Exploration of Candidate Genes Involved in the Biosynthesis, Regulation and Recognition of the Male-Produced Aggregation Pheromone of Halyomorpha halys
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Farnesyl Diphosphate (FDP) and Terpene Synthase (TPS) Genes in H. halys
2.2. Weighted Gene Co-Expression Network Analysis
2.3. Molecular Docking of HhTPS1 and HhCSP 5 in H. halys
3. Results
3.1. Identification of Key FDP and TPS Genes in the Aggregation Pheromone Biosynthesis Pathway
3.2. Co-Expression Network Analysis to Identify the HhTPS1 Co-Expressed Genes
3.3. Molecular Docking of HhTPS1 and HhCSP5
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D.H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Gariepy, T.; Mason, P.; Gillespie, D.; Talamas, E.; Haye, T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.E.; Bansal, R.; Benoit, J.B.; Blackburn, M.B.; Chao, H.; Chen, M.; Cheng, S.; Childers, C.; Dinh, H.; Doddapaneni, H.V.; et al. Brown marmorated stink bug, Halyomorpha halys (Stål), genome: Putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest. BMC Genom. 2020, 21, 227. [Google Scholar] [CrossRef]
- Weber, D.C.; Morrison, W.R.; Khrimian, A.; Rice, K.B.; Leskey, T.C.; Rodriguez-Saona, C.; Nielsen, A.L.; Blaauw, B.R. Chemical ecology of Halyomorpha halys: Discoveries and applications. J. Pest Sci. 2017, 90, 989–1008. [Google Scholar] [CrossRef]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Agnello, A.M.; Alston, D.G.; Andrews, H.; Beers, E.H.; Bergh, J.C.; Bessin, R.; Blaauw, B.R.; Buntin, G.D.; Burkness, E.C.; et al. Season-Long Monitoring of the Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Throughout the United States Using Commercially Available Traps and Lures. J. Econ. Entomol. 2019, 113, 159–171. [Google Scholar] [CrossRef]
- Short, B.D.; Khrimian, A.; Leskey, T.C. Pheromone-based decision support tools for management of Halyomorpha halys in apple orchards: Development of a trap-based treatment threshold. J. Pest Sci. 2017, 90, 1191–1204. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Foti, M.C.; Peri, E.; Maistrello, L.; Haye, T.; Conti, E. Native egg parasitoids recorded from the invasive Halyomorpha halys successfully exploit volatiles emitted by the plant–herbivore complex. J. Pest Sci. 2017, 90, 1087–1095. [Google Scholar] [CrossRef]
