Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Isolates in Terms of Efficacy against Cereal Ground Beetle Zabrus tenebrioides (Coleoptera: Carabidae): Morphometry and Principal Component Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Multiplication and Characterization of S. feltiae Isolates
2.2. Experimental Site
2.3. Assessment of Cereal Ground Beetle Abundance in Experimental Site
2.4. Detection of Local, Wild EPN in Experimental Site
2.5. EPN Inoculation in Field Studies
2.6. Study of EPN Persistence, Nematode Infectivity, and Control of Z. tenebrioides
2.7. Statistical and Data Analysis
3. Results
3.1. Presence of Local EPN Populations in the Experimental Site
3.2. Assessment of the Frequency of Z. tenebrioides in the Experimental Site
3.3. Assessment of the Efficacy of the Tested EPN Isolates against Z. tenebrioides
3.4. EPN Persistence and Infectivity in the Soil
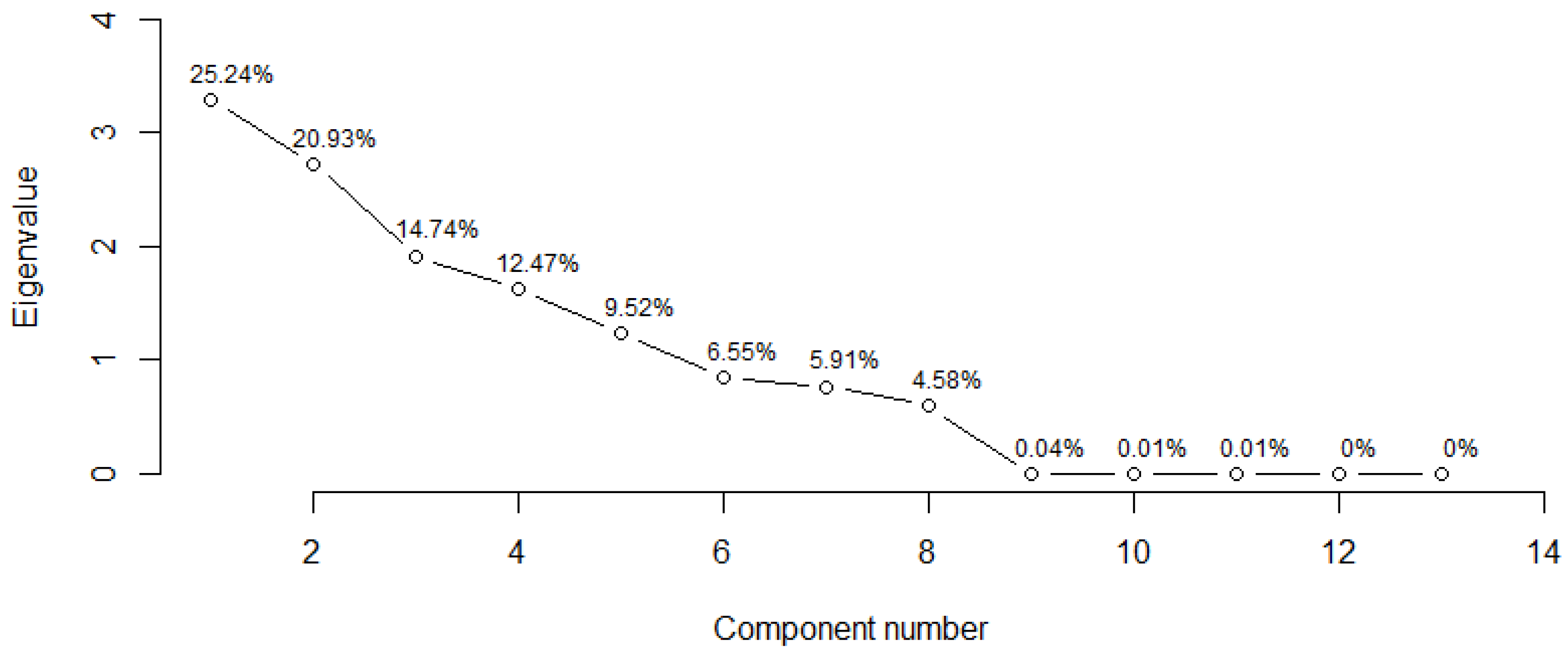
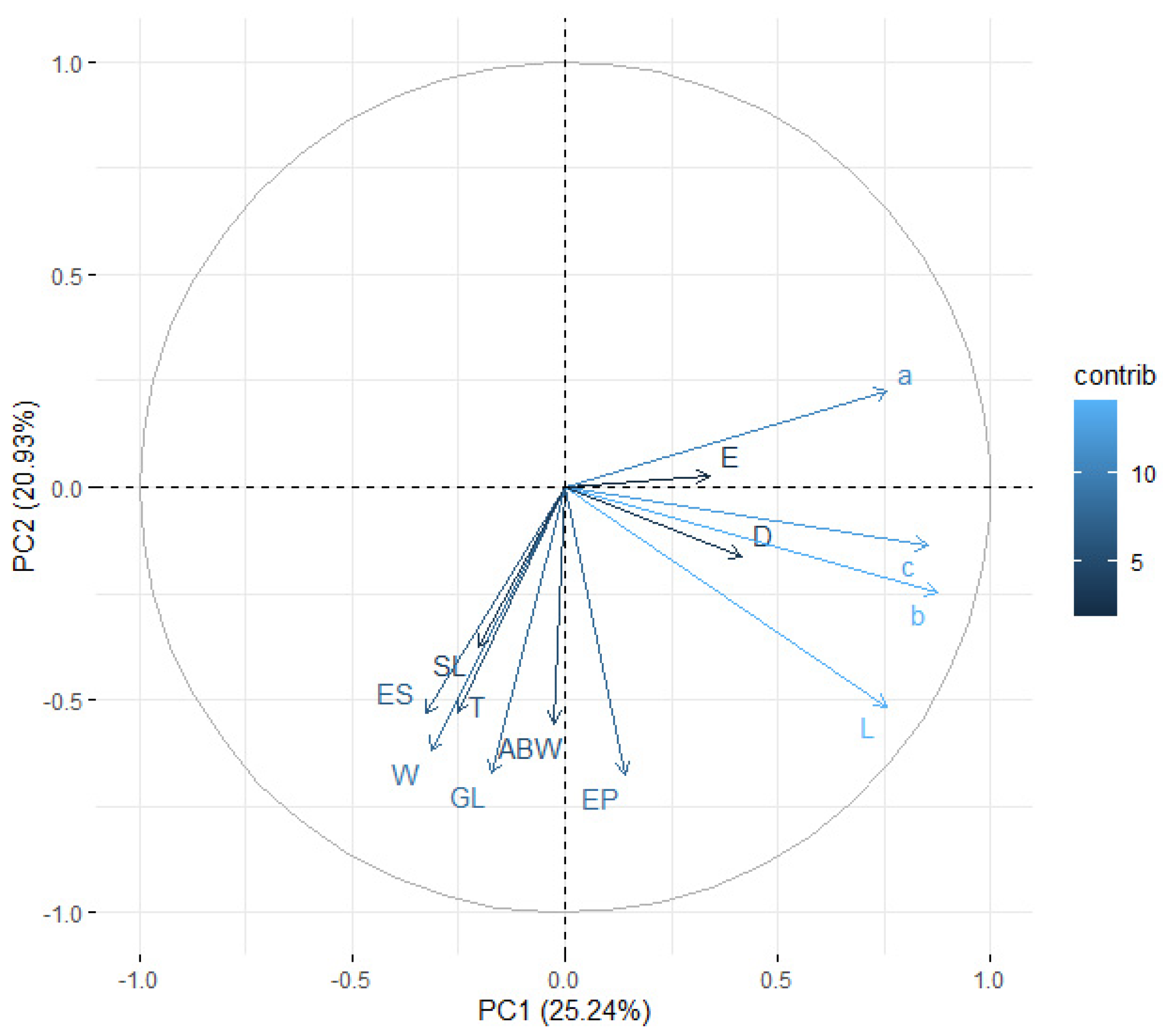
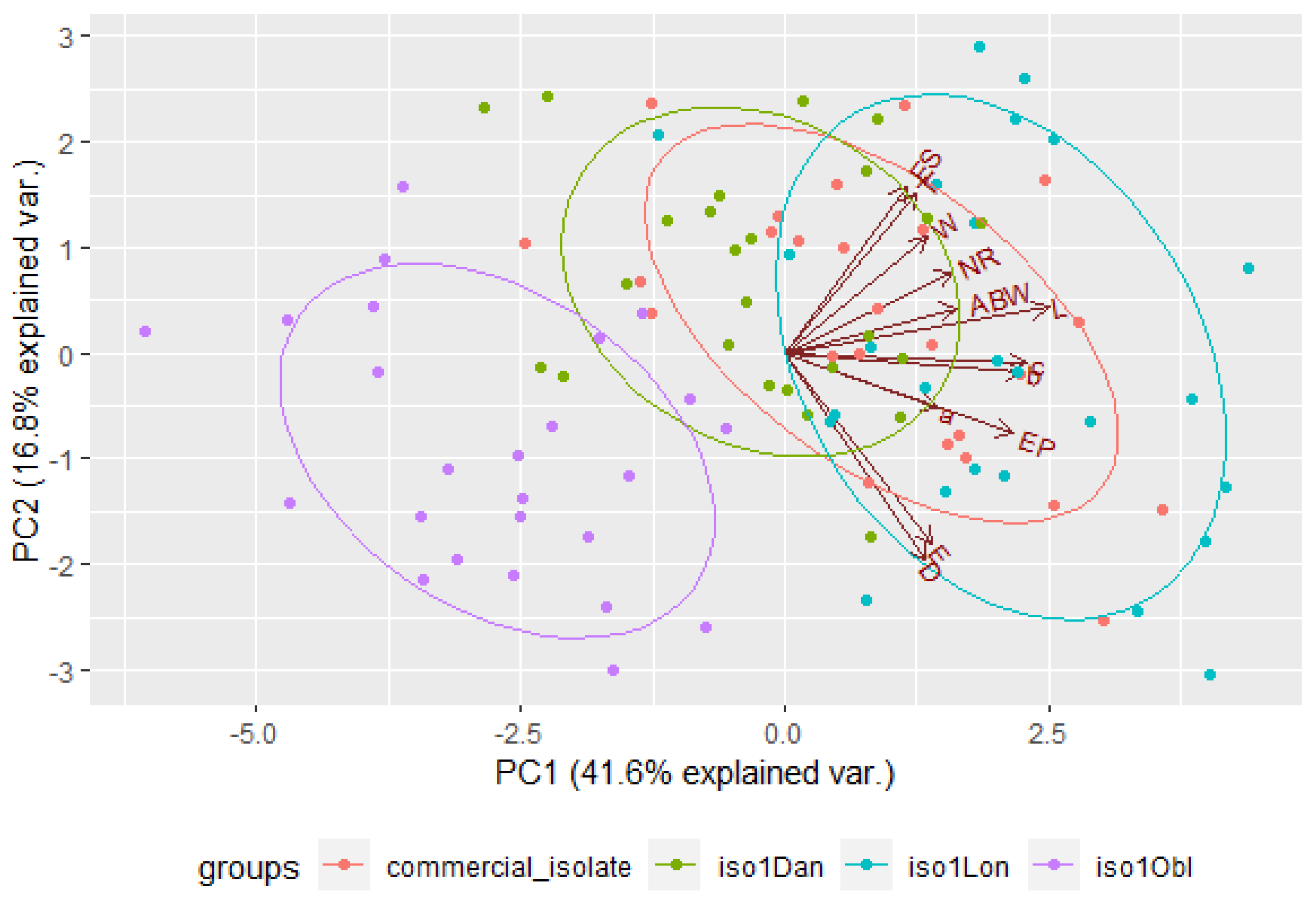
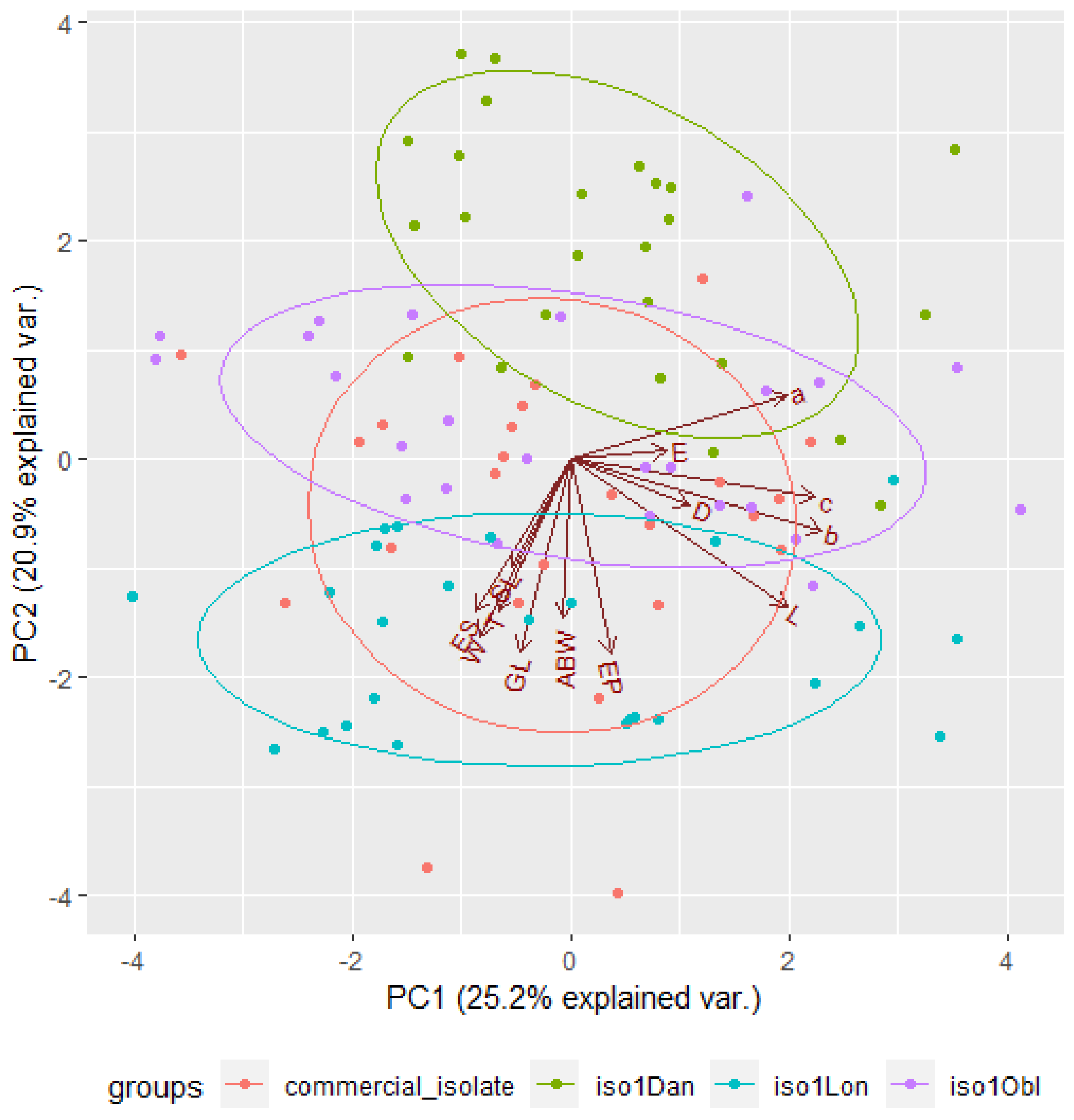
3.5. Distinction among EPN Isolates Using Morphometric Features and PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askary, T.H. Nematodes as Biocontrol Agents. In Sociology, Organic Farming, Climate Change and Soil Science; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 347–378. ISBN 978-90-481-3333-8. [Google Scholar]
- Silva, M.S.O.; Cardoso, J.F.M.; Ferreira, M.E.P.; Baldo, F.B.; Silva, R.S.A.; Chacon-Orozco, J.G.; Shapiro-Ilan, D.I.; Hazir, S.; Bueno, C.J.; Leite, L.G. An Assessment of Steinernema Rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus Levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy. Agriculture 2021, 11, 500. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, D.; Vk, C. Use of Steinernema and Heterorhabditis Nematodes for Control of White Grubs, Brahmina Coriacea Hope (Coleoptera: Scarabaeidae) in Potato Crop. Potato J. 2009, 36, 60–65. [Google Scholar]
- Vänninen, I. Control of Sciarid Flies with Steinernema Feltiae in Poinsettia Cutting Production. Int. J. Pest Manag. 2003, 49, 95–103. [Google Scholar] [CrossRef]
- FAO: Economic and Social Development Stream. Available online: https://www.fao.org/economic/es-home/en/ (accessed on 22 October 2022).
- Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 22 October 2022).
