus spp. (Lamiaceae) plants, thymol can be used as an effective fungicide, bactericide, and also acaricide, it being effective against Varroa spp. (Varroidae), an important ectoparasite of honeybees [65]. The isoflavone rotenone, extracted from the roots of some species of Derris, Lonchocarpus, and Tephrosia (Fabaceae), is a neurotoxic compound used against a wide spectrum of insects and in the control of fish populations [40]. The safety of rotenone for humans and the environment has been questioned due to its high toxicity towards mammals [47].
Another large class of plant secondary metabolites is that of polyketides, biosynthesized from acetyl-CoA. Annonins, classified as acetogenins, comprise an important group of polyketides that show a wide range of biological activities such as antimicrobial and pesticidal activities [66]. Effective against Coleoptera pests [67], annonins are extracted from the seeds of neotropical Annona (Annonaceae) trees [47].
Pyrethrin is one of the most marketed bioinsecticides. It is a mixture of compounds (Pyrethrins I, and Pyrethrins II) biosynthesized from Tanacetum cinerariifolium (Trevir.) Sch. Bip. (Asteraceae) [68]. Pyrethrin has neurotoxic action that interferes with the Na+/K+ exchange pump, causing paralysis and resulting in toxicity for several groups of pests [46]. The characteristic of pyrethrins, of being particularly labile to UV from sunlight, led in the 1970s and 1980s to the development of synthetic derivatives, the pyrethroids, which are widely used nowadays [49].
3. Risk Assessment of Biopesticides on Bees
Most of the regulatory risk assessment for plant protection products (PPPs) (pesticides and biopesticides) uses the western honeybee as a surrogate species for ecotoxicological testing of pollinators [69,70]. In recent years it has been realized that this approach is not enough for pollinator conservation [70,71]. Sensitivity to pesticides varies according to the bee species and other factors such as body size, level of sociality, seasonality, voltinism, floral specialization, nesting behaviour, food consumption rate, overwintering strategies, sex, and caste [27,33,72,73]. This leads to different ecological impacts from the use of pesticides. Therefore, risk assessments have recently been expanded to include other bee species such as bumblebees (Bombus spp.) [26,44,74,75], solitary bees (Osmia spp. and Megachile rotundata (Fabricius)) [8,26,44,76,77], and stingless bees [78]. Currently ground-nesting bees, which represent about 70% of bee species [1], have not been taken into consideration in the PPP risk assessment protocols due to their difficulty in breeding, management, and use in laboratory protocols [8]. Still, today, however, knowledge regarding the ecotoxicology of the non-Apis bee species is scarce and certainly needs to be increased [33,79,80].
To date, there are no specific regulations and protocols for testing the effects of biopesticides on bees. Therefore, the same protocols for the chemical pesticides developed by the Organisation for Economic Co-operation and Development (OECD) are used [37]. These protocols [74,81] include laboratory chronic and acute oral/contact toxicity tests to measure ecotoxicological parameters, such as LC50 and LD50, and to evaluate sublethal effects such as paralysis, movement alterations, and the presence of moribund specimens. However, sublethal effects caused by pesticides on bees are commonplace and still little studied, particularly regarding ecotoxicological tests about the effects of biopesticides on non-Apis bees [82,83,84].
In addition, there are few guidelines for the risk assessment of pesticides at field and semi-field levels with honeybees, bumblebees, and solitary bees [26,85], and no specific protocols for biopesticides. In general, the number of higher tier risk assessment studies on bees is low, both for synthetic pesticides (excluding neonicotinoids) and biopesticides.
We therefore highlight the need to (I) develop specific protocols to assess the lethal and sublethal effects of biopesticides on bees from different species; (II) increase the knowledge about species sensitivity distribution regarding chemical and biological pesticides for Apoidea, including ground-nesting solitary bees in the studies; and (III) develop new and better field and semi-field protocols both for synthetic and biopesticides.
4. Materials and Methods
The search for peer-reviewed English-language publications up to 2022 was conducted using Google Scholar, Scopus, and ResearchGate through the following keyword terms and their combination: “bioinsecticides”, “biopesticides”, “botanical insecticides”, “Azadirachtin”, “Essential oils”, “EOs”, “botanical extracts”, “Pyrethrins”, Pyrethrum”, “Nicotine”, AND “toxicity”, “exposure”, “effect”, AND “bee”, “social bee”, “honeybee”, “Apis”, “bumblebee”, “Bombus”, “stingless bee”, “Meliponini”, “solitary bee”, “Osmia”, “Megachile”. Additional studies from literature references were considered. In this review, papers about the toxicity on A. mellifera of botanical products used in beekeeping were not considered, as they are summarized in the recent revision of Ntalli et al. [39]. Table 1 includes and summarizes the studies and divides them following the different groups of Apoidea (social and solitary bees). Information is provided about the botanical substances tested, the category of assay (laboratory-assessed lethal (L) and/or sublethal (S) effects, field and semi-field), the type of treatment application (contact, topical, ingestion, fumigation, spray, crop spraying, crop granules, and "ingestion and topical" in the cases of tests with larvae), the target of the experiments (eggs, larvae, adults, colony, microcolony), the main effects reported by the results of the experiments, and the country in which the experiments were performed. The “Botanical substance” column in Table 1 includes the individual biopesticides tested in the different papers analysed. No papers were found in which synergistic effects between different substances were tested. Table 1 includes only article papers; however, some studies presented at conferences or symposia are discussed in the text. Most of the studies reviewed (53.7%) did not test a chemical insecticide as a positive control, and these studies are highlighted in Table 1 with a double asterisk (**). The toxicological parameters (LC50, LD50) of botanical biopesticides extrapolated from the analysed papers are reported in Table 2.
6. Effects of Botanical Biopesticides on Solitary Bees
The great majority of bee species in the world is solitary and belongs to seven different families (Stenotritidae, Colletidae, Andrenidae, Halictidae, Melittidae, Megachilidae, and Apidae) [7]. They exhibit a great variety of size, morphological characteristics, behaviour, nesting habitats, flight ranges, phenology, and nutritional requirements [1,7]. Eight species of solitary bees, mainly cavity-nesting species belonging to the genera Megachile Latreille and Osmia Panzer (Megachilidae), are managed for the pollination of crops around the world, and another 14 are potentially usable species [87]. Among these, there are a few ground nesting bee species such as the alkali bee, Nomia melanderi (Cockerell) (Halictidae), which are managed in North America, or Rhophitoides canus (Eversmann) (Halictidae) in Eastern Europe. The status of solitary bees is not well known throughout the world. In Europe, which hosts the best-known bee fauna, the latest IUCN Red List [23] assessed 60% of the species in the “data deficient” category, and the majority of the threatened species (45 spp.) are solitary. There is a clear need to improve our knowledge of the status of solitary bees in the world and to assess the risk to them of synthetic chemicals and alternative biopesticides.
The European Food Safety Authority (EFSA) suggested including the red mason bee, Osmia bicornis L., and the European orchard bee, Osmia cornuta (Latreille), as model organisms of solitary bees in the EU pesticide risk assessment scheme [26]. However, standardised test protocols to assess acute toxicity for solitary bees are still in development. The US EPA [44] has suggested the blue orchard bee, Osmia lignaria Say, and the alfalfa leafcutting bee, Megachile rotundata (Fabricius). The latter, to date widely managed for crop pollination in North America, is a Eurasian bee accidentally introduced into the US in the 1940s.
Knowledge regarding lethal and sublethal effects of botanical compounds on solitary bees is very scarce, and the few studies carried out show non-uniform laboratory protocols, with the use of different methods of application, and different life stages (eggs, larvae, newly emerged, adults of females and males). A product based on an extract of a small tropical tree, Quassia amara L. (Simaroubaceae) (Tecomag®), was found to be particularly toxic at field doses for Osmia cornuta eggs and larvae, with a mortality of more than 80% in a preliminary study conducted through the application of a drop of test solution in the provision of the eggs/larvae [157].
Studies conducted in North America, with adults of Osmia lignaria, showed low reduction in mortality with topical and ingestion treatment of Neem oil [116]. Slightly increased mortality was also registered in adults of Osmia cornifrons Radoszkowski with a treatment of wintergreen oil (Gaultheria procumbens L., Ericaceae) as a fumigant used for the control of Chaetodactylus krombeini Baker (Chaetodactylidae) [158]. Another study, conducted in Canada [57], tested botanical insecticides that could potentially be used to control a natural enemy of solitary bees, such as Pteromalus venustus Walker (Pteromalidae), a parasitoid of the alfalfa leafcutting bees, Megachile rotundata. This work tested fifteen plant powders against parasitoid and adult male bees in a contact experiment, highlighting a higher bee mortality with nutmeg powders [57].
The only field study [122] was conducted in Brazilian melon (Cucumis melo L.) fields, investigating the visitation rates of Halictus spp. (Halictidae) after treatments with azadirachtin. Halictus Latreille is a wide genus of bees that includes a scale of different social behaviours from solitary and semi-social to social species. Since the species was not specified in Tschoeke et al. [122], we reported this in the solitary bees category. Halictus bees showed reduced visitation intensity after treatment with a neem-based insecticide.
As with bumblebees, the LC50 or LD50 of botanical biopesticides for solitary bee species were not calculated in the studies reviewed in the literature (Table 2).

Table 1.