- Harris, C.; Abubeker, S.; Yu, M.; Leskey, T.; Zhang, A. Semiochemical Production and Laboratory Behavior Response of the Brown Marmorated Stink Bug, Halyomorpha Halys. PloS ONE 2015, 10, e0140876. [Google Scholar] [CrossRef]
- Helfrich, E.J.N.; Lin, G.M.; Voigt, C.A.; Clardy, J. Bacterial terpene biosynthesis: Challenges and opportunities for pathway engineering. Beilstein J. Org. Chem. 2019, 15, 2889–2906. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis of terpene pheromones in hemiptera/stink bugs. In Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 2021; pp. 269–284. [Google Scholar]
- Schmidt-Dannert, C. Biosynthesis of terpenoid natural products in fungi. Adv. Biochem. Eng./Biotechnol. 2015, 148, 19–61. [Google Scholar] [CrossRef]
- Keeling, C.I.; Weisshaar, S.; Lin, R.P.; Bohlmann, J. Functional plasticity of paralogous diterpene synthases involved in conifer defense. Proc. Natl. Acad. Sci. USA 2008, 105, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Lin, Y.J.; Pickett, J.A.; Birkett, M.A.; Wu, K.; Wang, G.; Zhou, J.J. Expression of lima bean terpene synthases in rice enhances recruitment of a beneficial enemy of a major rice pest. Plant Cell Environ. 2018, 41, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fu, N.; Zhou, J.; Wang, G. Functional characterization of (E)-β-caryophyllene synthase from lima bean and its up-regulation by spider mites and alamethicin. J. Integr. Agric. 2017, 16, 2231–2238. [Google Scholar] [CrossRef]
- Gilg, A.B.; Bearfield, J.C.; Tittiger, C.; Welch, W.H.; Blomquist, G.J. Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9760–9765. [Google Scholar] [CrossRef]
- Lancaster, J.; Lehner, B.; Khrimian, A.; Muchlinski, A.; Luck, K.; Köllner, T.G.; Weber, D.C.; Gundersen-Rindal, D.E.; Tholl, D. An IDS-Type Sesquiterpene Synthase Produces the Pheromone Precursor (Z)-α-Bisabolene in Nezara viridula. J. Chem. Ecol. 2019, 45, 187–197. [Google Scholar] [CrossRef]
