Factors Affecting the Flight Capacity of Two Woodwasp Species, Sirex noctilio F. (Hymenoptera: Siricidae) and Sirex nitobei M. (Hymenoptera: Siricidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Insect Measurement
2.3. Flight Mill Recordings and Bio-Assay Procedures
2.4. Statistical Analyses
3. Results
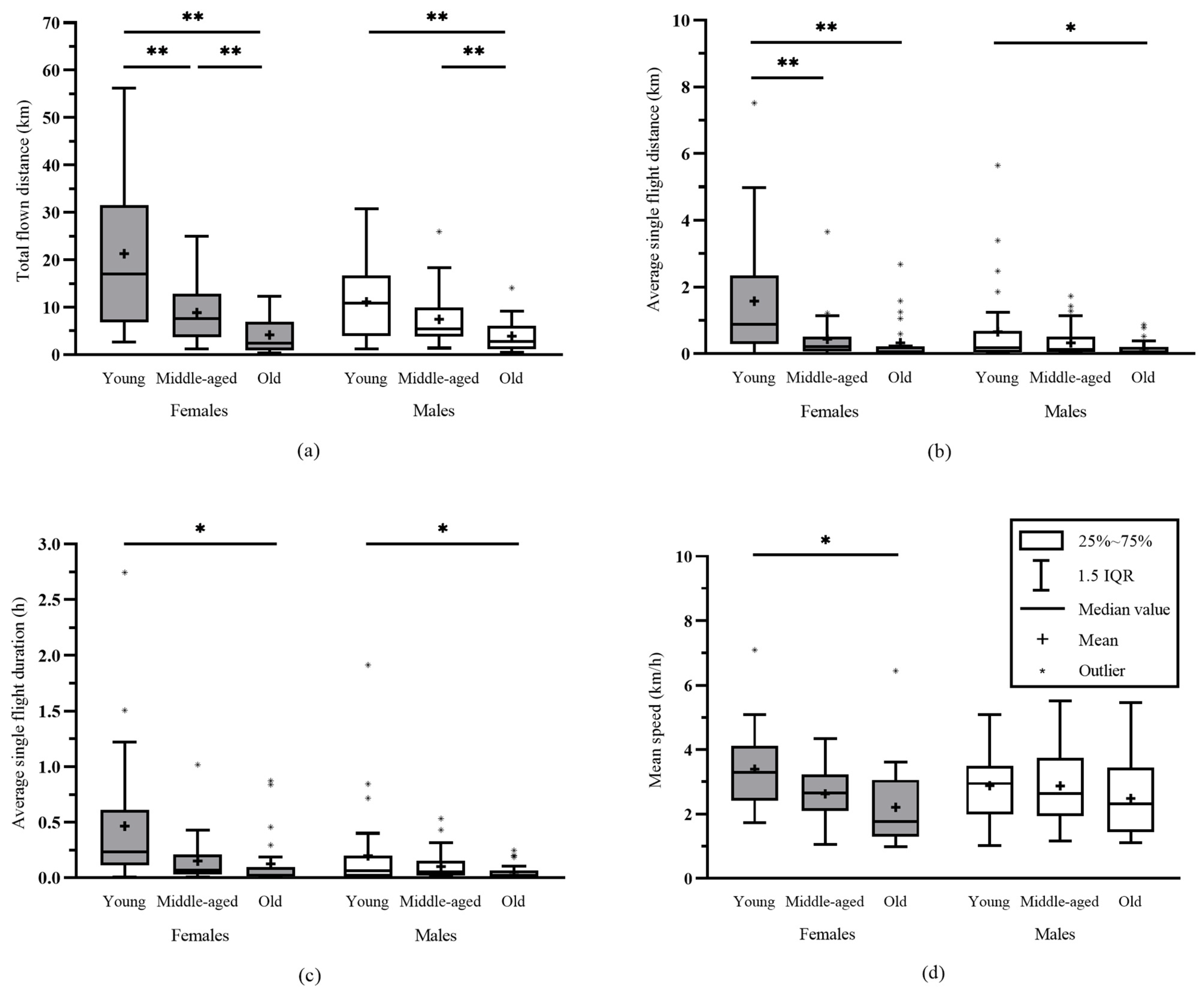
3.1. Effect of the Post-Eclosion-Day Age on Flight Capacity
3.2. Effect of Parasitism Status on Flight
3.3. Effect of Body Mass on Flight
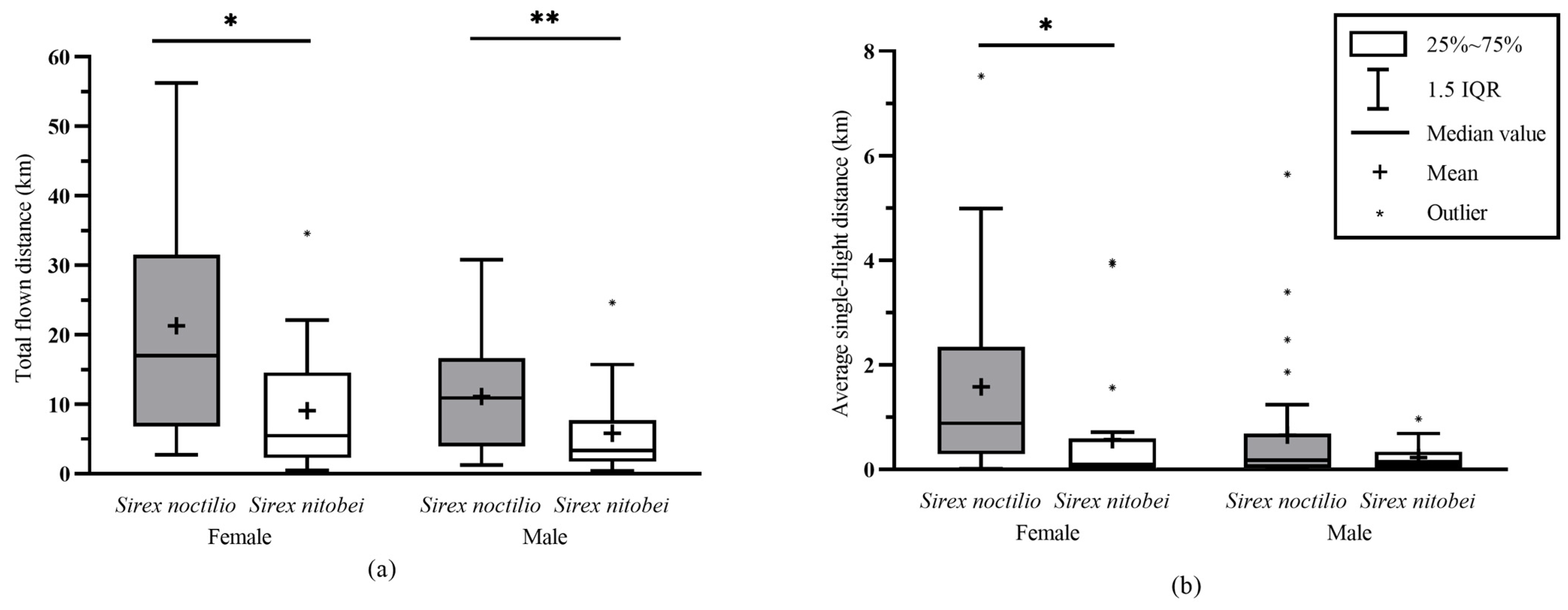
3.4. Comparison of the Flight Capacity of Sirex noctilio and Sirex nitobei
3.5. Main Factors Influencing Flight Capacity
4. Discussion
4.1. Flight Mill Techniques
4.2. PED Age and Flight Capacity
4.3. Body Mass and the Flight Capacity
4.4. Nematode Infestation and the Flight Capacity
4.5. Flight Capacity between Different Sexes
4.6. Flight Capacity between Two Sirex Species
4.7. Factors Affecting the Flight Capacity of Sirex noctilio and Sirex nitobei
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Flight Performance Parameters | Young | Middle-Aged | Old | K–W H Test | ||
|---|---|---|---|---|---|---|
| H | p | |||||
| Sirex noctilio females | Total flown distance (km) | 21.31 ± 3.28a | 8.88 ± 1.22b | 4.18 ± 0.75c | 27.258 | <0.001 ** |
| Average single-flight distance (km) | 1.58 ± 0.36a | 0.44 ± 0.15b | 0.33 ± 0.12bc | 18.186 | <0.001 ** | |
| Average single-flight duration (h) | 0.47 ± 0.12a | 0.15 ± 0.04ab | 0.12 ± 0.05bc | 15.633 | <0.001 ** | |
| Mean speed (km/h) | 3.4 ± 0.24a | 2.63 ± 0.17ab | 2.21 ± 0.24bc | 14.67 | 0.001 ** | |
| Sirex noctilio males | Total flown distance (km) | 11.13 ± 1.37a | 7.44 ± 0.95ab | 3.92 ± 0.6c | 19.776 | <0.001 * |
| Average single-flight distance (km) | 0.66 ± 0.2a | 0.33 ± 0.07ab | 0.15 ± 0.04bc | 7.731 | 0.021 * | |
| Average single-flight duration (h) | 0.2 ± 0.06a | 0.1 ± 0.02ab | 0.05 ± 0.01bc | 7.375 | 0.025 * | |
| Mean speed (km/h) | 2.89 ± 0.17 | 2.88 ± 0.2 | 2.49 ± 0.22 | 3.401 | 0.183 | |
| Sirex nitobei females | Total flown distance (km) | 9.07 ± 1.71 | 9.51 ± 1.13 | 5.96 ± 0.80 | 5.049 | 0.08 |
| Average single-flight distance (km) | 0.57 ± 0.22 | 0.48 ± 0.10 | 0.42 ± 0.42 | 1.822 | 0.402 | |
| Average single-flight duration (h) | 0.16 ± 0.05 | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.186 | 0.394 | |
| Mean speed (km/h) | 2.53 ± 0.22 | 2.59 ± 0.16 | 2.42 ± 0.80 | 3.888 | 0.824 | |
| Sirex nitobei males | Total flown distance (km) | 5.84 ± 0.98 | 6.00 ± 1.07 | 4.23 ± 0.74 | 1.777 | 0.411 |
| Average single-flight distance (km) | 0.23 ± 0.04 | 0.26 ± 0.09 | 0.18 ± 0.04 | 1.840 | 0.399 | |
| Average single-flight duration (h) | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.01 | 1.072 | 0.585 | |
| Mean speed (km/h) | 2.92 ± 0.14 | 2.68 ± 0.21 | 2.68 ± 0.18 | 2.555 | 0.279 | |