Laboratory, semi-field, and field studies testing the lethal (L) and sublethal (S) effects on social and solitary bees (Hymenoptera, Apoidea) of botanical biopesticides.
Table 1.
Laboratory, semi-field, and field studies testing the lethal (L) and sublethal (S) effects on social and solitary bees (Hymenoptera, Apoidea) of botanical biopesticides.
| Bee Species | Botanical Substance | Assay | Application | Target * | Effects | Country | Year | References |
|---|---|---|---|---|---|---|---|---|
| Social species | ||||||||
| Honeybees (Apis spp.) | ||||||||
| Apis cerana cerana | Azadirachtin | laboratory (L, S) | ingestion | adults | increase in mortality at the higher doses, anti-feeding and inhibition on the immune response | China | 2022 | [115] ** |
| Apis cerana indica | Annonin, azadirachtin | laboratory (L) and field | topical, crop spraying | adults | increase in mortality with both compounds, reduction of the number and speed of foraging bees with annonin | India | 2019 | [106] |
| Apis cerana indica | Azadirachtin | field | crop spraying | adults | reduction of the number of foraging bees | India | 2010 | [91] |
| Apis dorsata | Azadirachtin | field | crop spraying | adults | reduction of the number of foraging bees | India | 2010 | [91] |
| Apis mellifera | Azadirachtin | laboratory (L) | ingestion and topical | larvae, adults | increase in mortality, larvae more susceptible than adults | Brazil | 2016 | [118] ** |
| Apis mellifera | Sabadilla dust | laboratory (L) | contact | adults | increase in mortality | USA | 1958 | [95] |
| Apis mellifera | Aramite (blend of natural oils) | laboratory (L) | contact | adults | low increase in mortality | USA | 1952 | [96] |
| Apis mellifera | Formulations containing pyrethrins, rotenone, and pine oil, three formulations containing pyrethrins | laboratory (L, S) | spray | adults | increase in mortality and knockdown effects for all formulations | USA | 1990 | [126] |
| Apis mellifera | Aramite, pyrethrins, rotenone, ryania, and sabadilla dust | laboratory (L) | contact | adults | increase in mortality with sabadilla, medium and low increase in mortality with the other compounds | USA | 1954 | [94] |
| Apis mellifera | Pyrethrum | field | spray on cage and colony | adults, colony | low increase in mortality | USA | 1979 | [128] |
| Apis mellifera | Mentha piperita, Origanum vulgare, Thymus vulgaris, and Zingiber officinale EOs | laboratory (L, S) | topical, contact, ingestion | adults | increase in mortality with O. vulgare, and T. vulgaris, reduction of movements with O. vulgare | Brazil | 2020 | [102] ** |
| Apis mellifera | Neem oil, pyroligneous extract, and rotenone | laboratory (L) | topical, ingestion | adults | reduction in survival with rotenone on topical application | Brazil | 2012 | [97] |
| Apis mellifera | Azadirachtin | field | crop spraying | adults | no reduction of the numbers of foraging bees | USA | 2004 | [108] |
| Apis mellifera | Pyrethrum extract | laboratory (L) | fumigation | adults | no effects on mortality | USA | 1930 | [127] ** |
| Apis mellifera | Rotenone, and pyrethrum extract | laboratory (L) | spray | adults | increase in mortality | USA | 1932 | [124] ** |
| Apis mellifera | Azadirachtin | laboratory (L, S) | ingestion | adults | high mortality, effects on haemolymph amino acid composition | Egypt | 2015 | [114] |
| Apis mellifera | Neem oil | laboratory (L) | ingestion and topical | larvae | reduction in survival | India | 2022 | [119] ** |
| Apis mellifera | Neem oil | laboratory (L) | topical, ingestion | adults | reduction in survival in contact application | USA | 2005 | [116] |
| Apis mellifera | Pellitorine extracted from Piper tuberculatum | laboratory (L) and field | ingestion and topical | larvae, adults | high mortality at the highest rates | Brazil | 2003 | [105] ** |
| Apis mellifera | Azadirachtin | laboratory (L, S) | contact, ingestion | adults | increase in mortality, no repellent effects, reduction in flight ability | Brazil | 2020 | [117] |
| Apis mellifera | Neem seed extract | laboratory (S) and field | ingestion, crop spraying | adults | food repellency in laboratory bioassays, however no effects on the number of the foraging bees in the field | Canada | 1994 | [112] ** |
| Apis mellifera | Neem oil | field | ingestion and topical | larvae | increase in mortality at the higher concentration | Canada | 1996 | [113] ** |
| Apis mellifera | century plant, citronella, garlic, parsley, rue, and tobacco extracts | laboratory (S) and field | ingestion | adults | repellent effects in laboratory and in field | Brazil | 2004 | [104] ** |
| Apis mellifera | Pyrethrum extract, pyrethrum extract in nanoparticles | laboratory (L, S) | ingestion | adults | reduction in survival, morphological alterations in the epithelium of midgut | Brazil | 2019 | [129] |
| Apis mellifera | Azadirachtin | field | crop spraying | adults | reduction of the number of foraging bees | India | 2010 | [91] |
| Apis mellifera | Agave americana, Anadenanthera colubrina, and Nicotiana tabacum extracts | laboratory (L, S) | contact, ingestion | adults | increased mortality with N. tabacum, low increase in mortality with the other compounds, no effects on respiration rates or flight | Brazil | 2020 | [93] |
| Apis mellifera | Echinodorus grandiflorus, Matricaria chamomilla, Origanum majorana, and Punica granatum extracts | laboratory (L, S) | contact, ingestion, spray | adults | increase in mortality and reduction of the length of mesenteric cells with O. majorana and P. granatum | Brazil | 2020 | [103] ** |
| Apis mellifera | Neem oil | laboratory (L) | contact | adults | increase in mortality | India | 2017 | [107] |
| Apis mellifera | Neem seed extracts | laboratory (L, S) | ingestion and topical | larvae | effects on survival of larvae, no anti-feeding effects, morphological alteration | Germany | 1980 | [109] ** |
| Apis mellifera | Azadirachtin | laboratory (L, S) | ingestion and topical | larvae | increase in mortality, no anti-feeding effects, morphological alteration | Germany | 1982 | [110] ** |
| Apis mellifera | Geraniol and Cymbopogon martinii EO | laboratory (L, S) | topical, ingestion | adults | no effects on mortality, no effects on immune response, on behaviour, and locomotion | Brazil | 2018 | [99] |
| Apis mellifera | Neem seed extracts | field | crop spraying | colony | effects on the hatching observed in the smaller hives, morphological alteration, non-repellent effects on treated flowers | Germany | 1987 | [111] ** |
| Apis mellifera | Azadirachtin | semi-field | crop granules, crop spraying | colony | no effects on mortality, reduction in foraging activity and brood development with spray treatment | Czech Republic | 2005 | [120] ** |
| Apis mellifera | Pyrethrins and rotenone | laboratory (L) | topical, ingestion | adults | increased mortality with both compounds | UK | 1978 | [125] |
| Apis mellifera | Azadirachtin | field | ingestion | colony | colony overwintering failure, no effects on brood and queen development | UK | 2005 | [121] |
| Apis mellifera | Azadirachtin | field | crop spraying | adults | effects on flower visitation rates | Brazil | 2019 | [122] |
| Apis mellifera | Artemisia absinthium, and Eupatorium buniifolium EOs | laboratory (L) | topical, contact | adults | no effects on mortality in the topical test, increased mortality in the contact test | Uruguay | 2017 | [98] ** |
| Apis mellifera | Andiroba, citronella, eucalyptus, and neem oil, garlic extract, and rotenone | laboratory (L, S) | contact, ingestion and topical | larvae, adults | increase in mortality of bee larvae with andiroba, neem oil, and garlic extract, reduction of body mass of adults, reduction in walking activity and repellent effects in adult workers | Brazil | 2015 | [100] ** |
| Bumblebees (Bombus spp.) | ||||||||
| Bombus terrestris | Azadirachtin | laboratory (L, S) | ingestion | adults, microcolony | increase in mortality, repellent effects, reduction in egg-laying, in production of drones, and in ovarian length | Belgium | 2015 | [61] |
| Bombus terrestris | Azadirachtin | laboratory (L) | ingestion | adults | increase in mortality | Turkey | 2022 | [137] ** |