- Leskey, T.C.; Khrimian, A.; Weber, D.C.; Aldrich, J.C.; Short, B.D.; Lee, D.H.; Morrison, W.R., III. Behavioral responses of the invasive Halyomorpha halys (Stål) to traps baited with stereoisomeric mixtures of 10,11-epoxy-1-bisabolen-3-OL. J. Chem. Ecol. 2015, 41, 418–429. [Google Scholar] [CrossRef]
- Lancaster, J.; Khrimian, A.; Young, S.; Lehner, B.; Luck, K.; Wallingford, A.; Ghosh, S.K.B.; Zerbe, P.; Muchlinski, A.; Marek, P.E.; et al. De novo formation of an aggregation pheromone precursor by an isoprenyl diphosphate synthase-related terpene synthase in the harlequin bug. Proc. Natl. Acad. Sci. USA 2018, 115, E8634–E8641. [Google Scholar] [CrossRef]
- Sparks, M.E.; Rhoades, J.H.; Nelson, D.R.; Kuhar, D.; Lancaster, J.; Lehner, B.; Tholl, D.; Weber, D.C.; Gundersen-Rindal, D.E. A Transcriptome Survey Spanning Life Stages and Sexes of the Harlequin Bug, Murgantia histrionica. Insects 2017, 8, 55. [Google Scholar] [CrossRef]
- Ioannidis, P.; Lu, Y.; Kumar, N.; Creasy, T.; Daugherty, S.; Chibucos, M.C.; Orvis, J.; Shetty, A.; Ott, S.; Flowers, M.; et al. Rapid transcriptome sequencing of an invasive pest, the brown marmorated stink bug Halyomorpha halys. BMC Genom. 2014, 15, 738. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.E.; Shelby, K.S.; Kuhar, D.; Gundersen-Rindal, D.E. Transcriptome of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). PloS ONE 2014, 9, e111646. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, M.; Ban, L.; Song, L.M.; Liu, Y.; Pelosi, P.; Wang, G. Niemann-Pick C2 Proteins: A New Function for an Old Family. Front. Physiol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Paula, D.P.; Togawa, R.C.; Costa, M.M.; Grynberg, P.; Martins, N.F.; Andow, D.A. Identification and expression profile of odorant-binding proteins in Halyomorpha halys (Hemiptera: Pentatomidae). Insect Mol. Biol. 2016, 25, 580–594. [Google Scholar] [CrossRef]
- Sun, D.; Huang, Y.; Qin, Z.; Zhan, H.; Zhang, J.; Liu, Y.; Yang, S. Identification of Candidate Olfactory Genes in the Antennal Transcriptome of the Stink Bug Halyomorpha halys. Front. Physiol. 2020, 11, 876. [Google Scholar] [CrossRef]