| Total Flown Distance (km) | Average Single-Flight Distance (km) | Average Single-Flight Duration (h) | Mean Speed (km/h) | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Female | Male | Female | Male | Female | Male |
| Non-parasitized | 14.50 ± 3.20 | 9.26 ± 1.25 | 0.42 ± 0.1 | 0.39 ± 0.1 | 0.14 ± 0.04 | 0.1 ± 0.02 | 2.82 ± 0.26 | 3 ± 0.18 |
| Parasitized | 9.84 ± 1.38 | 6.41 ± 0.67 | 0.97 ± 0.22 | 0.38 ± 0.11 | 0.31 ± 0.07 | 0.13 ± 0.04 | 2.71 ± 0.16 | 2.6 ± 0.14 |
| U (M–W U test) | 639 | 966.5 | 776.5 | 1135 | 783.5 | 1179.5 | 673 | 924 |
| p (M–W U test) | 0.603 | 0.146 | 0.355 | 0.812 | 0.318 | 0.937 | 0.871 | 0.078 |
| Total Flown Distance (km) | Average Single-Flight Distance (km) | Average Single-Flight Duration (h) | Mean Speed (km/h) | |||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Non-parasitized | 10.64 ± 1.62 | 4.46 ± 0.83 | 0.82 ± 0.25 | 0.16 ± 0.03 | 0.24 ± 0.06 | 0.06 ± 0.01 | 2.64 ± 0.17 | 2.53 ± 0.12 |
| Parasitized | 7.8 ± 1.11 | 5.36 ± 0.68 | 0.38 ± 0.06 | 0.24 ± 0.05 | 0.13 ± 0.02 | 0.07 ± 0.01 | 2.5 ± 0.13 | 2.8 ± 0.14 |
| U (M–W U test) | 520 | 1060 | 560 | 1152.5 | 551.5 | 1190.5 | 625 | 1069 |
| p (M–W U test) | 0.061 | 0.28 | 0.145 | 0.66 | 0.122 | 0.86 | 0.435 | 0.309 |
| Sex | Flight Performance Parameters | Species | M–W U Test | |||
|---|---|---|---|---|---|---|
| Sirex noctilio | Sirex nitobei | U | z | p | ||
| Females | Total flown distance (km) | 21.31 ± 3.28 | 14.13 ± 2.88 | 161 | −2.932 | 0.003 * |
| Average single-flight distance (km) | 1.58 ± 0.36 | 1.30 ± 0.64 | 157 | −3.01 | 0.003 * | |
| Average single-flight duration (h) | 0.47 ± 0.12 | 0.21 ± 0.06 | 171.5 | 2.729 | 0.006 * | |
| Mean speed (km/h) | 3.40 ± 0.24 | 2.94 ± 0.24 | 174 | −2.68 | 0.007 * | |
| Males | Total flown distance (km) | 11.13 ± 1.37 | 5.68 ± 0.97 | 305 | −3.071 | 0.002 * |
| Average single-flight distance (km) | 0.66 ± 0.2 | 0.22 ± 0.04 | 467.5 | −0.988 | 0.323 | |
| Average single-flight duration (h) | 0.2 ± 0.06 | 0.07 ± 0.01 | 459 | −1.097 | 0.273 | |
| Mean speed (km/h) | 2.89 ± 0.17 | 2.91 ± 0.14 | 537 | −0.096 | 0.923 | |
References
- Madden, J.L. Sirex in Australasia. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Springer: Boston, MA, USA, 1988; pp. 407–429. ISBN 978-1-4899-0789-9. [Google Scholar]
- Farji-Brener, A.G.; Corley, J.C. Successful Invasions of Hymenopteran Insects into NW Patagonia. Ecol. Austral 1988, 8, 273–279. [Google Scholar]
- Tribe, G.D. The Woodwasp Sirex Noctilio Fabricius a Pest of Pinus Species, Now Established in South Africa. Afr. Entomol. 1995, 3, 216–217. [Google Scholar]
- Carnegie, A.J.; Matsuki, M.; Haugen, D.A.; Hurley, B.P.; Ahumada, R.; Klasmer, P.; Sun, J.; Iede, E.T. Predicting the Potential Distribution of Sirex Noctilio (Hymenoptera: Siricidae), a Significant Exotic Pest of Pinus Plantations. Ann. For. Sci. 2006, 63, 119–128. [Google Scholar] [CrossRef]