| Bombus terrestris | mixture of Perilla frutescens var. crispa extracts and phytoncide oil | laboratory (L, S) | contact | adults | high mortality, no effects on walking behaviour, reduction in gene expression | Republic of Korea | 2018 | [139] ** |
| Bombus terrestris | Azadirachtin | field | ingestion | adults | reduction in pollen foraging | Estonia | 2009 | [138] ** |
| Stingless bees (Meliponini) | ||||||||
| Melipona quadrifasciata | Azadirachtin | laboratory (L, S) | ingestion and topical | larvae | increase in mortality at higher doses, development of deformed pupae and adults | Brazil | 2015 | [153] ** |
| Melipona quadrifasciata | Azadirachtin | laboratory (L, S) | contact, ingestion | adults | no effects on mortality, no anti-feeding effects, modifications in walking behaviour, no effects on flight and respiration rate | Brazil | 2017 | [62] ** |
| Melipona quadrifasciata | Azadirachtin | laboratory (S) | ingestion | adults | reduction of gene expression of vitellogenin (Vg), increase of the number of haemocytes | Brazil | 2021 | [156] ** |
| Nannotrigona aff. testaceicornis | Lippia sidoides EO, and main compounds | laboratory (L, S) | topical | adults | low increase in mortality, low reduction in locomotion ability and flight orientation, avoidance effects | Brazil | 2021 | [149] |
| Nannotrigona testaceicornis | Andiroba, citronella, eucalyptus, and neem oil, garlic extract, and rotenone | laboratory (S) | contact | adults | no effects on handling behaviour | Brazil | 2010 | [152] ** |
| Partamona helleri | Azadirachtin | laboratory (L, S) | contact, ingestion | adults | no effects on mortality, anti-feeding effects, no effects on walking, flight and respiration rate | Brazil | 2017 | [62] ** |
| Partamona helleri | Azadirachtin | laboratory (L, S) | ingestion and topical | larvae of queens | increase of mortality at the higher doses, delayed development and production of deformed queens, no effects on walking behaviour, reduction in the ovarian morphometry | Brazil | 2018 | [63] |
| Partamona helleri | Agave americana, Anadenanthera colubrina, and Nicotiana tabacum extracts | laboratory (L, S) | contact, ingestion | adults | increased mortality with N. tabacum, low increase in mortality with the other compounds, no effects on respiration rates or flight | Brazil | 2020 | [93] |
| Partamona helleri | Azadirachtin | laboratory (L, S) | contact, ingestion | adults | low increase in mortality, no effects on overall group activity, reduction of flight take-off of worker | Brazil | 2015 | [154] |
| Plebeia sp. | Azadirachtin | field | crop spraying | adults | no effects on flower visitation rates | Brazil | 2019 | [122] |
| Scaptotrigona xanthotrica | Azadirachtin | laboratory (L, S) | contact, ingestion | adults | low increase in mortality, no effects on overall group activity, reduction of flight take-off of worker | Brazil | 2015 | [154] |
| Tetragonisca angustula | Azadirachtin | laboratory (S), and semi-field | contact | adults and colony | reduction in gene expression of esterase isoenzymes, changes in protein synthesis | Brazil | 2020 | [155] ** |
| Tetragonisca angustula | Corymbia citriodora EO | laboratory (L) | topical | adults | increase in mortality | Brazil | 2018 | [150] ** |
| Tetragonisca angustula | Artemisia annua EO | laboratory (L) | topical | adults | increase in mortality | Brazil | 2018 | [151] ** |
| Tetragonisca angustula | Andiroba, citronella, eucalyptus, and neem oil, garlic extract, and rotenone | laboratory (S) | contact | adults | no effects on handling behaviour | Brazil | 2010 | [152] ** |
| Trigona hyalinata | Mentha piperita, Origanum vulgare, Thymus vulgaris, and Zingiber officinale EOs | laboratory (L, S) | topical, contact, ingestion | adults | low increase in mortality, reduction in movements with oregano and thyme EOs | Brazil | 2020 | [102] ** |
| Trigona spinipes | Azadiracha indica, Lippiasidoides, Sapindus saponaria, Anonna squamosa, Cymbopogon winterianum, Corimbia citriodora, Jatropha curcas, Ricinus communis leaf and seed extracts | laboratory (L) | topical | adults | increase in mortality with A. indica, A. squamosa, and R. communis | Brazil | 2012 | [148] ** |
| Solitary species | ||||||||
| Halictus sp. *** | Azadirachtin | field | crop spraying | adults | reduction of flower visitation rates | Brazil | 2019 | [122] |
| Megachile rotundata | Ajwain, basil, cinnamon, clove, coriander, cumin, fenugreek, fennel, ginger, nutmeg, oregano, rosemary, sage, thyme, and turmeric powders (containing EOs) | laboratory (L) | contact | adult males | increase in mortality | Canada | 2020 | [57] ** |
| Osmia cornifrons | Wintergreen oil | laboratory (L) | topical, contact | adults | increase in mortality | USA | 2009 | [158] ** |
| Osmia cornuta | Quassia amara extract | laboratory (L) | contact | eggs, larvae | increase in mortality | Italy | 2003 | [157] |
| Osmia lignaria | Neem oil | laboratory (L) | topical, ingestion | adults | increase in mortality | USA | 2005 | [116] |
*: Most of the studies reviewed target adult worker bees belonging to different ages (newly emerged, foragers). The table specifies whether the target belongs to other castes (queen, male). **: Studies in which there is no positive control with chemical insecticides. ***: Halictus Latreille is a wide genus of bees that includes a scale of different social behaviours from solitary and semi-social to social species. Since the species was not specified in Tshoecke et al. (2019), we reported this in the solitary bees category.

Table 2.
Toxicological parameters (LC50, LD50) of botanical biopesticides extrapolated from the analysed papers. The values are reported as they were reported in the papers.
Table 2.
Toxicological parameters (LC50, LD50) of botanical biopesticides extrapolated from the analysed papers. The values are reported as they were reported in the papers.
| Group | Bee Species | Botanical Substance | Target | Application | Toxicological Parameters | References |
|---|---|---|---|---|---|---|
| HONEYBEES | Apis cerana indica | Annonin | adults | topical | LC50(%)(72 h): 1.22 | [106] |
| Azadirachtin | adults | topical | LC50(%)(72 h): 1.64 | |||
| Apis mellifera | Mentha piperita EO | adults | contact | LC50(%)(24 h): 13.35 | [102] | |
| adults | topical | LD50(%)(24 h): 12.58 | ||||
| Origanum vulgare EO | adults | contact | LC50(%)(24 h): 0.95 | |||
| adults | topical | LD50(%)(24 h): 2.03 | ||||
| Thymus vulgaris EO | adults | contact | LC50(%)(24 h): 2.61 | |||
| adults | topical | LD50(%)(24 h): 3.30 | ||||
| Zingiber officinale EO | adults | contact | LC50(%)(24 h): 22.01 | |||
| adults | topical | LD50(%)(24 h): 17.98 | ||||
| Pellitorine extracted from Piper tuberculatum | larvae | ingestion and topical | LD50 (μg a.i./bee)(96 h): 0.8048 | [105] | ||
| adults | ingestion | LD50 (μg a.i./bee)(96 h): 0.759 | ||||
| topical | LD50 (μg a.i./bee)(96 h): 0.357 | |||||
| Neem oil | I instar larvae | ingestion and topical | LD50 (μg a.i./g)(6 d): 37 | [113] | ||
| IV instar larvae | ingestion and topical | LD50 (μg a.i./g)(10 d): 61 | ||||
| Nicotine extracted from Nicotiana tabacum | adults | contact | LC50 (ng/bee)(48 h): 60.15 | [93] | ||
| adults | ingestion | LC50 (ng/bee)(48 h): 32.45 | ||||
| β-Caryophyllene extracted from Agave americana | adults | contact | LC50 (ng/bee)(48 h): 127.4 | |||
| adults | ingestion | LC50 (ng/bee)(48 h): 111.2 | ||||
| Lupeol extracted from Anadenanthera colubrina | adults | contact | LC50 (ng/bee)(48 h): 222.5 | |||
| adults | ingestion | LC50 (ng/bee)(48 h): 210.1 | ||||
| Cymbopogon martinii EO | adults | ingestion | LD50 (μg/bee)(24 h): 465 | [99] | ||
| adults | topical | LD50 (μg/bee)(24 h): 73 | ||||
| Geraniol | adults | ingestion | LD50 (μg/bee)(24 h): 290 | |||
| adults | topical | LD50 (μg/bee)(24 h): 43 | ||||
| Pyrethrins | adults | ingestion | LD50 (μg/bee): 0.29–0.13 | [125] | ||
| adults | topical | LD50 (μg/bee): 0.15 | ||||
| Rotenone | adults | ingestion | LD50 (μg/bee): >60 | |||
| adults | topical | LD50 (μg/bee): >30 | ||||
| Artemisia absinthium EO | adults | topical | LD50 (μg/bee)(24 h): 252 | [98] | ||
