Nationwide Screening for Bee Viruses in Apis mellifera Colonies in Egypt
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Samples
2.2. Varroa Mite Infestation Rate
2.3. RNA Extraction and Detection of Virus
2.4. Statistical Analysis
3. Results
3.1. Varroa Mite Presence per Sample and Infestation Rate
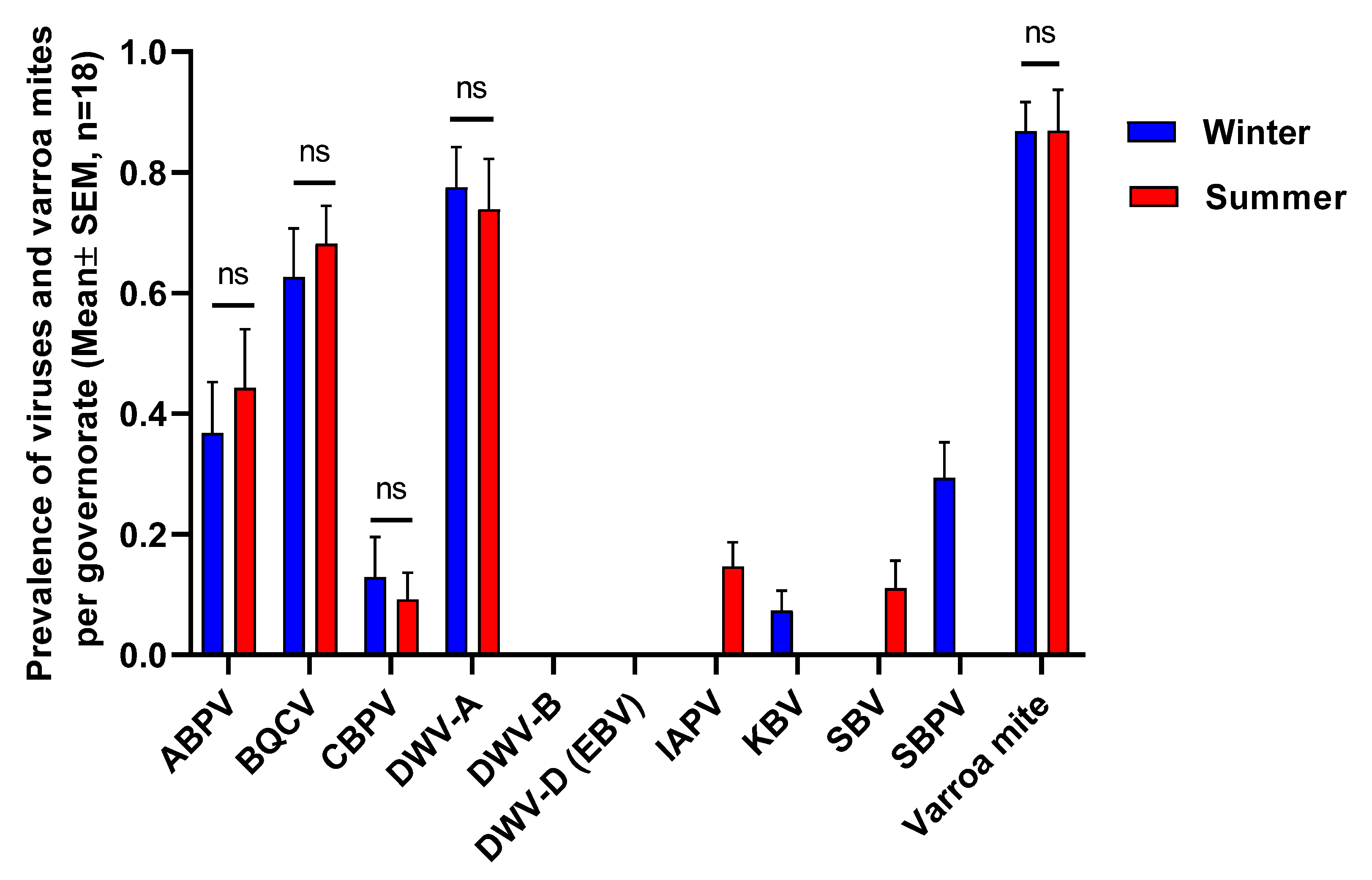
3.2. Prevalence of Honey Bee Viruses
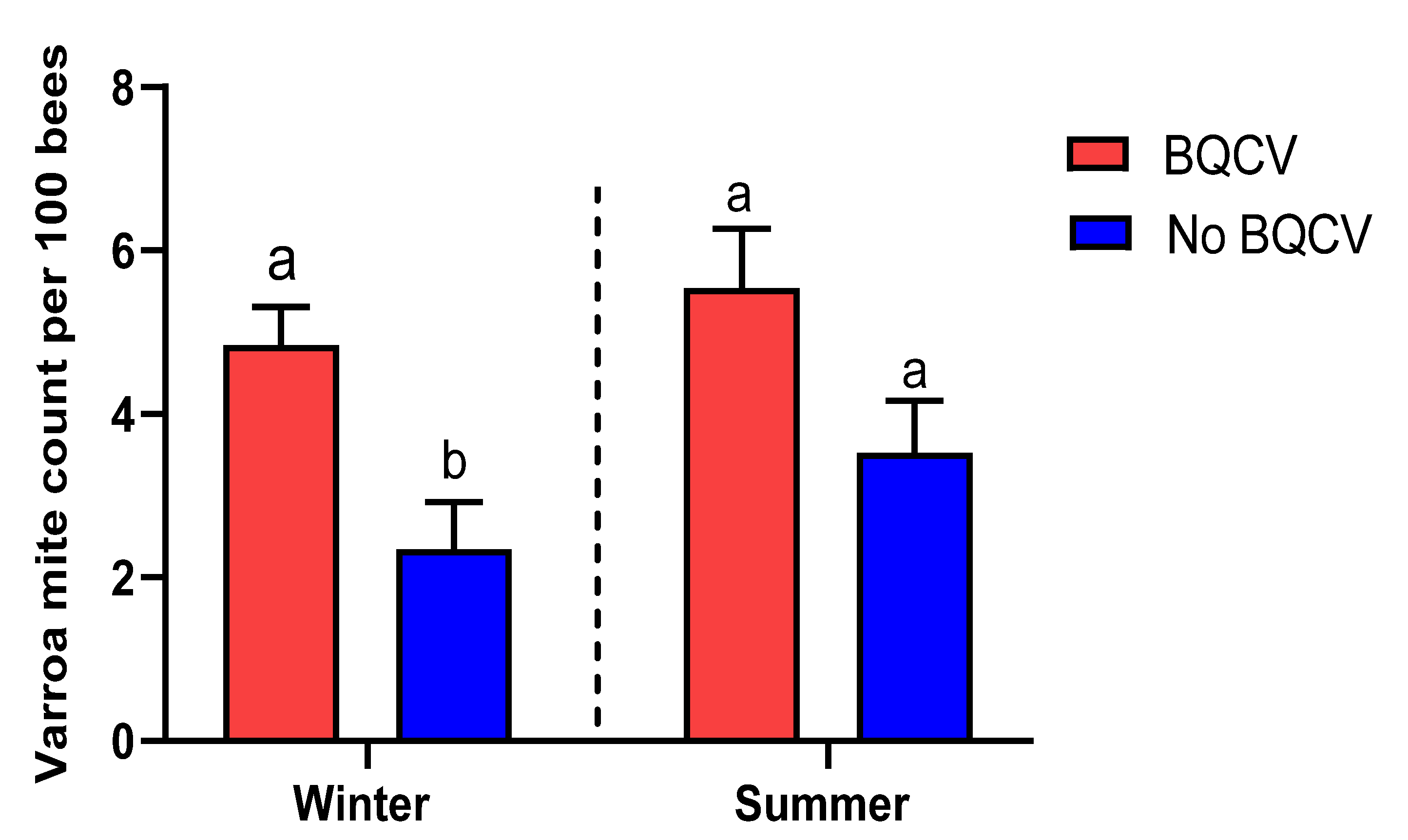
3.3. Association between Viral Prevalence and Varroa Infestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.V.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Tarpy, D.R.; Caron, D.M.; Rose, R.; Delaplane, K.S.; Baylis, K.; Lengerich, E.J.; et al. A National Survey of Managed Honey Bee 2013–2014 Annual Colony Losses in the USA. Apidologie 2015, 46, 292–305. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J.S. A Survey of Honey Bee Colony Losses in the United States, Fall 2008 to Spring 2009. J. Apic. Res. 2010, 49, 7–14. [Google Scholar] [CrossRef]
- van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed Honey Bee Colony Losses in Canada, China, Europe, Israel and Turkey, for the Winters of 2008–9 and 2009–10. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Singavarapu, B.; Paxton, R.J.; Wubet, T. Bees under Interactive Stressors: The Novel Insecticides Flupyradifurone and Sulfoxaflor along with the Fungicide Azoxystrobin Disrupt the Gut Microbiota of Honey Bees and Increase Opportunistic Bacterial Pathogens. Sci. Total Environ. 2022, 849, 157941. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Ahmed, H.R.; El-Wahed, A.A.A.; Saeed, A.; Algethami, A.F.; Attia, N.F.; Guo, Z.; Musharraf, S.G.; Khatib, A.; Alsharif, S.M.; et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Vet. Sci. 2022, 9, 199. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Doublet, V.; de Miranda, J.R.; Piot, N.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- Dalmon, A.; Diévart, V.; Thomasson, M.; Fouque, R.; Vaissière, B.E.; Guilbaud, L.; Conte, Y.L.; Henry, M. Possible Spillover of Pathogens between Bee Communities Foraging on the Same Floral Resource. Insects 2021, 12, 122. [Google Scholar] [CrossRef]
- Ding, G.; Fondevila, N.; Palacio, M.A.; Merke, J.; Martinez, A.; Camacho, B.; Aignasse, A.; Figini, E.; Rodriguez, G.; Lv, L.P.; et al. Prevalence of Honeybee Viruses in Different Regions of China and Argentina of the Scientific and Technical Review. Rev. Sci. Tech. Off. Int. Epiz 2016, 35, 35. [Google Scholar]
- Ball, B.V.; Bailey, L. Honey Bee Viruses. Encycl. Virol. 1999, 70, 743–749. [Google Scholar] [CrossRef]