- Ryan, K.; Hurley, B.P. Life History and Biology of Sirex Noctilio. In The Sirex Woodwasp and its Fungal Symbiont: Research and Management of a Worldwide Invasive Pest; Slippers, B., de Groot, P., Wingfield, M.J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 15–30. ISBN 978-94-007-1960-6. [Google Scholar]
- Li, D.P.; Shi, J.; Luo, Y.Q. Mutualism between the Eurasian Woodwasp, Sirex Noctilio (Hymenoptera: Siricidae) and Its Fungal Symbiont Amylostereum Areolatum (Russulales: Amylostereaceae). Acta Entomol. Sin. 2015, 58, 1019–1029. [Google Scholar]
- Masciocchi, M.; Corley, J. Distribution, Dispersal and Spread of the Invasive Social Wasp (Vespula Germanica) in Argentina. Austral. Ecol. 2013, 38, 162–168. [Google Scholar] [CrossRef]
- Bedding, R.A. Manipulating the Entomophagous-Mycetophagous Nematode, Deladenus Siricidicola, for Biological Control of the Wood Wasp, Sirex Noctilio in Australia. USDA For. Serv. Gen. Tech. Rep. WO 1979, 144–147. [Google Scholar]
- Villacide, J.M.; Corley, J.C. Parasitism and Dispersal Potential of Sirex Noctilio: Implications for Biological Control. Agric. For. Entomol. 2008, 10, 341–345. [Google Scholar] [CrossRef]
- Bruzzone, O.A.; Villacide, J.M.; Bernstein, C.; Corley, J.C. Flight Variability in the Woodwasp Sirex Noctilio (Hymenoptera: Siricidae): An Analysis of Flight Data Using Wavelets. J. Exp. Biol. 2009, 212, 731–737. [Google Scholar] [CrossRef]
- Gaudon, J.M.; Haavik, L.J.; MacQuarrie, C.J.K.; Smith, S.M.; Allison, J.D. Influence of Nematode Parasitism, Body Size, Temperature, and Diel Period on the Flight Capacity of Sirex Noctilio F. (Hymenoptera: Siricidae). J. Insect Behav. 2016, 29, 301–314. [Google Scholar] [CrossRef]
- Corley, J.C.; Villacide, J.M.; Bruzzone, O.A. Spatial Dynamics of a Sirex Noctilio Woodwasp Population within a Pine Plantation in Patagonia, Argentina. Entomol. Exp. Appl. 2007, 125, 231–236. [Google Scholar] [CrossRef]
- Haavik, L.J.; Allison, J.D.; MacQuarrie, C.J.K.; Nott, R.W.; Ryan, K.; de Groot, P.; Turgeon, J.J. Nonlethal Effects of Nematode Infection on Sirex Noctilio and Sirex Nigricornis (Hymenoptera: Siricidae). Environ. Entomol. 2016, 45, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, K. Woodwasps and Their Hymenopterous Parasitoids in Japanese Conifers. Kontyu 1978, 46, 498–508. [Google Scholar]
- Takeuchi, K. Insecta Japonica, Hymenoptera: Siricidae; Hokuryukan Publishing Co., Ltd.: Tokyo, Japan, 1962. [Google Scholar]
- Fukuda, H.; Kajimura, H.; Hijii, N. Fecundity of the Woodwasp, Sirex Nitobei Metstumura, in Relation to Its Body Size. J. Jpn. For. Soc. 1993, 75, 405–408. [Google Scholar]
- Zondag, R. A Non-Sterilizing Strain of Deladenus Siricidicola; Forestry Research Institute Report: Rotorua, New Zealand, 1975. [Google Scholar]