| adults | complete exposure | LD50 (mg/cm2)(24 h): 0.15 | ||||
| Eupatorium buniifolium EO | adults | topical | LD50 (μg/bee)(24 h): 197 | |||
| adults | complete exposure | LD50 (mg/cm2)(24 h): 0.26 | ||||
| STINGLESS BEES | Nannotrigona aff. testaceicornis | Lippia sidoides EO | adults | topical | LD50 (μg/bee)(24 h): 33.7 | [149] |
| Thymol (compound of Lippia sidoides EO) | adults | topical | LD50 (μg/bee)(24 h): 33.6 | |||
| ρ—cymene (compound of Lippia sidoides EO) | adults | topical | LD50 (μg/bee)(24 h): 198 | |||
| (E)—caryophyllene (compound of Lippia sidoides EO) | adults | topical | LD50 (μg/bee)(24 h): 306 | |||
| Partamona helleri | Nicotine extracted from Nicotiana tabacum | adults | contact | LC50 (ng/bee)(48 h): 44.32 | [93] | |
| adults | ingestion | LC50 (ng/bee)(48 h): 38.76 | ||||
| β-Caryophyllene extracted from Agave americana | adults | contact | LC50 (ng/bee)(48 h): 122.2 | |||
| adults | ingestion | LC50 (ng/bee)(48 h): 117.1 | ||||
| Lupeol extracted from Anadenanthera colubrina | adults | contact | LC50 (ng/bee)(48 h): 200.1 | |||
| adults | ingestion | LC50 (ng/bee)(48 h): 212.2 | ||||
| Trigona hyalinata | Mentha piperita EO | adults | contact | LC50(%)(24 h): 21.61 | [102] | |
| adults | topical | LD50(%)(24 h): 16.38 | ||||
| Origanum vulgare EO | adults | contact | LC50(%)(24 h): 7.14 | |||
| adults | topical | LD50(%)(24 h): 4.57 | ||||
| Thymus vulgaris EO | adults | contact | LC50(%)(24 h): 8.29 | |||
| adults | topical | LD50(%)(24 h): 6.53 | ||||
| Zingiber officinale EO | adults | contact | LC50(%)(24 h): 24.17 | |||
| adults | topical | LD50(%)(24 h): 32.65 |
7. Conclusions
Increasing awareness of the risks associated with synthetic pesticides is leading to a revaluation and increased production of studies on botanically derived products. These studies are undergoing a renaissance, especially in some countries such as India, China, and Brazil, where the number of papers on this topic has grown, particularly in recent decades [159]. Although botanicals are presented as eco-friendly alternatives with high selectivity and low persistence in the environment, the knowledge regarding their posing of risks to non-target organisms is still scarce, and studies in the literature indicate several detrimental effects on pollinators. Brazil appears to be the country from which the largest number of papers analysed came (n = 23), followed by the United States (n = 10) and India (n = 4). For several countries, especially in Europe, the efforts made to date on this topic are rather limited (Figure 1A). Brazil has the greatest biodiversity in the world, so many botanical biopesticides are constantly being tested [160,161,162,163]. In Brazil, beekeeping activities stand out, in addition to a vast population of native stingless bees. In this sense, efforts have been made to carry out selectivity and toxicity tests of these products on these pollinators [62,63]. In general, the toxicity of botanical biopesticides is lower than that of synthetic products. However, this review highlights how the products from different classes of botanical biopesticides can cause lethal effects and a wide variety of sublethal effects (Table 3) on different groups of bees, ranging from social to solitary species, although studies found in the literature focus on just a few model species. Indeed, the great majority of the analysed papers focused on honeybees, especially A. mellifera, while very few works focused on a few other model species, such as bumblebees, and stingless bees (Figure 1B and Figure 2). Despite neotropical stingless bees only recently being the subject of risk assessment regarding pesticides and biopesticides, there is a growing number of studies on botanical substances. On the other hand, for other groups such as solitary bees, the number of studies, and the number of species and substances tested, are still scarce (Figure 2). In the literature we found toxicological parameters (LC50 and LD50) of the different botanical pesticides only for honeybees and stingless bees, with a gap for bumblebees and solitary bees (Table 2). The toxicity of botanical biopesticides for bees varies greatly among different classes of botanicals and different formulations, and in this regard, the majority of the papers analysed focused on limonoids (azadirachtin) and essential oils (Figure 1C and Figure 2). Essential oils in general have been shown to be less toxic than other botanical products, although there are several exceptions. Alkaloid products such as sabadilla or ryania extracts cause lethal effects on honeybees and have not been tested on other bee species. Azadirachtin has proven to be one of the most studied botanical insecticides concerning bees, reporting lethal effects for several bee species, and a massive presence of sublethal effects (Table 3), this despite the detrimental effects varying significantly depending on the formulations used. The toxicity of some products for bees deserves further investigation with a focus on the sublethal effects, and an increase in field and semi-field studies, which have currently been carried out in a small proportion (Figure 2). None of the trials with alkaloids, EOs, or phenolics from the analysed papers were conducted under field or semi-field conditions (Figure 2). No synergistic effects between botanicals and between botanicals and chemicals pesticides have been investigated in bees, although this is an area of recent interest and attention [164,165,166]. Furthermore, of the total number of the studies reviewed (n = 54), a great proportion (53.7%, n = 29) do not include a chemical insecticide as the positive control in the experimental procedure. Including tests with a chemical insecticide group (control) can increase the potential for analysing and considering the data and can also facilitate understanding of the results. For this reason, it is important to highlight the need for protocols. In general, due to non-uniformly used methodologies, the results often cannot be compared. In addition, we have almost no information on their residues and persistence in bee matrices and thus the potential exposure level for bees in the field. The combination of these factors makes it complex to assess and discuss the actual safety of some of these products for bees. Therefore, the need has emerged for the development of new standardized protocols for the risk assessment of plant protection products regarding the different groups of bees. This is particularly true for stingless bees and solitary bees, there being protocols for honeybees in the literature but very few protocols for bumblebees (Table 4). New protocols are also required to evaluate the great variety of sublethal effects that could affect bees. In general, botanical biopesticides seem safer for bees than synthetic pesticides. However, some products need further evaluations, with the adoption of standardized biopesticide protocols, in order to assess the risks for different bee species, from social to solitary.
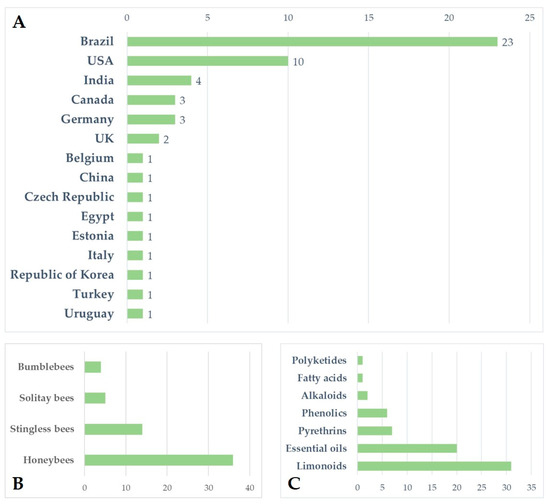
Figure 1.
(A) Number of analysed papers assessing the risk of botanical biopesticides for bees by country; (B) number of analysed papers regarding the different groups of bees (honeybees, bumblebees, stingless bees, and solitary bees); (C) number of analysed papers concerning the different classes of botanical biopesticides.

Table 3.
Classes of sublethal effects caused by botanical biopesticides reported in the reviewed studies on different groups of Hymenoptera Apoidea. In parentheses the total number of studies and the number of studies showing a significant detrimental effect.
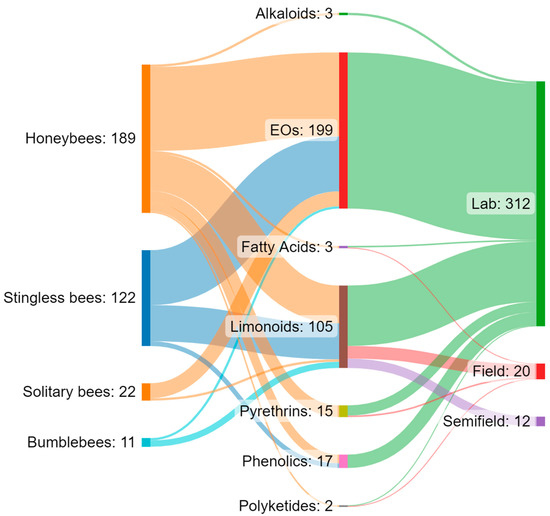
Figure 2.
Sankey diagram showing the interaction flows between the groups of bees, different categories of botanical biopesticides, and the type of assay. The reported values refer to the number of trials (n = 344) found from the analysed papers (n = 54).

Table 4.
Overview of the research gaps in the literature regarding the effects of botanical biopesticides and bees and future directions.