- Jalli, M.; Huusela, E.; Jalli, H.; Kauppi, K.; Niemi, M.; Himanen, S.; Jauhiainen, L. Effects of Crop Rotation on Spring Wheat Yield and Pest Occurrence in Different Tillage Systems: A Multi-Year Experiment in Finnish Growing Conditions. Front. Sustain. Food Syst. 2021, 5, 647335. [Google Scholar] [CrossRef]
- Jha, P.K.; Araya, A.; Stewart, Z.P.; Faye, A.; Traore, H.; Middendorf, B.J.; Prasad, P.V.V. Projecting Potential Impact of COVID-19 on Major Cereal Crops in Senegal and Burkina Faso Using Crop Simulation Models. Agric. Syst. 2021, 190, 103107. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of Climate Change on the Yield of Cereal Crops: A Review. Climate 2018, 6, 41. [Google Scholar] [CrossRef]
- You, L.; Rosegrant, M.W.; Wood, S.; Sun, D. Impact of Growing Season Temperature on Wheat Productivity in China. Agric. For. Meteorol. 2009, 149, 1009–1014. [Google Scholar] [CrossRef]
- Farrell, J.A.; Stufkens, M.W. Cereal Aphid Flights and Barley Yellow Dwarf Virus Infection of Cereals in Canterbury, New Zealand. N. Z. J. Crop Hortic. Sci. 1992, 20, 407–412. [Google Scholar] [CrossRef]
- Dhawan, A.K.; Peshin, R. Integrated Pest Management: Concept, Opportunities and Challenges. In Integrated Pest Management: Innovation-Development Process: Volume 1; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, Netherlands, 2009; pp. 51–81. ISBN 978-1-4020-8992-3. [Google Scholar]
- Olszak, R.W.; Pruszynski, S.; Lipa, J.J.; Dabrowski, Z.T. Rozwoj koncepcji i strategii wykorzystania metod oraz srodkow ochrony roslin. Prog. Plant Prot. 2000, 40, 40–50. [Google Scholar]
- Kaniuczak, Z.; Bereś, P. Występowanie oraz szkodliwość ważnych gospodarczo szkodników zbóż w gospodarstwach ekologicznych na Podkarpaciu w latach 2008–2010. J. Res. Appl. Agric. Eng. 2011, 56, 189–195. [Google Scholar]
- Mrówczynski, M.; Pruszyński, G.; Wachowiak, H.; Bereś, P. Nowe Zagrozenia Upraw Rolniczych Przez Szkodniki Ze Szczegolnym Uwzglednieniem Kukurydzy. Prog. Plant Prot. 2007, 47, 323–330. [Google Scholar]
- Mrówczynski, M.; Wachowiak, H.; Boroń, M. Szkodniki Zbóż—Aktualne Zagrożenia w Polsce. Prog. Plant Prot. 2005, 45, 929–932. [Google Scholar]
- Roik, K.; Strażyński, P.; Baran, M.; Bocianowski, J. Analiza Poziomu Zasiedlenia Pszenicy Ozimej Przez Mszycę Zbożową (Sitobion Avenae F.) w Różnych Rejonach Polski w Latach 2009–2018. Prog. Plant Prot. 2022, 62, 216–223. [Google Scholar]
- Skuhravá, M.; Skuhravý, V.; Skrzypczyńska, M.; Szadziewski, R. Gall Midges (Cecidomyiidae, Diptera) of Poland. Ann. Up. Silesian Mus. Bytom 2008, 16, 5–159. [Google Scholar]