- Bedding, R.A. Biology of Deladenus Siricidicola (Neotylenchidae) an Entomophagous-Mycetophagous Nematode Parasitic in Siricid Woodwasps. Nematologica 1972, 18, 482–493. [Google Scholar] [CrossRef]
- Bedding, R.A. Parasitic and Free-Living Cycles in Entomogenous Nematodes of the Genus Deladenus. Nature 1967, 214, 174–175. [Google Scholar] [CrossRef]
- Kroll, S.A.; Hajek, A.E.; Erin Morris, E.; Long, S.J. Parasitism of Sirex Noctilio by Non-Sterilizing Deladenus Siricidicola in Northeastern North America. Biol. Control 2013, 67, 203–211. [Google Scholar] [CrossRef]
- Kanzaki, N.; Tanaka, S.E.; Fitza, K.; Kosaka, H.; Slippers, B.; Kimura, K.; Tsuchiya, S.; Tabata, M. Deladenus Nitobei n. Sp. (Tylenchomorpha: Allantonematidae) Isolated from Sirex Nitobei (Hymenoptera: Siricidae) from Aomori, Japan, a New Member of the Siricidicola Superspecies. Nematology 2016, 18, 1199–1217. [Google Scholar] [CrossRef]
- Hurley, B.P.; Slippers, B.; Wingfield, M.J. A Comparison of Control Results for the Alien Invasive Woodwasp, Sirex Noctilio, in the Southern Hemisphere. Agric. Entomol. 2007, 9, 159–171. [Google Scholar] [CrossRef]
- Kanzaki, N.; Tanaka, S.E.; Ito, M.; Tanaka, K.; Slippers, B.; Tabata, M. Some Additional Bionomic Characters of Deladenus Nitobei. Nematology 2018, 20, 597–599. [Google Scholar] [CrossRef]
- Minter, M.; Pearson, A.; Lim, K.S.; Wilson, K.; Chapman, J.W.; Jones, C.M. The Tethered Flight Technique as a Tool for Studying Life-History Strategies Associated with Migration in Insects. Ecol. Entomol. 2018, 43, 397–411. [Google Scholar] [CrossRef]
- Snelling, E.P.; Seymour, R.S.; Matthews, P.G.D.; White, C.R. Maximum Metabolic Rate, Relative Lift, Wingbeat Frequency and Stroke Amplitude during Tethered Flight in the Adult Locust Locusta Migratoria. J. Exp. Biol. 2012, 215, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S.E. Assessing Insect Flight Behavior in the Laboratory: A Primer on Flight Mill Methodology and What Can Be Learned. Ann. Entomol. Soc. Am. 2019, 112, 182–199. [Google Scholar] [CrossRef]
- Jahant-Miller, C.; Miller, R.; Parry, D. Size-Dependent Flight Capacity and Propensity in a Range-Expanding Invasive Insect. Insect Sci. 2022, 29, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.C.; Villacide, J.M. Population Dynamics of Sirex Noctilio: Influence of Diapause, Spatial Aggregation and Flight Potential on Outbreaks and Spread. In The Sirex Woodwasp and Its Fungal Symbiont: Research and Management of a Worldwide Invasive Pest; Springer: Dordrecht, The Netherlands, 2012; pp. 51–64. ISBN 9789400719606. [Google Scholar]
- Evans, A.M. The Speed of Invasion: Rates of Spread for Thirteen Exotic Forest Insects and Diseases. Forests 2016, 7, 99. [Google Scholar] [CrossRef]