Author Contributions
Conceptualization, R.C., G.M. and M.A.P.L.; methodology, R.C.; validation, R.C., G.M., M.A.P.L. and L.Z. formal analysis, R.C., G.M. and M.A.P.L.; resources, R.C.; data curation, R.C.; writing—original draft preparation, R.C.; writing—review and editing, R.C., G.M., M.A.P.L., L.Z., F.S. and M.P.; supervision, M.A.P.L., L.Z. and G.M.; project administration, M.A.P.L., L.Z. and G.M.; funding acquisition, M.A.P.L., L.Z. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following: Capes Print program, research grant number 88887.571161/2020-00 to MAPL; PRIN 2020 project “Bio-inspired Plant Protection (BiPP)”, grant number 2020T58TA3; European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022) within the Agritech National Research Centre.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; p. 953. [Google Scholar]
- Orr, M.C.; Hughes, A.C.; Chesters, D.; Pickering, J.; Zhu, C.D.; Ascher, J.S. Global Patterns and Drivers of Bee Distribution. Curr. Biol. 2021, 31, 451–458.e4. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Kremen, C. The Value of Pollinator Species Diversity Most Crop-Visiting Species Are Needed to Ensure High Levels of Crop Pollination. Science 2018, 359, 741–742. [Google Scholar] [CrossRef]
- Porto, R.G.; de Almeida, R.F.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. Pollination Ecosystem Services: A Comprehensive Review of Economic Values, Research Funding and Policy Actions. Food Secur. 2020, 12, 1425–1442. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L. The Solitary Bees: Biology, Evolution, Conservation; Princeton University Press, Princeton and Oxford: Princeton, NJ, USA, 2019; p. 488. [Google Scholar] [CrossRef]
- Sgolastra, F.; Hinarejos, S.; Pitts-Singer, T.L.; Boyle, N.K.; Joseph, T.; Luckmann, J.; Raine, N.E.; Singh, R.; Williams, N.M.; Bosch, J. Pesticide Exposure Assessment Paradigm for Solitary Bees. Environ. Entomol. 2019, 48, 22–35. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 340, 1608–1611. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Conrad, K.F.; Warren, M.S.; Fox, R.; Parsons, M.S.; Woiwod, I.P. Rapid Declines of Common, Widespread British Moths Provide Evidence of an Insect Biodiversity Crisis. Biol. Conserv. 2006, 132, 279–291. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of Managed Honey Bees and Beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Bommarco, R.; Breeze, T.D.; Carvalheiro, L.G.; Franzén, M.; González-Varo; Schweiger, O. Status and Trends of European Pollinators; Pensoft Publishers: Sofia, Bulgaria, 2015; ISBN 9789546427625. [Google Scholar]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarl, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod Decline in Grasslands and Forests Is Associated with Landscape-Level Drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect Decline in the Anthropocene: Death by a Thousand Cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop Production in the USA Is Frequently Limited by a Lack of Pollinators. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; p. 86. ISBN 9789279445125. [Google Scholar]
- Kopec, K.; Burd, L.A. Pollinators In Peril: A Systematic Status Review of North American and Hawaiian Native Bees. Cent. Biol. Divers. 2017, 14. [Google Scholar]
- Goulson, D.; Nicholls, E. Anthropogenic Influences on Bee Foraging. Science 2022, 375, 970–972. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis mellifera, Bombus spp. and Solitary Bees). EFSA J. 2013, 11, 3295. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Botina, L.L.; Araújo, R.d.S.; Guedes, R.N.C.; Martins, G.F.; Lima, M.A.P. Artificial Intelligence-Aided Meta-Analysis of Toxicological Assessment of Agrochemicals in Bees. Front. Ecol. Evol. 2022, 10, 845608. [Google Scholar] [CrossRef]
- Mayer, D.F.; Lunden, J.D. Effects of Imidacloprid Insecticide on Three Bee Pollinators. Hortic. Sci. 1997, 29, 93–97. [Google Scholar]
- Blacquière, T.; Smagghe, G.; Van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in Bees: A Review on Concentrations, Side-Effects and Risk Assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- Siviter, H.; Richman, S.K.; Muth, F. Field-Realistic Neonicotinoid Exposure Has Sub-Lethal Effects on Non-Apis Bees: A Meta-Analysis. Ecol. Lett. 2021, 24, 2586–2597. [Google Scholar] [CrossRef]
- Domenica, A.; Maria, A.; Stefania, B.; Alessio, I.; Alberto, L.; Tunde, M.; Rachel, S.; Csaba, S.; Benedicte, V.; Alessia, V. Neonicotinoids and Bees: The Case of the European Regulatory Risk Assessment. Sci. Total Environ. 2017, 579, 966–971. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and Pesticide Regulation: Lessons from the Neonicotinoid Experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Arena, M.; Sgolastra, F. A Meta-Analysis Comparing the Sensitivity of Bees to Pesticides. Ecotoxicology 2014, 23, 324–334. [Google Scholar] [CrossRef]
- Abati, R.; Sampaio, A.R.; Maciel, R.M.A.; Colombo, F.C.; Libardoni, G.; Battisti, L.; Lozano, E.R.; Ghisi, N.d.C.; Costa-Maia, F.M.; Potrich, M. Bees and Pesticides: The Research Impact and Scientometrics Relations. Environ. Sci. Pollut. Res. 2021, 28, 32282–32298. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Viñuela, E.; Zappalà, L.; Desneux, N. The Non-Target Impact of Spinosyns on Beneficial Arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef]
- Borges, S.; Alkassab, A.T.; Collison, E.; Hinarejos, S.; Jones, B.; McVey, E.; Roessink, I.; Steeger, T.; Sultan, M.; Wassenberg, J. Overview of the Testing and Assessment of Effects of Microbial Pesticides on Bees: Strengths, Challenges and Perspectives. Apidologie 2021, 52, 1256–1277. [Google Scholar] [CrossRef]
- Cappa, F.; Baracchi, D.; Cervo, R. Biopesticides and Insect Pollinators: Detrimental Effects, Outdated Guidelines, and Future Directions. Sci. Total Environ. 2022, 837, 155714. [Google Scholar] [CrossRef]
- Erler, S.; Eckert, J.H.; Steinert, M.; Alkassab, A.T. Impact of Microorganisms and Entomopathogenic Nematodes Used for Plant Protection on Solitary and Social Bee Pollinators: Host Range, Specificity, Pathogenicity, Toxicity, and Effects of Experimental Parameters. Environ. Pollut. 2022, 302, 119051. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Spochacz, M.; Adamski, Z. The Role of Botanical Treatments Used in Apiculture to Control Arthropod Pests. Apidologie 2022, 53, 27. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural Products That Have Been Used Commercially as Crop Protection Agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Nakasu, E.Y.T.; Williamson, S.M.; Edwards, M.G.; Fitches, E.C.; Gatehouse, J.A.; Wright, G.A.; Gatehouse, A.M.R. Novel Biopesticide Based on a Spider Venom Peptide Shows No Adverse Effects on Honeybees. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140619. [Google Scholar] [CrossRef]
- Jacobsson, E.; Andersson, H.S.; Strand, M.; Peigneur, S.; Eriksson, C.; Lodén, H.; Shariatgorji, M.; Andrén, P.E.; Lebbe, E.K.M.; Rosengren, K.J.; et al. Peptide Ion Channel Toxins from the Bootlace Worm, the Longest Animal on Earth. Sci. Rep. 2018, 8, 4596. [Google Scholar] [CrossRef]
- US EPA—US Environmental Protection Agency; Health Canada; California Department of Pesticide Regulation. Guidance for Assessing Pesticide Risk to Bees; US EPA: Washington, DC, USA, 2014.
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-Target Effects of Essential Oil-Based Biopesticides for Crop Protection: Impact on Natural Enemies, Pollinators, and Soil Invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J. Biofertil. Biopestic. 2015, 6, 100–129. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Ujvàry, I. Nicotine and Other Insecticidal Alkaloids. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 29–69. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mossa, A.T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-Pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Campolo, O.; Cherif, A.; Ricupero, M.; Siscaro, G.; Grissa-Lebdi, K.; Russo, A.; Cucci, L.M.; Di Pietro, P.; Satriano, C.; Desneux, N.; et al. Citrus Peel Essential Oil Nanoformulations to Control the Tomato Borer, Tuta absoluta: Chemical Properties and Biological Activity. Sci. Rep. 2017, 7, 13036. [Google Scholar] [CrossRef]
- Sciortino, M.; Scurria, A.; Lino, C.; Pagliaro, M.; D’Agostino, F.; Tortorici, S.; Ricupero, M.; Biondi, A.; Zappalà, L.; Ciriminna, R. Silica-Microencapsulated Orange Oil for Sustainable Pest Control. Adv. Sustain. Syst. 2021, 5, 2000280. [Google Scholar] [CrossRef]