- Brutscher, L.M.; McMenamin, A.J.; Flenniken, M.L. The Buzz about Honey Bee Viruses. PLoS Pathog. 2016, 12, e1005757. [Google Scholar] [CrossRef]
- Yañez, O.; Piot, N.; Dalmon, A.; de Miranda, J.R.; Chantawannakul, P.; Panziera, D.; Amiri, E.; Smagghe, G.; Schroeder, D.; Chejanovsky, N. Bee Viruses: Routes of Infection in Hymenoptera. Front. Microbiol. 2020, 11, 943. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef]
- Mráz, P.; Hýbl, M.; Kopecký, M.; Bohatá, A.; Hoštičková, I.; Šipoš, J.; Vočadlová, K.; Čurn, V. Screening of Honey Bee Pathogens in the Czech Republic and Their Prevalence in Various Habitats. Insects 2021, 12, 1051. [Google Scholar] [CrossRef]
- Punko, R.N.; Currie, R.W.; Nasr, M.E.; Hoover, S.E. Epidemiology of Nosema spp. and the Effect of Indoor and Outdoor Wintering on Honey Bee Colony Population and Survival in the Canadian Prairies. PLoS ONE 2021, 16, e0258801. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Codling, G.; Giesy, J.P.; Safer, A. Beekeeping and the Need for Pollination from an Agricultural Perspective in Egypt. Bee World 2018, 95, 107–112. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey Bee Colony Losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed Wing Virus Is a Recent Global Epidemic in Honeybees Driven by Varroa Mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef]
- Abd-El-Samie, E.M.; Basuny, N.K.; Seyam, H. Molecular Characterization of Viruses Found in Honeybee (Apis mellifera) Colonies Infested with Varroa Destructor and Nosema Cerana in Egypt. Mol. Cell. Probes 2021, 57, 101731. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.-P.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard Methods for Virus Research in Apis mellifera. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- Fries, I.; Aarhus, A.; Hansen, H.; Korpela, S. Comparison of Diagnostic Methods for Detection of Low Infestation Levels of Varroa Jacobsoni in Honey-Bee (Apis mellifera) Colonies. Exp. Appl. Acarol. 1991, 10, 279–287. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard Methods for Varroa Research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Tehel, A.; Vu, Q.; Bigot, D.; Gogol-Döring, A.; Koch, P.; Jenkins, C.; Doublet, V.; Theodorou, P.; Paxton, R. The Two Prevalent Genotypes of an Emerging Infectious Disease, Deformed Wing Virus, Cause Equally Low Pupal Mortality and Equally High Wing Deformities in Host Honey Bees. Viruses 2019, 11, 114. [Google Scholar] [CrossRef]
- Tehel, A.; Streicher, T.; Tragust, S.; Paxton, R.J. Experimental Cross Species Transmission of a Major Viral Pathogen in Bees Is Predominantly from Honeybees to Bumblebees. Proc. R. Soc. B Biol. Sci. 2022, 289, 20212255. [Google Scholar] [CrossRef]