- Hurej, M.; Twardowski, J.; Chrzanowska-Drożdż, B. THRIPS (Thysanoptera) occuring in ears of Triticum Durum DESF. in conditions of different protection level. Acta Sci. Pol. Agric. 2010, 9, 3–10. [Google Scholar]
- Ulrich, W.; Czarnecki, A.; Kruszyński, T. Occurrence of pest species of the genus oulema (Coleoptera: Chrysomelidae) in cereal fields in northern poland. Electron. J. Pol. Agric. Univ. 2004, 7, 4. [Google Scholar]
- Walczak, F. Ważne Szkodniki Zbóż i Terminy Ich Zwalczania. Wieś Jutra 2010, 4, 30–34. [Google Scholar]
- Szyszko, J. Mozliwosci Wykorzystania Biegaczowatych [Carabidae, Col.] Do Oceny Zaawansowania Procesow Sukcesyjnych w Srodowisku Lesnym-Aspekty Gospodarcze. Sylwan 2002, 146, 45–59. [Google Scholar]
- Georgescu, E.; Rîșnoveanu, L.; Toader, M.; Ionescu, A.M.; Gărgăriță, R.; Cană, L. Actual Problems Concerning Protection of the Wheat Crops against Cereal Ground Beetle (Zabrus Tenebrioides Goeze) Attack in South-East of the Romania. Sci. Pap.-Ser. Agron. 2017, 60, 256–263. [Google Scholar]
- Jasim, S.A.; Yasin, G.; Cartono, C.; Sevbitov, A.; Shichiyakh, R.A.; Al-Husseini, Y.; Mustafa, Y.F.; Jalil, A.T.; Iswanto, A.H. Survey of Ground Beetles Inhabiting Agricultural Crops in South-East Kazakhstan. Braz. J. Biol. Rev. Brasleira Biol. 2022, 84, e260092. [Google Scholar] [CrossRef]
- Korbas, M.; Horoszkiewicz-Janka, J.; Mrówczyński, M. Metodyka Integrowanej Ochrony Pszenicy Ozimej i Jarej Dla Doradców; Instytut Ochrony Roślin-PIB: Poznań, Poland, 2017; ISBN 978-83-64655-33-3. [Google Scholar]
- Kosewska, A.; Nijak, K. Analiza Struktury Zgrupowań Biegaczowatych (Col., Carabidae) w Integrowaniej i Ekologicznej Uprawie Ziemniaka. Komunikat. Biul. Inst. Hod. Aklim. Roślin 2012, 265, 157–164. [Google Scholar]
- Strażyński, P.; Horoszkiewicz-Janka, J.; Mrówczyński, M.; Przybył, J.; Węgorek, P.; Kierzek, R.; Korbas, M.; Matysiak, K.; Grabiński, J.; Zamojska, J.; et al. Metodyka Integrowanej Ochrony Żyta Dla Doradców; Instytut Ochrony Roślin–PIB: Poznań, Poland, 2020; ISBN 978-83-64655-64-7. [Google Scholar]
- Tratwal, A.; Bereś, P.; Korbas, M.; Danielewicz, J.; Jajor, E.; Horoszkiewicz, J.; Jakubowska, M.; Roik, K.; Baran, M.; Strażyński, P.; et al. Poradnik Sygnalizatora Ochrony Zbóż; Instytut Ochrony Roślin-PIB: Poznań, Poland, 2017; ISBN 978-83-64655-29-6. [Google Scholar]
- Collins, P.J.; Schlipalius, D.I. Insecticide Resistance. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 169–182. ISBN 978-3-662-56125-6. [Google Scholar]