- Ong, M.; Chomistek, N.; Dayment, H.; Goerzen, W.; Baines, D. Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects 2020, 11, 359. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Chang, K.S.; Park, C.; Ahn, Y.J. Larvicidal Activity of Asarum Heterotropoides Root Constituents against Insecticide-Susceptible and-Resistant Culex pipiens Pallens and Aedes aegypti and Ochlerotatus togoi. J. Agric. Food Chem. 2010, 58, 10001–10006. [Google Scholar] [CrossRef] [PubMed]
- Buxton, T.; Takahashi, S.; Takakura, M.; Niwata, I.; Owusu, E.O.; Kim, C.-S. Insecticidal Activities of Pellitorine Isolated from Zanthoxylum zanthoxyloides Roots against Sitophilus oryzae L. (Coleoptera : Curculionidae). J. Entomol. Zool. Stud. 2017, 5, 163–168. [Google Scholar]
- Morgan, E.D. Azadirachtin, a Scientific Gold Mine. Bioorg. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.F.; De Meyer, L.; Guedes, R.N.C.; Smagghe, G. Lethal and Sublethal Effects of Azadirachtin on the Bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, R.C.; Tomé, H.V.V.; Barbosa, W.F.; Guedes, R.N.C.; Lima, M.A.P. Azadirachtin-Induced Antifeeding in Neotropical Stingless Bees. Apidologie 2017, 48, 275–285. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Barbosa, W.F.; Martins, G.F.; Lima, M.A.P. The Reduced-Risk Insecticide Azadirachtin Poses a Toxicological Hazard to Stingless Bee Partamona helleri (Friese, 1900) Queens. Chemosphere 2018, 201, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Imdorf, A.; Kilchenmann, V.; Bogdanov, S.; Bachofen, B.; Beretta, C. Toxic Effects of Thymol, Camphor, Menthol and Eucalyptol on Varroa jacobsoni Oud and Apis mellifera L. in a Laboratory Test. Apidologie 1995, 26, 27–31. [Google Scholar] [CrossRef]
- Ocampo, D.; Ocampo, R. Bioactividad de La Família Annonaceae. Rev. Univ. Caldas 2006, 1, 135–155. [Google Scholar]
- Ribeiro, L.P.; Zanardi, O.Z.; Gonçalves, G.L.P.; Ansante, T.F.; Yamamoto, P.T.; Vendramim, J.D. Toxicity of an Annonin-Based Commercial Bioinsecticide Against Three Primary Pest Species of Stored Products. Neotrop. Entomol. 2018, 47, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Jeran, N.; Grdiša, M.; Varga, F.; Šatović, Z.; Liber, Z.; Dabić, D.; Biošić, M. Pyrethrin from Dalmatian Pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.): Biosynthesis, Biological Activity, Methods of Extraction and Determination. Phytochem. Rev. 2021, 20, 875–905. [Google Scholar] [CrossRef]
- Heard, M.S.; Baas, J.; Dorne, J.L.; Lahive, E.; Robinson, A.G.; Rortais, A.; Spurgeon, D.J.; Svendsen, C.; Hesketh, H. Comparative Toxicity of Pesticides and Environmental Contaminants in Bees: Are Honey Bees a Useful Proxy for Wild Bee Species? Sci. Total Environ. 2017, 578, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, A.; Galic, N.; Feken, M.; Thompson, H.; Sgolastra, F.; Pitts-Singer, T.; Elston, C.; Pamminger, T.; Hinarejos, S. Assessment of the Vulnerability to Pesticide Exposures Across Bee Species. Environ. Toxicol. Chem. 2021, 40, 2640–2651. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J. Wild Bee Toxicity Data for Pesticide Risk Assessments. Data 2019, 4, 98. [Google Scholar] [CrossRef]
- Brittain, C.; Potts, S.G. The Potential Impacts of Insecticides on the Life-History Traits of Bees and the Consequences for Pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Lima, M.A.P.; Martins, G.F.; Oliveira, E.E.; Guedes, R.N.C. Agrochemical-Induced Stress in Stingless Bees: Peculiarities, Underlying Basis, and Challenges. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2016, 202, 733–747. [Google Scholar] [CrossRef]
- OECD. Bumblebee, Acute Contact Toxicity Test; OECD: Paris, France, 2017; Volume 11. [Google Scholar]
- Gradish, A.E.; Van Der Steen, J.; Scott-Dupree, C.D.; Cabrera, A.R.; Cutler, G.C.; Goulson, D.; Klein, O.; Lehmann, D.M.; Lückmann, J.; O’Neill, B.; et al. Comparison of Pesticide Exposure in Honey Bees (Hymenoptera: Apidae) and Bumble Bees (Hymenoptera: Apidae): Implications for Risk Assessments. Environ. Entomol. 2019, 48, 12–21. [Google Scholar] [CrossRef]
- Auteri, D.; Arce, A.; Ingels, B.; Marchesi, M.; Neri, F.M.; Rundlöf, M.; Wassenberg, J. Analysis of the Evidence to Support the Definition of Specific Protection Goals for Bumble Bees and Solitary Bees. EFSA Support. Publ. 2022, 19, 7125E. [Google Scholar] [CrossRef]
- Uhl, P.; Awanbor, O.; Schulz, R.S.; Brühl, C.A. Is Osmia bicornis an Adequate Regulatory Surrogate? Comparing Its Acute Contact Sensitivity to Apis mellifera. PLoS ONE 2019, 14, e0201081. [Google Scholar] [CrossRef]
- Cham, K.O.; Nocelli, R.C.F.; Borges, L.O.; Viana-Silva, F.E.C.; Tonelli, C.A.M.; Malaspina, O.; Menezes, C.; Rosa-Fontana, A.S.; Blochtein, B.; Freitas, B.M.; et al. Pesticide Exposure Assessment Paradigm for Stingless Bees. Environ. Entomol. 2019, 48, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Franke, L.A.; Rehberg, C.; Wollmann, C.; Stahlschmidt, P.; Jeker, L.; Brühl, C.A. Interspecific Sensitivity of Bees towards Dimethoate and Implications for Environmental Risk Assessment. Sci. Rep. 2016, 6, 34439. [Google Scholar] [CrossRef] [PubMed]
- Bireley, R.; Borges, S.; Cham, K.; Epstein, D.; Garber, K.; Hart, C.; Hou, W.; Ippolito, A.; Pistorius, J.; Poulsen, V.; et al. Preface: Workshop on Pesticide Exposure Assessment Paradigm for Non-Apis Bees. Environ. Entomol. 2019, 48, 1–3. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 213: Honeybees, Acute Oral Toxicity Test; OECD Guidel. Test. Chem.: Paris, France, 1998; p. 8. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Mommaerts, V.; Smagghe, G. Side-Effects of Pesticides on the Pollinator Bombus: An Overview; In-Tech: Warwick, UK, 2011. [Google Scholar] [CrossRef]
- Lima, M.A.P.; Cutler, G.C.; Mazzeo, G.; Hrncir, M. Editorial: The Decline of Wild Bees: Causes and Consequences. Front. Ecol. Evol. 2022, 10, 1027169. [Google Scholar] [CrossRef]
- OECD. Test No. 75: Guidance Document on the Honey Bee (Apis mellifera L.) Brood Test under Semi-Field Conditions; OECD: Paris, France, 2007. [Google Scholar]
- Kumar Gupta, R.; Reybroeck, W.; Van Veen, J.W.; Gupta, A. Beekeeping for Poverty Alleviation and Livelihood Security: Vol. 1: Technological Aspects of Beekeeping; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789401791991. [Google Scholar]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A.; et al. Global Trends in the Number and Diversity of Managed Pollinator Species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Radloff, S.; Hepburn, C.; Hepburn, R.; Fuchs, S.; Hadisoesilo, S.; Tan, K.; Engel, M.S.; Kuznetsov, V. Population Structure and Classification of Apis cerana. Apidologie 2010, 41, 589–601. [Google Scholar] [CrossRef]
- Crane, E. Recent Research on the World History of Beekeeping. Bee World 1999, 80, 174–186. [Google Scholar] [CrossRef]
- IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; IPBES: Bonn, Germany, 2016. [Google Scholar]
- Pandey, A. Suitability Assessment of Neem Formulations as Biopesticide against Honey Bees and Other Pollinators. J. Ecophysiol. Occup. Health 2010, 10, 49–52. [Google Scholar]
- Detzel, A.; Wink, M. Attraction, Deterrence or Intoxication of Bees (Apis mellifera) by Plant Allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Cunha Pereira, R.; Faria Barbosa, W.; Pereira Lima, M.A.; Vieira, J.O.L.; Carvalho Guedes, R.N.; Rodrigues da Silva, B.K.; Dias Barbosa, G.M.; Lemes Fernandes, F. Toxicity of Botanical Extracts and Their Main Constituents on the Bees Partamona helleri and Apis mellifera. Ecotoxicology 2020, 29, 246–257. [Google Scholar] [CrossRef]
- Atkins, E.L.; Anderson, L.D. Toxicity of Bees on Contaminated Surfaces. The Series. Separate Containers Are Used for Toxicological Studies with Honeybees. The Pesticide Evaluation Is given in Detail, and Toxicity of Pesticide Dusts to Honeybees 1 Formation Concerning the Effect O. J. Econ. Entomol. 1954, 47, 969–972. [Google Scholar] [CrossRef]
- Anderson, L.D.; Atkins, E.L. Toxicity of Pesticides to Honey Bees in Laboratory and Field Tests in Southern California, 1955–1956. J. Econ. Entomol. 1958, 51, 103–108. [Google Scholar] [CrossRef]
- Anderson, L.D.; Tuft, T.O. Toxicity of Several New Insecticides to Honey Bees. J. Econ. Entomol. 1952, 45, 466–469. [Google Scholar] [CrossRef]