- Iede, E.T.; Penteado, S.R.C.; Filho, W.R. The Woodwasp Sirex Noctilio in Brazil: Monitoring and Control. In The Sirex Woodwasp and its Fungal Symbiont; Springer: Dordrecht, The Netherlands, 2012; pp. 217–228. ISBN 9789400719606. [Google Scholar]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-Range Seasonal Migration in Insects: Mechanisms, Evolutionary Drivers and Ecological Consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef]
- Mollet, P.; Kéry, M.; Gardner, B.; Pasinelli, G.; Royle, J.A. Estimating Population Size for Capercaillie (Tetrao Urogallus L.) with Spatial Capture-Recapture Models Based on Genotypes from One Field Sample. PLoS ONE 2015, 10, e0129020. [Google Scholar] [CrossRef]
- Schtickzelle, N.; Baguette, M.; Boulengé, É. le Modelling Insect Demography from Capture–Recapture Data: Comparison between the Constrained Linear Models and the Jolly–Seber Analytical Method. Can. Entomol. 2003, 135, 313–323. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Naranjo, S.E.; Iii, L.H.W. Tethered and Untethered Flight by Lygus Hesperus and Lygus Lineolaris (Heteroptera: Miridae). Environ. Entomol. 2004, 33, 1389–1400. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Campbell, J.F.; Throne, J.E. Effects of Rearing Density, Age, Sex, and Food Deprivation on Flight Initiation of the Red Flour Beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2011, 104, 443–451. [Google Scholar] [CrossRef]
- Hocking, B. The Intrinsic Range and Speed of Flight of Insects. Ecol. Entomol. 1953, 104, 223–345. [Google Scholar]
- Yang, F.; Luo, Y.; Shi, J. The Influence of Geographic Population, Age, and Mating Status on the Flight Activity of the Asian Gypsy Moth Lymantria Dispar (Lepidoptera: Erebidae) in China. Appl. Entomol. Zool. 2017, 52, 265–270. [Google Scholar] [CrossRef]
- Service, M.W. Mark-Recapture Techniques and Adult Dispersal. In Mosquito Ecology: Field Sampling Methods; Service, M.W., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 652–751. ISBN 978-94-011-1868-2. [Google Scholar]
- Kutsch, W.; Stevenson, P. Time-Correlated Flights of Juvenile and Mature Locusts: A Comparison between Free and Tethered Animals. J. Insect Physiol. 1981, 27, 455–459. [Google Scholar] [CrossRef]
- McAnelly, M.L.; Rankin, M.A. Migration in the Grasshopper Melanoplus Sanguinipes (Fab.). II. Interactions between Flight and Reproduction. Biol. Bull. 1986, 170, 378–392. [Google Scholar] [CrossRef]
- Riley, J.R.; Downham, M.C.A.; Cooter, R.J. Comparison of the Performance of Cicadulina Leafhoppers on Flight Mills with That to Be Expected in Free Flight. Entomol. Exp. Appl. 1997, 83, 317–322. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Bauer, L.S.; Poland, T.M.; Windell, K.N. Flight Performance of Agrilus Planipennis (Coleoptera: Buprestidae) on a Flight Mill and in Free Flight. J. Insect Behav. 2010, 23, 128–148. [Google Scholar] [CrossRef]