- Forsgren, E.; de Miranda, J.R.; Isaksson, M.; Wei, S.; Fries, I. Deformed Wing Virus Associated with Tropilaelaps mercedesae Infesting European Honey Bees (Apis mellifera). Exp. Appl. Acarol. 2009, 47, 87–97. [Google Scholar] [CrossRef]
- Paxton, R.J.; Schäfer, M.O.; Nazzi, F.; Zanni, V.; Annoscia, D.; Marroni, F.; Bigot, D.; Laws-Quinn, E.R.; Panziera, D.; Jenkins, C.; et al. Epidemiology of a Major Honey Bee Pathogen, Deformed Wing Virus: Potential Worldwide Replacement of Genotype A by Genotype B. Int. J. Parasitol. Parasites Wildl. 2022, 18, 157–171. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Brettell, L.E.; Chejanovsky, N.; Childers, A.K.; Dalmon, A.; Deboutte, W.; de Graaf, D.C.; Doublet, V.; Gebremedhn, H.; Genersch, E.; et al. Cold Case: The Disappearance of Egypt Bee Virus, a Fourth Distinct Master Strain of Deformed Wing Virus Linked to Honeybee Mortality in 1970’s Egypt. Virol. J. 2022, 19, 12. [Google Scholar] [CrossRef]
- Lanzi, G.; De Miranda, J.R.; Boniotti, M.B.; Cameron, C.E.; Lavazza, A.; Capucci, L.; Camazine, S.M.; Rossi, C. Molecular and Biological Characterization of Deformed Wing Virus of Honeybees (Apis mellifera L.). J. Virol. 2006, 80, 4998–5009. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global Honey Bee Viral Landscape Altered by a Parasitic Mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef]
- Ongus, J.R.; Peters, D.; Bonmatin, J.M.; Bengsch, E.; Vlak, J.M.; van Oers, M.M. Complete Sequence of a Picorna-like Virus of the Genus Iflavirus Replicating in the Mite Varroa destructor. J. Gen. Virol. 2004, 85, 3747–3755. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a Honey Bee Pathogen: First Report of a Third Master Variant of the Deformed Wing Virus Quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef]
- Allen, M.; Ball, B. The Incidence and World Distribution of Honey Bee Viruses. Bee World 1996, 77, 141–162. [Google Scholar] [CrossRef]
- Manley, R.; Temperton, B.; Doyle, T.; Gates, D.; Hedges, S.; Boots, M.; Wilfert, L. Knock-on Community Impacts of a Novel Vector: Spillover of Emerging DWV-B from Varroa-infested Honeybees to Wild Bumblebees. Ecol. Lett. 2019, 22, 1306–1315. [Google Scholar] [CrossRef]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed Wing Virus: Replication and Viral Load in Mites (Varroa destructor). J. Gen. Virol. 2009, 90, 463–467. [Google Scholar] [CrossRef]