- Efrom, C.F.S.; Redaelli, L.R.; Meirelles, R.N.; Ourique, C.B. Side-Effects of Pesticides Used in the Organic System of Production on Apis mellifera Linnaeus, 1758. Brazilian Arch. Biol. Technol. 2012, 55, 47–53. [Google Scholar] [CrossRef]
- Umpiérrez, M.L.; Paullier, J.; Porrini, M.; Garrido, M.; Santos, E.; Rossini, C. Potential Botanical Pesticides from Asteraceae Essential Oils for Tomato Production: Activity against Whiteflies, Plants and Bees. Ind. Crops Prod. 2017, 109, 686–692. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Melo, C.R.; Lima, A.P.S.; Santana, E.D.R.; de Oliveira, B.M.S.; Oliveira, J.W.S.; Vieira, J.S.; Blank, A.F.; et al. Apis mellifera (Insecta: Hymenoptera) in the Target of Neonicotinoids: A One-Way Ticket? Bioinsecticides Can Be an Alternative. Ecotoxicol. Environ. Saf. 2018, 163, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Santana Júnior, P.A.; Ramos, R.S.; Martins, J.C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci. 2015, 15, 137. [Google Scholar] [CrossRef]
- Ruffinengo, S.; Eguaras, M.; Floris, I.; Faverin, C.; Bailac, P.; Ponzi, M. LD50 and Repellent Effects of Essential Oils from Argentinian Wild Plant Species on Varroa destructor. J. Econ. Entomol. 2005, 98, 651–655. [Google Scholar] [CrossRef]
- da Silva, I.M.; Zanuncio, J.C.; Brügger, B.P.; Soares, M.A.; Zanuncio, A.J.V.; Wilcken, C.F.; Tavares, W.d.S.; Serrão, J.E.; Sediyama, C.S. Selectivity of the Botanical Compounds to the Pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 2020, 10, 4820. [Google Scholar] [CrossRef]
- Potrich, M.; da Silva, R.T.L.; Maciel, R.M.A.; Costa-Maia, F.M.; Lozano, E.R.; Rossi, R.M.; Martins, J.R.; Dallacort, S. Are Plant Extracts Safe for Honey Bees (Apis mellifera)? J. Apic. Res. 2020, 59, 844–851. [Google Scholar] [CrossRef]
- Nicodemo, D.; Nogueira Couto, R.H. Use of Repellents for Honeybees (Apis mellifera L.) in Vitro in the Yellow Passion-Fruit (Passiflora edulis Deg) Crop and in Confined Beef Cattle Feeders. J. Venom. Anim. Toxins Incl. Trop. Dis. 2004, 10, 77–85. [Google Scholar] [CrossRef]
- Miranda, J.; Navickiene, H.M.D.; Nogueira-Couto, R.; de Bortoli, S.A.; Kato, M.; Bolzani, V.D.; Furlan, M. Susceptibility of Apis mellifera (Hymenoptera: Apidae) to Pellitorine, an Amide Isolated from Piper tuberculatum (Piperaceae). Apidologie 2003, 34, 409–415. [Google Scholar] [CrossRef]
- Challa, G.K.; Firake, D.M.; Behere, G.T. Bio-Pesticide Applications May Impair the Pollination Services and Survival of Foragers of Honey Bee, Apis cerana Fabricius in Oilseed Brassica. Environ. Pollut. 2019, 249, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Ratnakar, V.; Rao, S.R.K.; Sridevi, D.; Vidyasagar, B. Contact Toxicity of Certain Conventional Insecticides to European Honeybee, Apis mellifera Linnaeus. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3359–3365. [Google Scholar] [CrossRef]
- Elzen, P.J.; Elzen, G.W.; Lester, G.E. Compatibility of an Organically Based Insect Control Program with Honey Bee (Hymenoptera: Apidae) Pollination in Cantaloupes. J. Econ. Entomol. 2004, 97, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Rembold, H.; Sharma, G.K.; Czoppelt, C. Evidence of Growth Disruption in Insects without Feeding Inhibition by Neem Seed Fractions. J. Plant Dis. Prot. 1980, 87, 290–297. [Google Scholar]
- Rembold, H.; Sharma, G.K.; Czoppelt, C.; Schmutterer, H. Azadirachtin: A Potent Insect Growth Regulator of Plant Origin. Z. Angew. Entomol. 1982, 93, 12–17. [Google Scholar] [CrossRef]
- Schmutterer, H.; Holst, H. Untersuchungen Über Die Wirkung Des Angereicherten Und Formulierten Niemsamenextrakts AZT-VR-K Auf Die Honigbiene Apis mellifera L. J. Appl. Entomol. 1987, 103, 208–213. [Google Scholar] [CrossRef]
- Naumann, K.; Isman, M.B.; Currie, R.W. Evaluation of the Repellent Effects of a Neem Insecticide on Foraging Honey Bees and Other Pollinators. Can. Entomol. 1994, 126, 225–230. [Google Scholar] [CrossRef]
- Naumann, K.; Isman, M.B. Toxicity of a Neem (Azadirachta indica A. Juss) Insecticide to Larval Honey Bees. Am. Bee J. 1996, 136, 518–520. [Google Scholar]
- Hassona, N.M.; Kordy, A.M. Relationship between Toxicity of Certain Pesticides to the Honey Bee, Apis mellifera L. (Hymenoptera: Apidea) Foragers and Their Haemolymph Amino Acids. Middle East J. Appl. Sci. 2015, 5, 19–25. [Google Scholar]
- Zhao, K.; Wu, H.; Hou, R.; Wu, J.; Wang, Y.; Huang, S.; Cheng, D.; Xu, H.; Zhang, Z. Effects of Sublethal Azadirachtin on the Immune Response and Midgut Microbiome of Apis cerana cerana (Hymenoptera: Apidae). Ecotoxicol. Environ. Saf. 2022, 229, 113089. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, E.; Bosch, J.; Kemp, W.P.; Maini, S. Assessing Delayed and Acute Toxicity of Five Formulated Fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Naiara Gomes, I.; Ingred Castelan Vieira, K.; Moreira Gontijo, L.; Canto Resende, H. Honeybee Survival and Flight Capacity Are Compromised by Insecticides Used for Controlling Melon Pests in Brazil. Ecotoxicology 2020, 29, 97–107. [Google Scholar] [CrossRef]
- Amaral, R.L.; Venzon, M.; Filho, S.M.; Lima, M.A.P. Does Ingestion of Neem-Contaminated Diet Cause Mortality of Honey Bee Larvae and Foragers? J. Apic. Res. 2016, 54, 405–410. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, R.; Singh, A. Impact of Neem Oil on Developmental Stages of Honey Bee Apis mellifera L. Indian J. Entomol. 2022, 84, 783–787. [Google Scholar] [CrossRef]
- Shawki, M.A.-A.; Táborský, V.; Kamler, F.; Kazda, J. Effect of Two NeemAzalTM Formulations on Honeybees under Semi-Field Conditions. Plant Prot. Sci. 2005, 41, 63–72. [Google Scholar] [CrossRef]
- Thompson, H.M.; Wilkins, S.; Battersby, A.H.; Waite, R.J.; Wilkinson, D. The Effects of Four Insect Growth-Regulating (IGR) Insecticides on Honeybee (Apis mellifera L.) Colony Development, Queen Rearing and Drone Sperm Production. Ecotoxicology 2005, 14, 757–769. [Google Scholar] [CrossRef]
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.C.; Sarmento, R.A.; Santos, G.R. Botanical and Synthetic Pesticides Alter the Flower Visitation Rates of Pollinator Bees in Neotropical Melon Fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef]
- Calderone, N.W.; Nasr, M.E. Evaluation of a Formic Acid Formulation for the Fall Control of Varroa jacobsoni (Acari: Varroidae) in Colonies of the Honey Bee Apis mellifera (Hymenoptera: Apidae) in a Temperate Climate. J. Econ. Entomol. 1999, 92, 526–533. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Schmit, J.B. A Comparison between Rotenone and Pyrethrins as Contact Insecticides. J. Econ. Entomol. 1932, 25, 918–922. [Google Scholar] [CrossRef]
- Stevenson, J.H. The Acute Toxicity of Unformulated Pesticides to Worker Honey Bees (Apis mellifera L.). Plant Pathol. 1978, 27, 38–40. [Google Scholar] [CrossRef]
- Appel, A.G. Knockdown Efficiency and Materials’ Compatibility of Wasp and Hornet Spray Formulations to Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 1990, 83, 1925–1931. [Google Scholar] [CrossRef]
- Ginsburg, J.M. Test To Determine Toxicity Of Pyrethrum Vapors To Honeybees. J. Agric. Res. 1930, 40, 1053–1057. [Google Scholar]
- Caron, D.M. Effects of Some ULV Mosquito Abatement Insecticides on Honey Bees. J. Econ. Entomol. 1979, 72, 148–151. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Domingues, C.E.C.; de Melo, N.F.S.; Roat, T.C.; Malaspina, O.; Jones-Costa, M.; Silva-Zacarin, E.C.M.; Fraceto, L.F. Nanopesticide Based on Botanical Insecticide Pyrethrum and Its Potential Effects on Honeybees. Chemosphere 2019, 236, 124282. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Bumblebees: Behaviour, Ecology, and Conservation; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of Widespread Decline in North American Bumble Bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Rollin, O.; Vray, S.; Dendoncker, N.; Michez, D.; Dufrêne, M.; Rasmont, P. Drastic Shifts in the Belgian Bumblebee Community over the Last Century. Biodivers. Conserv. 2020, 29, 2553–2573. [Google Scholar] [CrossRef]
- Rasmont, P.; Coppée, A.; Michez, D.; De Meulemeester, M.T. An Overview of the Bombus terrestris (L. 1758) Subspecies (Hymenoptera: Apidae). Ann. Société Entomol. Fr. 2008, 44, 243–250. [Google Scholar] [CrossRef]
- Velthuis, H.H.W.; Van Doorn, A. A Century of Advances in Bumblebee Domestication and the Economic and Environmental Aspects of Its Commercialization for Pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Sterk, G.; Cuylaerts, J.; Kolokytha, P. Lethal and Sublethal Effects of Several Formulations of Azadirachtin on IPM Impact R&D Colonies of the Bumblebee Bombus terrestris (Hymenoptera: Apidae). Hazards Pestic. Bees 2018, 462, 39–45. [Google Scholar] [CrossRef]