- Hominick, W.M. Biogeography. Entomopathog. Nematol. 2002, 1, 115–143. [Google Scholar]
- Hominick, W.M.; Reid, A.P.; Bohan, D.A.; Briscoe, B.R. Entomopathogenic Nematodes: Biodiversity, Geographical Distribution and the Convention on Biological Diversity. Biocontrol Sci. Technol. 1996, 6, 317–332. [Google Scholar] [CrossRef]
- Nguyen, K.B. Chapter 3. Methodology, Morphology And Identification. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Brill: Leiden, The Netherlands, 2007; pp. 59–119. ISBN 978-90-474-2239-6. [Google Scholar]
- Bhat, A.H.; Sharma, L.; Chaubey, A.K. Characterisation of Steinernema Surkhetense and Its Symbiont Xenrorhabdus Stockiae and A Note on Its Geographical Distribution Characterisation of Steinernema Surkhetense and Its Symbiont Xenrorhabdus Stockiae and A Note on Its Geographical Distribution. Egypt. Acad. J. Biol. Sci. Entomol. 2020, 13, 105–122. [Google Scholar] [CrossRef]
- Grinang, J.; Das, I.; Ng, P.K.L. Geometric Morphometric Analysis in Female Freshwater Crabs of Sarawak (Borneo) Permits Addressing Taxonomy-Related Problems. PeerJ 2019, 7, e6205. [Google Scholar] [CrossRef]
- Putra, W.; Said, S.; Arifin, J. Principal Component Analysis (PCA) of Body Measurements and Body Indices in the Pasundan Cows. Black Sea J. Agric. 2020, 3, 49–55. [Google Scholar]
- Putra, W.; Ilham, F. Principal Component Analysis of Body Measurements and Body Indices and Their Correlation with Body Weight in Katjang Does of Indonesia. J. Dairy Veter- Anim. Res. 2019, 8, 124–134. [Google Scholar] [CrossRef]
- Stock, S.P.; Gardner, S.L.; Wu, F.F.; Kaya, H.K. Characterization of Two Steinernema Scapterisci Populations (Nemata: Steinernematidae) Using Morphology and Random Amplified Polymorphic DNA Markers. J. Helminthol. Soc. Wash. 1995, 62, 242–249. [Google Scholar]
- Winstanley, T.; Clements, K. Morphological Re-Examination and Taxonomy of the Genus Macropodus (Perciformes, Osphronemidae). Zootaxa 2008, 1908, 1–27. [Google Scholar] [CrossRef]
- Grewal, P.S.; Ehlers, R.-U.; Shapiro-Ilan, D.I. Nematodes as Biocontrol Agents; CABI: Wallingford, UK, 2005; ISBN 1-84593-142-4. [Google Scholar]
- Shapiro-Ilan, D.I.; Gouge, D.H.; Koppenhöfer, A.M. Factors Affecting Commercial Success: Case Studies in Cotton, Turf and Citrus. In Entomopathogenic Nematology; CABI: Wallingford, UK, 2002; pp. 333–355. [Google Scholar]