- Möckel, N.; Gisder, S.; Genersch, E. Horizontal Transmission of Deformed Wing Virus: Pathological Consequences in Adult Bees (Apis mellifera) Depend on the Transmission Route. J. Gen. Virol. 2011, 92, 370–377. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Direct Evidence for Infection of Varroa Destructor Mites with the Bee-Pathogenic Deformed Wing Virus Variant B, but Not Variant A, via Fluorescence In Situ Hybridization Analysis. J. Virol. 2021, 95, e01786-20. [Google Scholar] [CrossRef]
- Kevill, J.L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A Lethal to Honey Bees (Apis mellifera): A Colony Level Survey of DWV Variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 2019, 11, 426. [Google Scholar] [CrossRef]
- Zioni, N.; Soroker, V.; Chejanovsky, N. Replication of Varroa Destructor Virus 1 (VDV-1) and a Varroa Destructor Virus 1–Deformed Wing Virus Recombinant (VDV-1–DWV) in the Head of the Honey Bee. Virology 2011, 417, 106–112. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Chen, Y.; Madella, S.; Nessa, A.; VanEngelsdorp, D.; Evans, J.D. Recent Spread of Varroa Destructor Virus-1, a Honey Bee Pathogen, in the United States. Sci. Rep. 2017, 7, 17447. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.E.; Nguyen, L.T.K.; Noh, J.H.; Koh, H.B.; Jean, Y.H.; Kweon, C.H.; Kang, S.W. Prevalence and Distribution of Six Bee Viruses in Korean Apis Cerana Populations. J. Invertebr. Pathol. 2012, 109, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.T.; Thi Kim Lien, N.; Thuy Linh, M.; Le, T.H.; Hoa, N.T.; Hong Thai, P.; Reddy, K.E.; Yoo, M.S.; Kim, Y.H.; Cho, Y.S.; et al. Prevalencia de Virus de Abeja En Poblaciones de Apis Cerana En Vietnam. J. Apic. Res. 2016, 55, 379–385. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and Seasonal Variations of Six Bee Viruses in Apis mellifera L. and Varroa Destructor Mite Populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef]
- Kojima, Y.; Toki, T.; Morimoto, T.; Yoshiyama, M.; Kimura, K.; Kadowaki, T. Infestation of Japanese Native Honey Bees by Tracheal Mite and Virus from Non-Native European Honey Bees in Japan. Microb. Ecol. 2011, 62, 895–906. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Deng, J.; Wen, Z.; Wang, S.; Chen, Y.; Hu, F.; Zheng, H. Seasonal Variation of Viral Infections between the Eastern Honey Bee (Apis Cerana) and the Western Honey Bee (Apis mellifera). Microbiologyopen 2021, 10, e1162. [Google Scholar] [CrossRef]
- D’Alvise, P.; Seeburger, V.; Gihring, K.; Kieboom, M.; Hasselmann, M. Seasonal Dynamics and Co-occurrence Patterns of Honey Bee Pathogens Revealed by High-throughput RT-qPCR Analysis. Ecol. Evol. 2019, 9, 10241–10252. [Google Scholar] [CrossRef]
- Welch, A.; Drummond, F.; Tewari, S.; Averill, A.; Burand, J.P. Presence and Prevalence of Viruses in Local and Migratory Honeybees (Apis mellifera) in Massachusetts. Appl. Environ. Microbiol. 2009, 75, 7862–7865. [Google Scholar] [CrossRef]