- Sterk, G.; Hannegraaf, J.; Kolokytha, P. Effects of Chemical and Biological Plant Protection Products on R&D Colonies of the Buff-Tailed Bumblebee Bombus terrestris. In Proceedings of the Hazards of Pesticides to Bees, 14th International Symposium of the ICP-PR Bee Protection Group, Bern, Switzerland, 23–25 October 2019. [Google Scholar]
- Demirozer, O.; Uzun, A.; Yanik, G.; Bulus, I.Y.; Gosterit, A. Investigation of the Efficacy of Some Biopesticides by Food Exposure on Bombus terrestris L. (Hymenoptera: Apidae). J. Apic. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Koskor, E.; Muljar, R.; Drenkhan, K.; Karise, R.; Bender, A.; Viik, E.; Luik, A.; Mänd, M. The Chronic Effect of the Botanical Insecticide Neem EC on the Pollen Forage of the Bumble Bee Bombus terrestris L. Agron. Res. 2009, 7, 341–346. [Google Scholar]
- Kim, S.; Lee, J.K.; Song, Y.J.; Kang, S.C.; Kim, B.; Choi, I.J.; Lee, D.H. Evaluating Natural Compounds as Potential Insecticides against Three Economically Important Pests, Bemisia tabaci (Hemiptera: Aleyrodidae), Frankliniella occidentalis (Thysanoptera: Thripidae), and Myzus persicae (Hemiptera: Aphididae), on Greenhouse Swee. Appl. Biol. Chem. 2018, 61, 313–323. [Google Scholar] [CrossRef]
- Rasmussen, C.; Thomas, J.C.; Engel, M.S. A New Genus of Eastern Hemisphere Stingless Bees (Hymenoptera: Apidae), with a Key to the Supraspecific Groups of Indomalayan and Australasian Meliponini. Am. Mus. Novit. 2017, 3888, 33. [Google Scholar] [CrossRef]
- Cortopassi-Laurino, M.V.L.I.-F.; Roubik, D.W.; Dollin, A.; Heard, T.; Aguilar, I.; Venturieri, C.G.; Eardley, C.; Nogueira-Neto, P. Global Meliponiculture: Challenges and Opportunities. Apidologie 2006, 37, 275–292. [Google Scholar] [CrossRef]
- Christopher Brown, J.; Albrecht, C. The Effect of Tropical Deforestation on Stingless Bees of the Genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in Central Rondonia, Brazil. J. Biogeogr. 2001, 28, 623–634. [Google Scholar] [CrossRef]
- Samejima, H.; Marzuki, M.; Nagamitsu, T.; Nakasizuka, T. The Effects of Human Disturbance on a Stingless Bee Community in a Tropical Rainforest. Biol. Conserv. 2004, 120, 577–587. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, A.; Mora, F.; Porter-Boland, L.; MRamírez, I.M.; Del-Val, E. Stingless Bees (Apidae: Meliponini) at Risk in Western Mexico. Biotropica 2022, 54, 829–838. [Google Scholar] [CrossRef]
- Toledo-Hernández, E.; Peña-Chora, G.; Hernández-Velázquez, V.M.; Lormendez, C.C.; Toribio-Jiménez, J.; Romero-Ramírez, Y.; León-Rodríguez, R. The Stingless Bees (Hymenoptera: Apidae: Meliponini): A Review of the Current Threats to Their Survival. Apidologie 2022, 53, 8. [Google Scholar] [CrossRef]
- Barbosa, W.F.; Smagghe, G.; Guedes, R.N.C. Pesticides and Reduced-Risk Insecticides, Native Bees and Pantropical Stingless Bees: Pitfalls and Perspectives. Pest Manag. Sci. 2015, 71, 1049–1053. [Google Scholar] [CrossRef]
- Tomé, H.V.; Ramos, G.S.; Araújo, M.F.; Santana, W.C.; Santos, G.R.; Guedes, R.N.; Maciel, C.D.; Newland, P.L.; Oliveira, E.E. Agrochemical Synergism Imposes Higher Risk to Neotropical Bees than to Honeybees. R. Soc. Open Sci. 2017, 18, 160866. [Google Scholar] [CrossRef]
- Correia-Oliveira, M.E.; Poderoso, J.C.M.; Ferreira, A.F.; De Olinda, R.A.; Ribeiro, G.T. Impact of Aqueous Plant Extracts on Trigona spinipes (Hymenoptera: Apidae). Sociobiology 2012, 59, 849–858. [Google Scholar]
- Matos, W.B.; Santos, A.C.C.; Lima, A.P.S.; Santana, E.D.R.; Silva, J.E.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Potential Source of Ecofriendly Insecticides: Essential Oil Induces Avoidance and Cause Lower Impairment on the Activity of a Stingless Bee than Organosynthetic Insecticides, in Laboratory. Ecotoxicol. Environ. Saf. 2021, 209, 111764. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.V.; Farias, E.d.S.; Santos, A.A.; Filomeno, C.A.; dos Santos, I.B.; Barbosa, L.C.A.; Picanço, M.C. Selection of an Essential Oil from Corymbia and Eucalyptus Plants against Ascia monuste and Its Selectivity to Two Non-Target Organisms. Crop Prot. 2018, 110, 207–213. [Google Scholar] [CrossRef]
- Seixas, P.T.L.; Demuner, A.J.; Alvarenga, E.S.; Barbosa, L.C.A.; Marques, A.; Farias, E.d.S.; Picanço, M.C. Bioactivity of Essential Oils from Artemisia against Diaphania hyalinata and Its Selectivity to Beneficial Insects. Sci. Agric. 2018, 75, 519–525. [Google Scholar] [CrossRef]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Bacci, L.; Silva, G.A.; Da Silva Benevenute, J. Impact of Botanical Insecticides on Indigenous Stingless Bees (Hymenoptera: Apidae). Sociobiology 2010, 56, 713–725. [Google Scholar]
- Barbosa, W.F.; Tomé, H.V.V.; Bernardes, R.C.; Siqueira, M.A.L.; Smagghe, G.; Guedes, R.N.C. Biopesticide-Induced Behavioral and Morphological Alterations in the Stingless Bee Melipona quadrifasciata. Environ. Toxicol. Chem. 2015, 34, 2149–2158. [Google Scholar] [CrossRef]
- Tome, H.V.V.; Barbosa, W.F.; Corrêa, A.S.; Gontijo, L.M.; Martins, G.F.; Guedes, R.N.C. Reduced-Risk Insecticides in Neotropical Stingless Bee Species: Impact on Survival and Activity. Ann. Appl. Biol. 2015, 167, 186–196. [Google Scholar] [CrossRef]
- Oliveira, C.V.P.; Sinópolis Gigliolli, A.A.; Galhardo, D.; Moreira, D.R.; Ronqui, L.; Santos, S.A.; Arnault Toledo, V.A.; Ruvolo-Takasusuki, M.C.C. Effects Of Biopesticides In Tetragonisca angustula Latreille (Hymenoptera: Meliponinae) Pollinators. Arq. Ciências Veterinárias Zool. UNIPAR 2020, 23, e2301. [Google Scholar] [CrossRef]
- Viana, T.A.; Barbosa, W.F.; Lourenço, A.P.; Santana, W.C.; Campos, L.O.; Martins, G.F. Changes in Innate Immune Response and Detoxification in Melipona quadrifasciata (Apinae: Meliponini) on Oral Exposure to Azadirachtin and Spinosad. Apidologie 2021, 52, 252–261. [Google Scholar] [CrossRef]
- Tesoriero, D.; Maccagnani, B.; Santi, F.; Celli, G. Toxicity of Three Pesticides on Larval Instars of Osmia cornuta: Preliminary Results. Bull. Insectology 2003, 56, 169–171. [Google Scholar]
- White, J.B.; Park, Y.L.; West, T.P.; Tobin, P.C. Assessment of Potential Fumigants to Control Chaetodactylus krombeini (Acari: Chaetodactylidae) Associated with Osmia cornifrons (Hymenoptera: Megachilidae). J. Econ. Entomol. 2009, 102, 2090–2095. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical Insecticide Research: Many Publications, Limited Useful Data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Santana, C.B.; Souza, J.G.L.; Toledo, A.G.; Alves, L.F.A.; Alves, D.S.; Corrêa, J.M.; Pinto, F.G.S. Antimicrobial and Insecticidal Effects of Essential Oil and Plant Extracts of Myrcia oblongata Dc in Pathogenic Bacteria and Alphitobius diaperinus. Brazilian J. Biol. 2022, 82, e233425. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Vilela Bertolucci, S.K.; Carvalho, G.A. Toxicity of Essential Oils and Pure Compounds of Lamiaceae Species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and Their Safety for the Nontarget Organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Prot. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Stenger, L.D.; Abati, R.; Pawlak, I.G.; Varpechoski, G.O.; De Souza Vismara, E.; Barbosa, L.R.; Wagner Júnior, A.; Lozano, E.R.; Potrich, M. Toxicity of Essential Oil of Eugenia uniflora (L.) to Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) and Selectivity to the Parasitoid Cleruchoides noackae (Lin & Hubert) (Hymenoptera: Mymaridae). Crop Prot. 2021, 147, 105693. [Google Scholar] [CrossRef]
- Altoe, M.D.; Lima, J.D.A.; Potrich, M.; Battisti, L.; Lozano, E.R. Insecticidal and Repellent Effects of Essential Oil Eugenia uniflora L. (Myrtaceae) on Sitophilus zeamais Mots. (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2022, 7. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic Mortality between a Neonicotinoid Insecticide and an Ergosterol-Biosynthesis-Inhibiting Fungicide in Three Bee Species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C. Lethal and Sublethal Synergistic Effects of a New Systemic Pesticide, Flupyradifurone (Sivantow), on Honeybees. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190433. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J.F. Agrochemicals Interact Synergistically to Increase Bee Mortality. Nature 2021, 596, 389–392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).