- Matuska-Łyżwa, J.; Żarnowiec, P.; Kaca, W. Comparison of Biological Activity of Field Isolates of Steinernema Feltiae with a Commercial S. Feltiae Biopesticide. Product. Insects 2021, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Matuska-Łyżwa, J. Method of Multiplication of Entomopathogenic Nematodes for the Plant Protection Research Purposes. WUP Patent PL 212617 B1, April 2012. [Google Scholar]
- Fan, X.; Hominick, W.M. Efficiency of the Galleria (Wax Moth) Baiting Technique for Recovering Infective Stages of Entomopathogenic Rhabditids (Steinernematidae and Heterorhabditidae) from Sand and Soil. Rev. Nématol 1991, 14, 381–387. [Google Scholar]
- Billard, L.; Diday, E. Symbolic Data Analysis: Conceptual Statistics and Data Mining (Wiley Series in Computational Statistics); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 0-470-09016-2. [Google Scholar]
- Izenman, A.J. Modern Multivariate Statistical Techniques: Regression, Classification, and Manifold Learning, 1st ed.; Springer Publishing Company, Incorporated: Berlin/Heidelberg, Germany, 2008; ISBN 0-387-78188-9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; 2022. [Google Scholar]
- Vu, V.Q. Ggbiplot: A Ggplot2 Based Biplot; 2011. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; 2020. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, Illinois, 2021. [Google Scholar]
- Gulzar, S.; Wakil, W.; Shapiro-Ilan, D.I. Potential Use of Entomopathogenic Nematodes against the Soil Dwelling Stages of Onion Thrips, Thrips Tabaci Lindeman: Laboratory, Greenhouse and Field Trials. Biol. Control 2021, 161, 104677. [Google Scholar] [CrossRef]
- Georgis, R.; Koppenhöfer, A.M.; Lacey, L.A.; Bélair, G.; Duncan, L.W.; Grewal, P.S.; Samish, M.; Tan, L.; Torr, P.; van Tol, R.W.H.M. Successes and Failures in the Use of Parasitic Nematodes for Pest Control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Levy, N.; Faigenboim, A.; Salame, L.; Molina, C.; Ehlers, R.-U.; Glazer, I.; Ment, D. Characterization of the Phenotypic and Genotypic Tolerance to Abiotic Stresses of Natural Populations of Heterorhabditis Bacteriophora. Sci. Rep. 2020, 10, 10500. [Google Scholar] [CrossRef]
- Taşkesen, Y.; Yüksel, E.; Canhilal, R. Field Performance of Entomopathogenic Nematodes against the Larvae of Zabrus Spp. Clairville, 1806 (Coleoptera: Carabidae). Uluslar. Tarım Ve Yaban Hayatı Bilim. Derg. 2021, 7, 429–437. [Google Scholar] [CrossRef]
- Mráček, Z.; Bečvár, S.; Kindlmann, P.; Webster, J. Infectivity and Specificity of Canadian and Czech Isolates of Steinernema Kraussei (Steiner, 1923) to Some Insect Pests at Low Temperatures in the Laboratory. Nematologica 1998, 44, 437–448. [Google Scholar]