- Jones, H.B.C.; Lim, K.S.; Bell, J.R.; Hill, J.K.; Chapman, J.W. Quantifying Interspecific Variation in Dispersal Ability of Noctuid Moths Using an Advanced Tethered Flight Technique. Ecol. Evol. 2016, 6, 181–190. [Google Scholar] [CrossRef]
- Kent, J.W., Jr.; Rankin, M.A. Heritability and Physiological Correlates of Migratory Tendency in the Grasshopper Melanoplus Sanguinipes. Physiol. Entomol. 2001, 26, 371–380. [Google Scholar] [CrossRef]
- Hughes, J.; Dorn, S. Sexual Differences in the Flight Performance of the Oriental Fruit Moth. Entomol. Exp. Appl. 2002, 103, 171–182. [Google Scholar] [CrossRef]
- Boggs, C.L.; Freeman, K.D. Larval Food Limitation in Butterflies: Effects on Adult Resource Allocation and Fitness. Oecologia 2005, 144, 353–361. [Google Scholar] [CrossRef]
- Senger, S.E.; Roitberg, B.D.; Thistlewood, H.M.A. Relative Flight Responses of Rhagoletis Indifferens as Influenced by Crowding, Sex, and Resources. Entomol. Exp. Appl. 2007, 123, 91–100. [Google Scholar] [CrossRef]
- David, G.; Giffard, B.; Piou, D.; Jactel, H. Dispersal Capacity of Monochamus Galloprovincialis, the European Vector of the Pine Wood Nematode, on Flight Mills. J. Appl. Entomol. 2014, 138, 566–576. [Google Scholar] [CrossRef]
- Cui, J.; Li, S.; Zhao, P.; Zou, F. Flight Capacity of Adult Culex Pipiens Pallens (Diptera: Culicidae) in Relation to Gender and Day-Age. J. Med. Entomol. 2013, 50, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.G.; Morey, J.L.; McKimm, R.J. The Sirex Wasp in Victoria; Department of Conservation Forests and Lands: Melbourne, Australia, 1987. [Google Scholar]
- Zondag, R.; Nuttall, M.J. Sirex Noctilio Fabricius (Hymenoptera: Siricidae). In Forest and Timber Insects in New Zealand; New Zealand Forest Service: Rotorua, New Zealand, 1977; Volume 20, pp. 1–7. [Google Scholar]
- Dudley, R.; Srygley, R. Flight Physiology of Neotropical Butterflies: Allometry of Airspeeds during Natural Free Flight. J. Exp. Biol. 1994, 191, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y. Longevity, Flight Ability and Reproductive Performance of the Diamondback Moth, Plutella Xylostella (L.) (Lepidoptera: Yponomeutidae), Related to Adult Body Size. Res. Popul. Ecol. 1995, 37, 269–277. [Google Scholar] [CrossRef]
- Pignata, M.I.B.; Diniz-Filho, J.A.F. Phylogenetic Autocorrelation and Evolutionary Constraints in Worker Body Size of Some Neotropical Stingless Bees (Hymenoptera: Apidae). Heredity 1996, 76, 222–228. [Google Scholar] [CrossRef]
- Jervis, M.; Ferns, P. The Timing of Egg Maturation in Insects: Ovigeny Index and Initial Egg Load as Measures of Fitness and of Resource Allocation. Oikos 2004, 107, 449–461. [Google Scholar] [CrossRef]
- Elliott, C.G.; Evenden, M.L. Factors Influencing Flight Potential of Choristoneura Conflictana. Physiol. Entomol. 2009, 34, 71–78. [Google Scholar] [CrossRef]