- Rebholz, Z.; Lancaster, J.; Larose, H.; Khrimian, A.; Luck, K.; Sparks, M.E.; Gendreau, K.L.; Shewade, L.; Köllner, T.G.; Weber, D.C.; et al. Ancient origin and conserved gene function in terpene pheromone and defense evolution of stink bugs and hemipteran insects. Insect Biochem. Mol. Biol. 2023, 152, 103879. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lindert, S.; Zhu, W.; Wang, K.; McCammon, J.A.; Oldfield, E. Taxodione and arenarone inhibit farnesyl diphosphate synthase by binding to the isopentenyl diphosphate site. Proc. Natl. Acad. Sci. USA 2014, 111, E2530–E2539. [Google Scholar] [CrossRef]
- Tomaselli, S.; Crescenzi, O.; Sanfelice, D.; Ab, E.; Wechselberger, R.; Angeli, S.; Scaloni, A.; Boelens, R.; Tancredi, T.; Pelosi, P.; et al. Solution structure of a chemosensory protein from the desert locust Schistocerca gregaria. Biochemistry 2006, 45, 10606–10613. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. Publ. Protein Soc. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [PubMed]
- Gagnon, J.K.; Law, S.M.; Brooks, C.L., III. Flexible CDOCKER: Development and application of a pseudo-explicit structure-based docking method within CHARMM. J. Comput. Chem. 2016, 37, 753–762. [Google Scholar] [CrossRef]
- Liu, C.L.; Xue, K.; Yang, Y.; Liu, X.; Li, Y.; Lee, T.S.; Bai, Z.; Tan, T. Metabolic engineering strategies for sesquiterpene production in microorganism. Crit. Rev. Biotechnol. 2022, 42, 73–92. [Google Scholar] [CrossRef]
- Takahashi, S.; Yeo, Y.; Greenhagen, B.T.; McMullin, T.; Song, L.; Maurina-Brunker, J.; Rosson, R.; Noel, J.P.; Chappell, J. Metabolic engineering of sesquiterpene metabolism in yeast. Biotechnol. Bioeng. 2007, 97, 170–181. [Google Scholar] [CrossRef]
- Choo, Y.M.; Xu, P.; Hwang, J.K.; Zeng, F.; Tan, K.; Bhagavathy, G.; Chauhan, K.R.; Leal, W.S. Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc. Natl. Acad. Sci. USA 2018, 115, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fu, N.; Li, D.; Chang, H.; Qu, C.; Wang, R.; Xu, Y.; Luo, C. Identification of an Alarm Pheromone-Binding Chemosensory Protein From the Invasive Sycamore Lace Bug Corythucha ciliata (Say). Front. Physiol. 2018, 9, 354. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Dewer, Y.; Qu, C.; Yang, Z.; Tian, J.; Luo, C. Discrimination of Oviposition Deterrent Volatile β-Ionone by Odorant-Binding Proteins 1 and 4 in the Whitefly Bemisia tabaci. Biomolecules 2019, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Li, D.; Dewer, Y.; Niu, C.; Li, F.; Luo, C. Identification and functional characterization of odorant-binding proteins 69a and 76a of Drosophila suzukii. Heliyon 2021, 7, e06427. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular Basis of Alarm Pheromone Detection in Aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Z.; Tang, R.; Zhang, J.P.; Yang, S.Y.; Chen, G.H.; He, K.L.; Wang, Z.Y.; Zhang, F. Behavioral Evidence and Olfactory Reception of a Single Alarm Pheromone Component in Halyomorpha halys. Front. Physiol. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
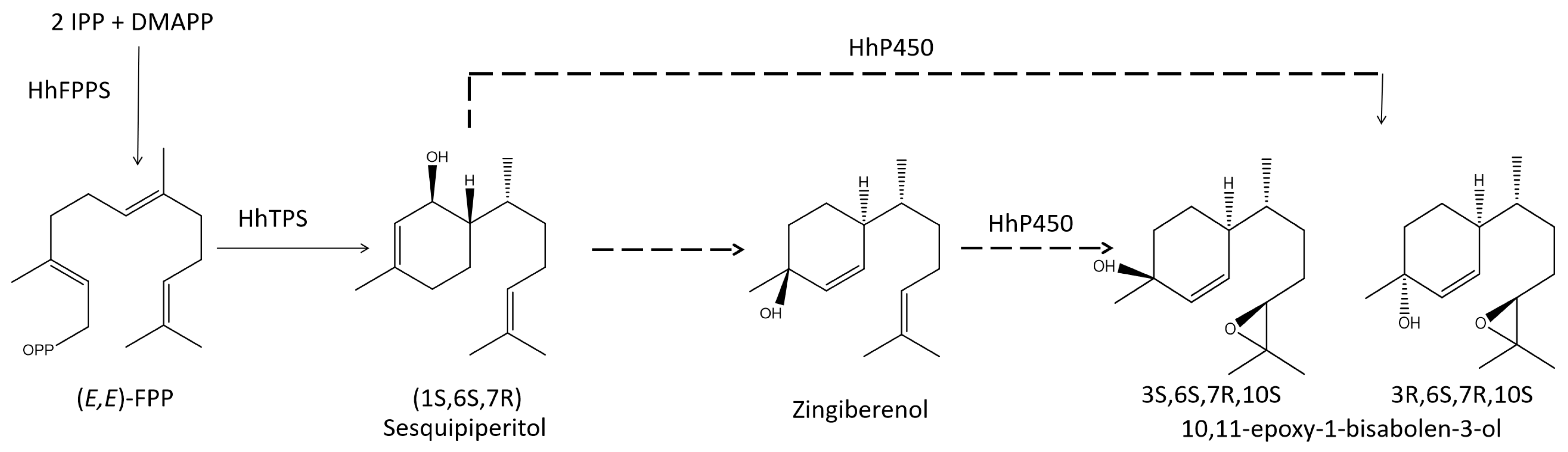
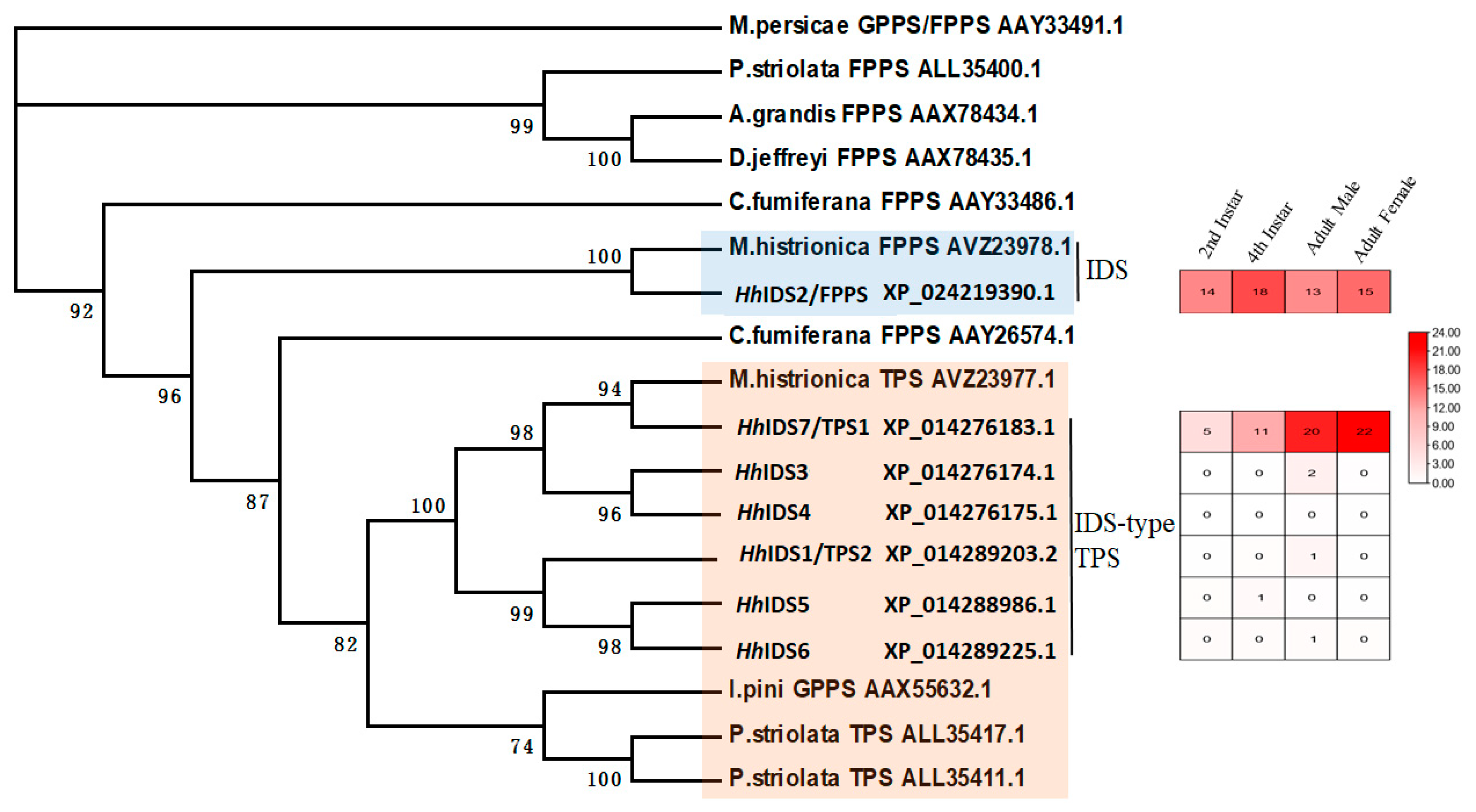
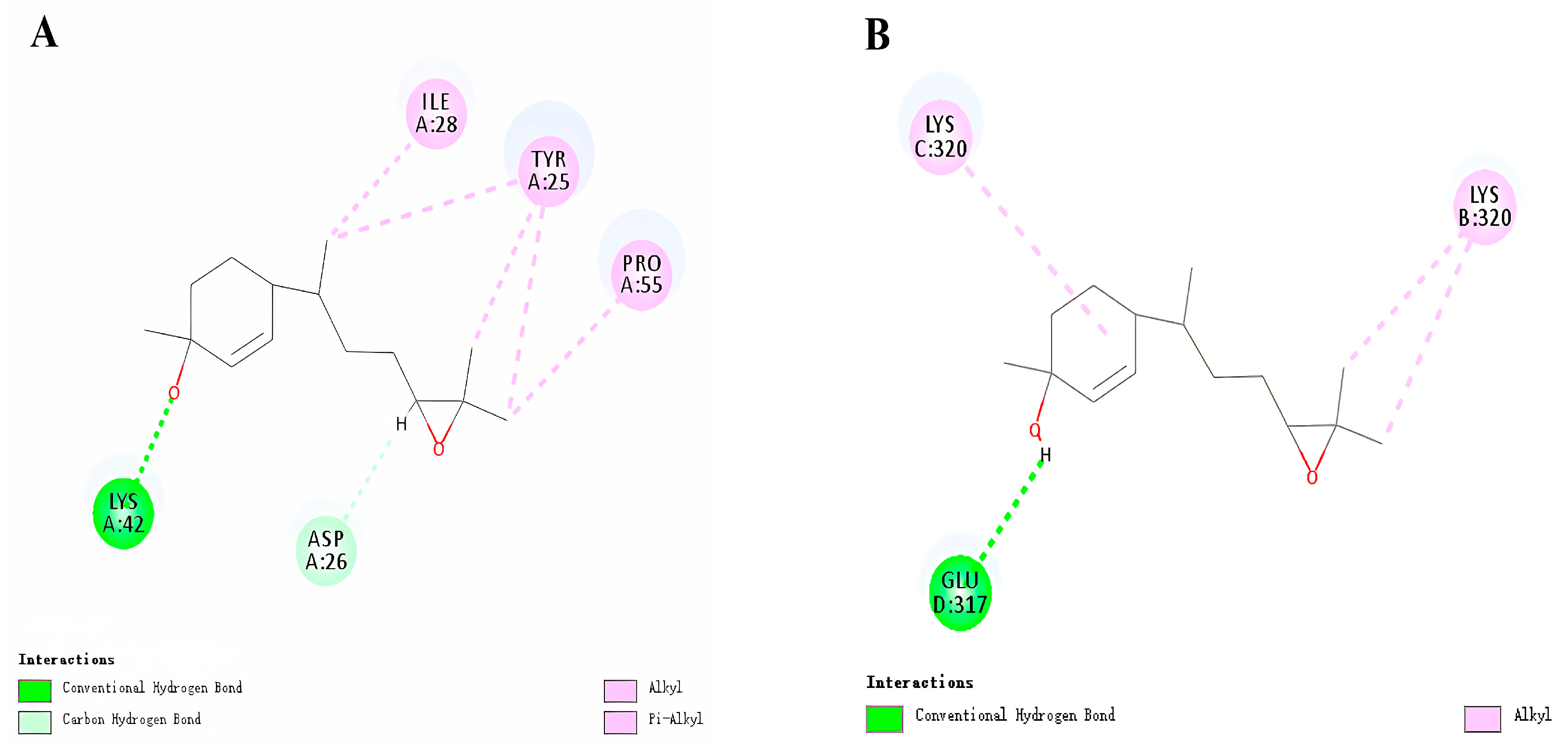
| Potential Function | Coexpressed Genes | Weight Values | Annotation |
|---|---|---|---|
| Synthase genes in aggregation pheromone biosynthesis pathway | XM_024359849.1 | 0.030 | cytochrome P450 6a14 (LOC106686595) |
| XM_014425704.2 | 0.080 | cytochrome P450 6j1 (LOC106683935) | |
| XM_014435358.2 | 0.068 | cytochrome P450 6a2-like (LOC106690077) | |
| XM_024361356.1 | 0.128 | cytochrome P450 6j1-like (LOC106678797) | |
| XM_024361357.1 | 0.184 | cytochrome P450 6j1-like (LOC106678797) | |
| XM_024362540.1 | 0.176 | cytochrome P450 6a2-like (LOC106678035) | |
| XM_014428854.1 | 0.092 | cytochrome P450 4C1 (LOC106685872) | |
| Transcriptional factors in aggregation pheromone biosynthesis pathway | XM_024361675.1 | 0.040 | zinc finger protein 711-like (LOC106678825) |
| XM_024362041.1 | 0.211 | zinc finger MIZ domain-containing protein 1-like (LOC106691938) | |
| XM_024359956.1 | 0.177 | zinc finger FYVE domain-containing protein 1-like (LOC106682986) | |
| XM_024360254.1 | 0.175 | putative homeodomain transcription factor (LOC106692479) | |
| XM_014438343.2 | 0.11 | zinc finger BED domain-containing protein 4-like (LOC106692406) | |
| XM_014438440.2 | 0.206 | RING finger protein 37 (LOC106692477) | |
| XM_014438969.1 | 0.039 | transcriptional regulatory protein AlgP (LOC106692793) | |