- Dillon, A.B.; Rolston, A.N.; Meade, C.V.; Downes, M.J.; Griffin, C.T. Establishment, Persistence, and Introgression of Entomopathogenic Nematodes in a Forest Ecosystem. Ecol. Appl. 2008, 18, 735–747. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Escuer, M.; Robertson, L.; Gutiérrez, C. Morphological and Ecological Characterization of Steinernema Feltiae (Rhabditida: Steinernematidae) Rioja Strain Isolated from Bibio Hortulanus (Diptera: Bibionidae) in Spain. J. Nematol. 2006, 38, 68–75. [Google Scholar]
- Stock, S.P.; Mrácek, Z.; Webster, J. Morphological Variation between Allopatric Populations of Steinernema Kraussei (Steiner, 1923) (Rhabditida: Steinernematidae). Nematology 2000, 2, 143–152. [Google Scholar] [CrossRef]
- Achinelly, M.F.; Eliceche, D.P.; Belaich, M.N.; Ghiringhelli, P.D. Variability Study of Entomopathogenic Nematode Populations (Heterorhabditidae) from Argentina. Braz. J. Biol. 2016, 77, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Dolinski, C.; Kamitani, F.; Machado, I.; Winter, C. Molecular and Morphological Characterization of Heterorhabditid Entomopathogenic Nematodes from the Tropical Rainforest in Brazil. Mem. Inst. Oswaldo Cruz. 2008, 103, 150–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, A.H.; Chaubey, A.K.; Shokoohi, E.; William Mashela, P. Study of Steinernema Hermaphroditum (Nematoda, Rhabditida), from the West Uttar Pradesh, India. Acta Parasitol. 2019, 64, 720–737. [Google Scholar] [CrossRef] [PubMed]


| Parameter | p-Value Shapiro–Wilk Test | Median | Mean | Standard Deviation (SD) |
|---|---|---|---|---|
| Live larvae of Z. tenebrioides | 0.08991 | 3 | 2.925 | 0.6857 |
| Plants damaged by Z. tenebrioides | 0.09016 | 5 | 4.725 | 1.2401 |
| Nematode Isolates | Parameter | Median | Mean | SD |
|---|---|---|---|---|
| Commercial isolate | Live larvae | 2 | 2.25 | 0.9105 |
| Plants damaged | 3 | 2.95 | 0.9987 | |
| iso1Dan | Live larvae | 2 | 2.05 | 0.6863 |
| Plants damaged | 3 | 2.85 | 0.7452 | |
| iso1Lon | Live larvae | 2 | 1.85 | 0.7452 |
| Plants damaged | 3 | 2.75 | 0.7864 | |
| iso1Obl | Live larvae | 3 | 3.25 | 0.9666 |
| Plants damaged | 5 | 5.25 | 1.0196 |
| Nematode Isolates | No. Live Larvae of Z. tenebrioides | No. Plants Damaged by Z. tenebrioides |
|---|---|---|
| Commercial isolate | 0.0158 | 0.0022 |
| iso1Dan | 0.0011 | 0.0012 |
| iso1Lon | 0.0012 | 0.0094 |
| iso1Obl | 0.0193 | 0.0110 |