- Araújo, E.D.; Costa, M.; Chaud-Netto, J.; Fowler, H.G. Body Size and Flight Distance in Stingless Bees (Hymenoptera: Meliponini): Inference of Flight Range and Possible Ecological Implications. Braz. J. Biol. 2004, 64, 563–568. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Parasites Hinder Monarch Butterfly Flight: Implications for Disease Spread in Migratory Hosts. Ecol. Lett. 2005, 8, 290–300. [Google Scholar] [CrossRef]
- Atkins, M.D. A Study of the Flight of the Douglas-Fir Beetle Dendroctonus Pseudotsugae Hopk. (Coleoptera: Scolytidae): III Flight Capacity. Can. Entomol. 1961, 93, 467–474. [Google Scholar] [CrossRef]
- Akbulut, S.; Linit, M.J. Flight Performance of Monochamus Carolinensis (Coleoptera: Cerambycidae) with Respect to Nematode Phoresis and Beetle Characteristics. Environ. Entomol. 1999, 28, 1014–1020. [Google Scholar] [CrossRef]
- Bedding, R.A.; Akhurst, R.J. Use of the Nematode Deladenus Siricidicola in the Biological Control of Sirex Noctilio in Australia. Aust. J. Entomol. 1974, 13, 129–135. [Google Scholar] [CrossRef]
- Bedding, R.A. Nematode Parasites of Hymenoptera. In Plant and Insect Nematodes; Marcel Dekker: New York, NY, USA, 1984; pp. 755–795. [Google Scholar]
- Sappington, T.W.; Fescemyer, H.W.; Showers, W.B. Lipid and Carbohydrate Utilization during Flight of the Migratory Moth, Agrotis Ipsilon (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 1995, 29, 397–414. [Google Scholar] [CrossRef]
- Coll, M.; Yuval, B. Larval Food Plants Affect Flight and Reproduction in an Oligophagous Insect Herbivore. Environ. Entomol. 2004, 33, 1471–1476. [Google Scholar] [CrossRef]
- Betts, C.R.; Wootton, R.J. Wing Shape and Flight Behaviour in Butterflies (Lepidoptera: Papilionoidea and Hesperioidea): A Preliminary Analysis. J. Exp. Biol. 1988, 138, 271–288. [Google Scholar] [CrossRef]
- Hill, J.K.; Thomas, C.D.; Lewis, O.T. Flight Morphology in Fragmented Populations of a Rare British Butterfly, Hesperia Comma. Biol. Conserv. 1999, 87, 277–283. [Google Scholar] [CrossRef]
- Berwaerts, K.; van Dyck, H.; Aerts, P. Does Flight Morphology Relate to Flight Performance? An Experimental Test with the Butterfly Pararge Aegeria. Funct. Ecol. 2002, 16, 484–491. [Google Scholar] [CrossRef]
- Dudley, R. Biomechanics of Flight in Neotropical Butterflies: Morphometrics and Kinematics. J. Exp. Biol. 1990, 150, 37–53. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Villacide, J.M.; Garnas, J.R.; Croft, P.; Carnegie, A.J.; Liebhold, A.M.; Corley, J.C. Temperature Explains Variable Spread Rates of the Invasive Woodwasp Sirex Noctilio in the Southern Hemisphere. Biol. Invasions 2014, 16, 329–339. [Google Scholar] [CrossRef]
| Dependent Variable | Model | Coefficients | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | F | p | Independent Variables | Standardized β | t | p | ||