| XM_014433039.2 | 0.083 | transcription factor kayak (LOC106688534) | |
| XM_014433901.1 | 0.055 | zinc finger autosomal protein-like (LOC106689113) | |
| XM_014435086.2 | 0.108 | zinc finger autosomal protein-like (LOC106689884) | |
| XM_014435137.2 | 0.215 | zinc finger protein 711-like (LOC106689915) | |
| XM_014430570.2 | 0.054 | zinc finger protein 420 (LOC106686950) | |
| XM_014431700.2 | 0.137 | coiled-coil-helix-coiled-coil-helix domain-containing protein 1 (LOC106687684) | |
| XM_014425878.2 | 0.058 | coiled-coil-helix-coiled-coil-helix domain-containing protein 2-like (LOC106684044) | |
| XM_014427582.2 | 0.053 | zinc finger MYM-type protein 1 (LOC106685089) | |
| XM_014428786.2 | 0.077 | zinc finger protein 629-like (LOC106685840) | |
| XM_014432021.2 | 0.222 | transcription factor A, mitochondrial (LOC106687869) | |
| Recognition and transport aggregation pheromones | XM_024358492.1 | 0.178 | odorant receptor 85b-like (LOC106692425) |
| XM_014421632.2 | 0.124 | chemosensory protein 5 [Nezara viridula] (LOC106681357) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, F.; Dewer, Y.; Zhang, J.; Li, F. Exploration of Candidate Genes Involved in the Biosynthesis, Regulation and Recognition of the Male-Produced Aggregation Pheromone of Halyomorpha halys. Insects 2023, 14, 163. https://doi.org/10.3390/insects14020163
Wu C, Zhang F, Dewer Y, Zhang J, Li F. Exploration of Candidate Genes Involved in the Biosynthesis, Regulation and Recognition of the Male-Produced Aggregation Pheromone of Halyomorpha halys. Insects. 2023; 14(2):163. https://doi.org/10.3390/insects14020163
Chicago/Turabian StyleWu, Chunyan, Feng Zhang, Youssef Dewer, Jinping Zhang, and Fengqi Li. 2023. "Exploration of Candidate Genes Involved in the Biosynthesis, Regulation and Recognition of the Male-Produced Aggregation Pheromone of Halyomorpha halys" Insects 14, no. 2: 163. https://doi.org/10.3390/insects14020163
APA StyleWu, C., Zhang, F., Dewer, Y., Zhang, J., & Li, F. (2023). Exploration of Candidate Genes Involved in the Biosynthesis, Regulation and Recognition of the Male-Produced Aggregation Pheromone of Halyomorpha halys. Insects, 14(2), 163. https://doi.org/10.3390/insects14020163