- Berényi, O.; Bakonyi, T.; Derakhshifar, I.; Köglberger, H.; Nowotny, N. Occurrence of Six Honeybee Viruses in Diseased Austrian Apiaries. Appl. Environ. Microbiol. 2006, 72, 2414–2420. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Nicolaisen, M.; Kryger, P. Incidence of Acute Bee Paralysis Virus, Black Queen Cell Virus, Chronic Bee Paralysis Virus, Deformed Wing Virus, Kashmir Bee Virus and Sacbrood Virus in Honey Bees (Apis mellifera) in Denmark. Apidologie 2008, 39, 310–314. [Google Scholar] [CrossRef]
- Haddad, N.; Brake, M.; Migdadi, H.; De Miranda, J.R. First Detection of Honey Bee Viruses in Jordan by RT-PCR. Jordan J. Agric. Sci. 2008, 4, 242–247. [Google Scholar]
- Teixeira, E.W.; Chen, Y.; Message, D.; Pettis, J.; Evans, J.D. Virus Infections in Brazilian Honey Bees. J. Invertebr. Pathol. 2008, 99, 117–119. [Google Scholar] [CrossRef]
- Bakonyi, T.; Grabensteiner, E.; Kolodziejek, J.; Rusvai, M.; Topolska, G.; Ritter, W.; Nowotny, N. Phylogenetic Analysis of Acute Bee Paralysis Virus Strains. Appl. Environ. Microbiol. 2002, 68, 6446–6450. [Google Scholar] [CrossRef]
- BALL, B.V.; ALLEN, M.F. The Prevalence of Pathogens in Honey Bee (Apis mellifera) Colonies Infested with the Parasitic Mite Varroa Jacobsoni. Ann. Appl. Biol. 1988, 113, 237–244. [Google Scholar] [CrossRef]
- Kulincevic, J.; Ball, B.V.; Mladjan, V. Viruses in Honey Bee Colonies Infested with Varroa Jacobsoni: First Findings in Yugoslavia. Acta Vet. 1990, 40, 37–42. [Google Scholar]
- Faucon, J.P.; Vitu, C.; Russo, P.; Vignoni, M. Diagnostic de La Paralysie Aiguë: Application à l’épidémiologie Des Maladies Virales de l’abeille En France En 1990. Apidologie 1992, 23, 139–146. [Google Scholar] [CrossRef]
- Hung, A.C.F.; Ball, B.V.; Adams, J.R.; Shimanuki, H.; Knox, D.A. A Scientific Note on the Detection of American Strains of Acute Paralysis Virus and Kashmir Bee Virus in Dead Bees in One US Honey Bee (Apis mellifera L.) Colony. Apidologie 1996, 27, 55–56. [Google Scholar] [CrossRef]
- O’Shea-Wheller, T.A.; Rinkevich, F.D.; Danka, R.G.; Simone-Finstrom, M.; Tokarz, P.G.; Healy, K.B. A Derived Honey Bee Stock Confers Resistance to Varroa Destructor and Associated Viral Transmission. Sci. Rep. 2022, 12, 4852. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Paxton, R.J. Mode of Transmission Determines the Virulence of Black Queen Cell Virus in Adult Honey Bees, Posing a Future Threat to Bees and Apiculture. Viruses 2020, 12, 535. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Chen, Y.; Pettis, J.S.; Feldlaufer, M.F. Detection of Multiple Viruses in Queens of the Honey Bee Apis mellifera L. J. Invertebr. Pathol. 2005, 90, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Hammond, J.; Hsu, H.; Evans, J.; Feldlaufer, M. Multiple Virus Infections in the Honey Bee and Genome Divergence of Honey Bee Viruses. J. Invertebr. Pathol. 2004, 87, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Frey, E.; Rosenkranz, P.; Paxton, R.J. The Virulent, Emerging Genotype B of Deformed Wing Virus Is Closely Linked to Overwinter Honeybee Worker Loss. Sci. Rep. 2017, 7, 5242. [Google Scholar] [CrossRef] [PubMed]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal Analysis of the Honey Bee Microbiome Reveals Four Novel Viruses and Seasonal Prevalence of Known Viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef]
- Piot, N.; Schweiger, O.; Meeus, I.; Yañez, O.; Straub, L.; Villamar-Bouza, L.; De la Rúa, P.; Jara, L.; Ruiz, C.; Malmstrøm, M.; et al. Honey Bees and Climate Explain Viral Prevalence in Wild Bee Communities on a Continental Scale. Sci. Rep. 2022, 12, 1904. [Google Scholar] [CrossRef]
- Döke, M.A.; Frazier, M.; Grozinger, C.M. Overwintering Honey Bees: Biology and Management. Curr. Opin. Insect Sci. 2015, 10, 185–193. [Google Scholar] [CrossRef]
- Locke, B.; Conte, Y.L.; Crauser, D.; Fries, I. Host Adaptations Reduce the Reproductive Success of Varroa Destructor in Two Distinct European Honey Bee Populations. Ecol. Evol. 2012, 2, 1144–1150. [Google Scholar] [CrossRef]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa Destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef]
- McMahon, D.P.; Fürst, M.A.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R.J. A Sting in the Spit: Widespread Cross-Infection of Multiple RNA Viruses across Wild and Managed Bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandel, M.; Paxton, R.J.; Al Naggar, Y. Nationwide Screening for Bee Viruses in Apis mellifera Colonies in Egypt. Insects 2023, 14, 172. https://doi.org/10.3390/insects14020172
Kandel M, Paxton RJ, Al Naggar Y. Nationwide Screening for Bee Viruses in Apis mellifera Colonies in Egypt. Insects. 2023; 14(2):172. https://doi.org/10.3390/insects14020172
Chicago/Turabian StyleKandel, Mohamed, Robert J. Paxton, and Yahya Al Naggar. 2023. "Nationwide Screening for Bee Viruses in Apis mellifera Colonies in Egypt" Insects 14, no. 2: 172. https://doi.org/10.3390/insects14020172
APA StyleKandel, M., Paxton, R. J., & Al Naggar, Y. (2023). Nationwide Screening for Bee Viruses in Apis mellifera Colonies in Egypt. Insects, 14(2), 172. https://doi.org/10.3390/insects14020172