| Sirex noctilio females | Total flown distance | 0.683 | 80.668 | <0.001 | Age cohorts | −0.675 | −9.392 | <0.001 |
| Body mass | 0.269 | 3.748 | <0.001 | |||||
| Average single-flight distance | 0.556 | 95.356 | <0.001 | Age cohorts | −0.746 | −9.765 | <0.001 | |
| Average single-flight duration | 0.594 | 111.39 | <0.001 | Age cohorts | −0.771 | −10.554 | <0.001 | |
| Mean speed | 0.49 | 73.01 | <0.001 | Age cohorts | −0.7 | −8.545 | <0.001 | |
| Sirex noctilio males | Total flown distance | 0.737 | 132.829 | <0.001 | Body mass | 0.549 | 9.878 | <0.001 |
| Age cohorts | −0.507 | −9.122 | <0.001 | |||||
| Average single-flight distance | 0.715 | 119.382 | <0.001 | Body mass | 0.552 | 7.601 | <0.001 | |
| Age cohorts | −0.374 | −5.143 | <0.001 | |||||
| Average single-flight duration | 0.457 | 40.017 | <0.001 | Body mass | 0.474 | 5.799 | <0.001 | |
| Age cohorts | −0.333 | −4.072 | <0.001 | |||||
| Mean speed | 0.294 | 39.94 | <0.001 | Body mass | 0.542 | 6.32 | <0.001 | |
| Sirex nitobei females | Total flown distance | 0.417 | 52.202 | <0.001 | Body mass | 0.646 | 7.225 | <0.001 |
| Average single-flight distance | 0.4 | 48.677 | <0.001 | Body mass | 0.632 | 6.977 | <0.001 | |
| Average single-flight duration | 0.358 | 40.65 | <0.001 | Body mass | 0.598 | 6.376 | <0.001 | |
| Mean speed | 0.528 | 81.594 | <0.001 | Body mass | 0.726 | 9.033 | <0.001 | |
| Sirex nitobei males | Total flown distance | 0.309 | 41.549 | <0.001 | Body mass | 0.556 | 6.446 | <0.001 |
| Average single-flight distance | 0.378 | 56.565 | <0.001 | Body mass | 0.615 | 7.521 | <0.001 | |
| Average single-flight duration | 0.124 | 13.217 | <0.001 | Body mass | 0.353 | 3.636 | <0.001 | |
| Mean speed | 0.552 | 114.488 | <0.001 | Body mass | 0.743 | 10.7 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shi, J.; Ren, L.; Luo, Y. Factors Affecting the Flight Capacity of Two Woodwasp Species, Sirex noctilio F. (Hymenoptera: Siricidae) and Sirex nitobei M. (Hymenoptera: Siricidae). Insects 2023, 14, 236. https://doi.org/10.3390/insects14030236
Liu X, Shi J, Ren L, Luo Y. Factors Affecting the Flight Capacity of Two Woodwasp Species, Sirex noctilio F. (Hymenoptera: Siricidae) and Sirex nitobei M. (Hymenoptera: Siricidae). Insects. 2023; 14(3):236. https://doi.org/10.3390/insects14030236
Chicago/Turabian StyleLiu, Xiaobo, Juan Shi, Lili Ren, and Youqing Luo. 2023. "Factors Affecting the Flight Capacity of Two Woodwasp Species, Sirex noctilio F. (Hymenoptera: Siricidae) and Sirex nitobei M. (Hymenoptera: Siricidae)" Insects 14, no. 3: 236. https://doi.org/10.3390/insects14030236
APA StyleLiu, X., Shi, J., Ren, L., & Luo, Y. (2023). Factors Affecting the Flight Capacity of Two Woodwasp Species, Sirex noctilio F. (Hymenoptera: Siricidae) and Sirex nitobei M. (Hymenoptera: Siricidae). Insects, 14(3), 236. https://doi.org/10.3390/insects14030236