| Commercial Isolate | iso1Dan | iso1Lon | iso1Obl | |
|---|---|---|---|---|
| Commercial isolate | — | 0.5382 | 0.1701 | 0.0031 |
| iso1Dan | 0.9425 | — | 0.3690 | 0.0002 |
| iso1Lon | 0.7398 | 0.7697 | — | 0.0001 |
| iso1Obl | 0.0000 | 0.0000 | 0.0000 | — |
| Days | Commercial Isolate | iso1Dan | iso1Lon | iso1Obl |
|---|---|---|---|---|
| 14 days | 97% | 100% | 100% | 92% |
| 60 days | 93% | 99% | 100% | 83% |
| L | W | EP | NR | ES | T | ABW | a | b | c | D | E | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | 1 | — | — | — | — | — | — | — | — | — | — | — |
| W | 0.484 | 1 | — | — | — | — | — | — | — | — | — | — |
| EP | 0.531 | 0.306 | 1 | — | — | — | — | — | — | — | — | — |
| NR | 0.418 | 0.336 | 0.470 | 1 | — | — | — | — | — | — | — | — |
| ES | 0.409 | 0.324 | 0.392 | 0.401 | 1 | — | — | — | — | — | — | — |
| T | 0.441 | 0.305 | 0.382 | 0.427 | 0.409 | 1 | — | — | — | — | — | — |
| ABW | 0.422 | 0.398 | 0.474 | 0.446 | 0.272 | 0.363 | 1 | — | — | — | — | — |
| a | 0.627 | −0.375 | 0.289 | 0.141 | 0.149 | 0.190 | 0.090 | 1 | — | — | — | — |
| b | 0.924 | 0.396 | 0.416 | 0.291 | 0.031 | 0.307 | 0.346 | 0.625 | 1 | — | — | — |
| c | 0.938 | 0.418 | 0.440 | 0.301 | 0.298 | 0.102 | 0.323 | 0.622 | 0.905 | 1 | — | — |
| D | 0.230 | 0.067 | 0.726 | 0.179 | −0.347 | 0.084 | 0.277 | 0.180 | 0.395 | 0.221 | 1 | — |
| E | 0.255 | 0.115 | 0.782 | 0.208 | 0.132 | −0.276 | 0.250 | 0.170 | 0.225 | 0.389 | 0.699 | 1 |
| L | W | EP | ES | T | ABW | SL | GL | a | b | c | D | E | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| W | 0.138 | 1 | — | — | — | — | — | — | — | — | — | — | — |
| EP | 0.261 | 0.206 | 1 | — | — | — | — | — | — | — | — | — | — |
| ES | 0.140 | 0.266 | 0.385 | 1 | — | — | — | — | — | — | — | — | — |
| T | 0.206 | 0.187 | 0.313 | 0.250 | 1 | — | — | — | — | — | — | — | — |
| ABW | 0.183 | 0.266 | 0.255 | 0.168 | 0.216 | 1 | — | — | — | — | — | — | — |
| SL | 0.015 | 0.217 | 0.115 | 0.270 | 0.035 | 0.144 | 1 | — | — | — | — | — | — |
| GL | 0.149 | 0.312 | 0.351 | 0.335 | 0.313 | 0.289 | 0.288 | 1 | — | — | — | — | — |
| a | 0.493 | −0.788 | −0.005 | −0.164 | −0.048 | −0.132 | −0.184 | −0.180 | 1 | — | — | — | — |
| b | 0.889 | 0.006 | 0.075 | −0.328 | 0.078 | 0.100 | −0.107 | −0.010 | 0.549 | 1 | — | — | — |
| c | 0.783 | 0.005 | 0.037 | −0.035 | −0.445 | 0.031 | −0.005 | −0.061 | 0.484 | 0.766 | 1 | — | — |
| D | 0.119 | −0.045 | 0.593 | −0.515 | 0.069 | 0.089 | −0.126 | 0.034 | 0.140 | 0.354 | 0.065 | 1 | — |
| E | −0.015 | −0.032 | 0.415 | 0.034 | −0.733 | −0.030 | 0.048 | −0.052 | 0.040 | −0.024 | 0.450 | 0.359 | 1 |
| -Value | KMO | |
|---|---|---|
| Infective juveniles | <2.2 | 0.6515 |
| Adult first-stage males | <2.2 | 0.5471 |
| Principal Component | Infective Juveniles | First-Stage Adult Males | ||||
|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | |
| 1 | 4.9861 | 41.5509 | 41.5509 | 3.2807 | 25.2360 | 25.2360 |
| 2 | 2.0182 | 16.8181 | 58.3690 | 2.7207 | 20.9288 | 46.1648 |
| 3 | 1.7501 | 14.5845 | 72.9535 | 1.9168 | 14.7448 | 60.9096 |
| 4 | 1.1261 | 9.3840 | 82.3375 | 1.6207 | 12.4673 | 73.3768 |
| 5 | 1.0005 | 8.3376 | 90.6751 | 1.2376 | 9.5198 | 82.8966 |
| 6 | 0.5920 | 4.9331 | 95.6081 | 0.8519 | 6.5534 | 89.4500 |
| 7 | 0.5231 | 4.3590 | 99.9671 | 0.7680 | 5.9079 | 95.3578 |
| 8 | 0.0027 | 0.0222 | 99.9893 | 0.5960 | 4.5849 | 99.9428 |
| 9 | 0.0005 | 0.0038 | 99.9931 | 0.0047 | 0.0362 | 99.9790 |
| 10 | 0.0004 | 0.0032 | 99.9963 | 0.0014 | 0.0109 | 99.9900 |
| 11 | 0.0003 | 0.0022 | 99.9985 | 0.0007 | 0.0055 | 99.9954 |
| 12 | 0.0002 | 0.0015 | 100 | 0.0004 | 0.0028 | 99.9982 |
| 13 | — | — | — | 0.0002 | 0.0018 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuska-Łyżwa, J.; Wodecka, B.; Kaca, W. Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Isolates in Terms of Efficacy against Cereal Ground Beetle Zabrus tenebrioides (Coleoptera: Carabidae): Morphometry and Principal Component Analysis. Insects 2023, 14, 150. https://doi.org/10.3390/insects14020150
Matuska-Łyżwa J, Wodecka B, Kaca W. Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Isolates in Terms of Efficacy against Cereal Ground Beetle Zabrus tenebrioides (Coleoptera: Carabidae): Morphometry and Principal Component Analysis. Insects. 2023; 14(2):150. https://doi.org/10.3390/insects14020150
Chicago/Turabian StyleMatuska-Łyżwa, Joanna, Barbara Wodecka, and Wiesław Kaca. 2023. "Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Isolates in Terms of Efficacy against Cereal Ground Beetle Zabrus tenebrioides (Coleoptera: Carabidae): Morphometry and Principal Component Analysis" Insects 14, no. 2: 150. https://doi.org/10.3390/insects14020150
APA StyleMatuska-Łyżwa, J., Wodecka, B., & Kaca, W. (2023). Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Isolates in Terms of Efficacy against Cereal Ground Beetle Zabrus tenebrioides (Coleoptera: Carabidae): Morphometry and Principal Component Analysis. Insects, 14(2), 150. https://doi.org/10.3390/insects14020150






